Development of a New Swine Model Resembling Human Empty Nose Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Surgical Procedure
2.3. Hematological Analysis
2.4. Scanning Electron Microscopy (SEM)
2.5. Tissue Processing
2.6. Histological Analysis
2.7. Immunohistochemistry (IHC) and Immunofluorescence (IF) Analysis
2.8. Quantitative and Statistical Analysis
3. Results
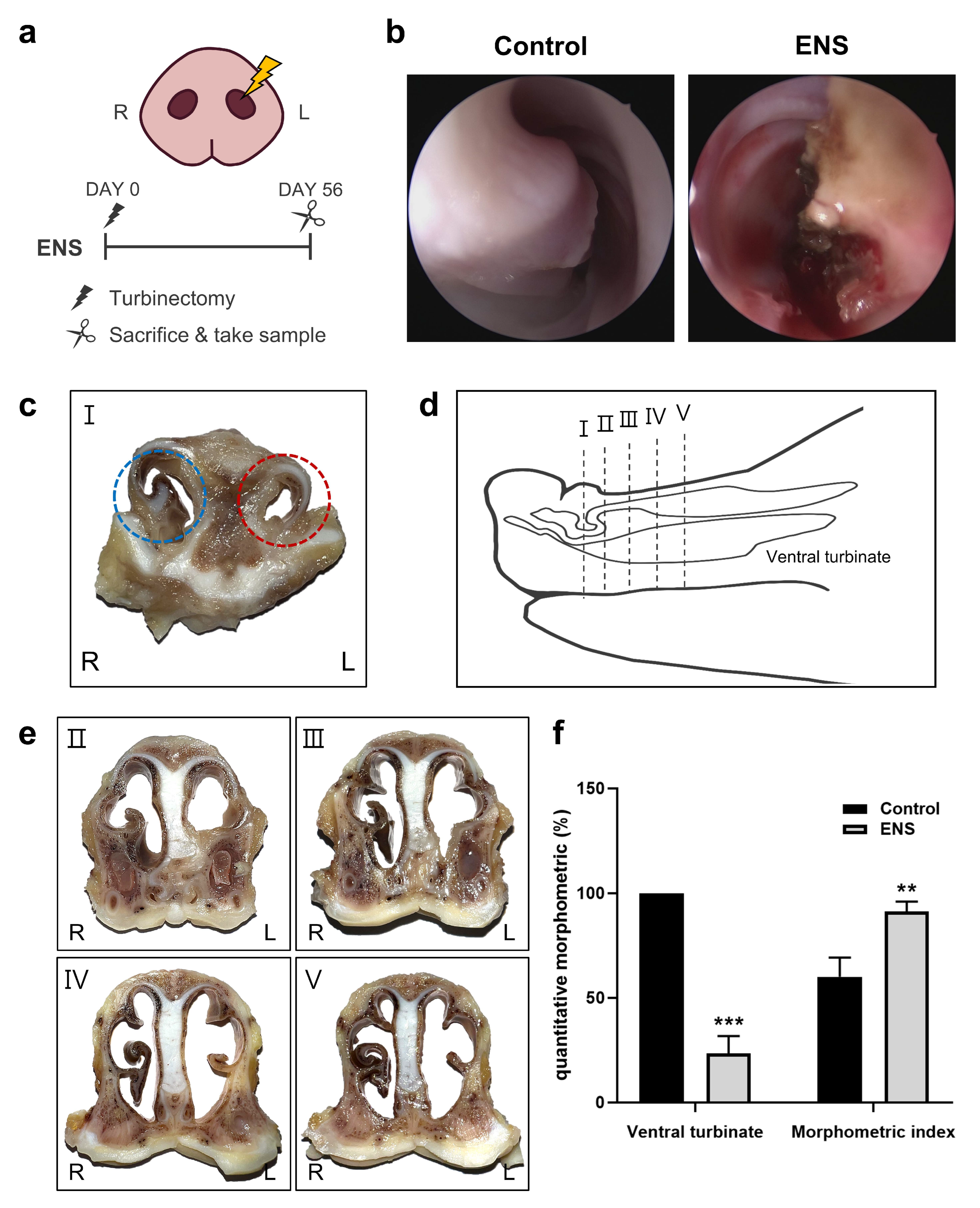
3.1. Morphological Changes in the Nasal Cavity in the ENS Model
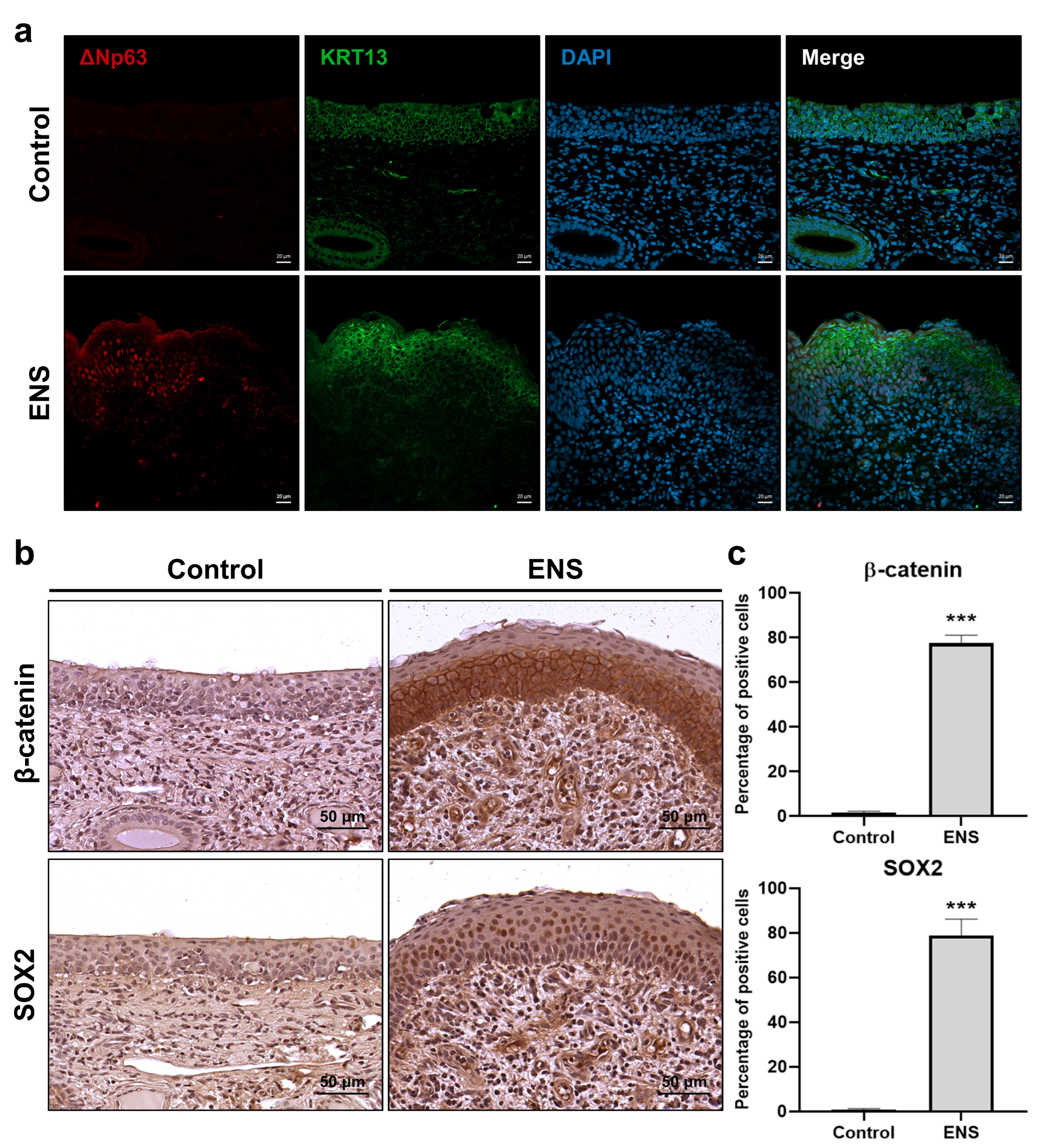
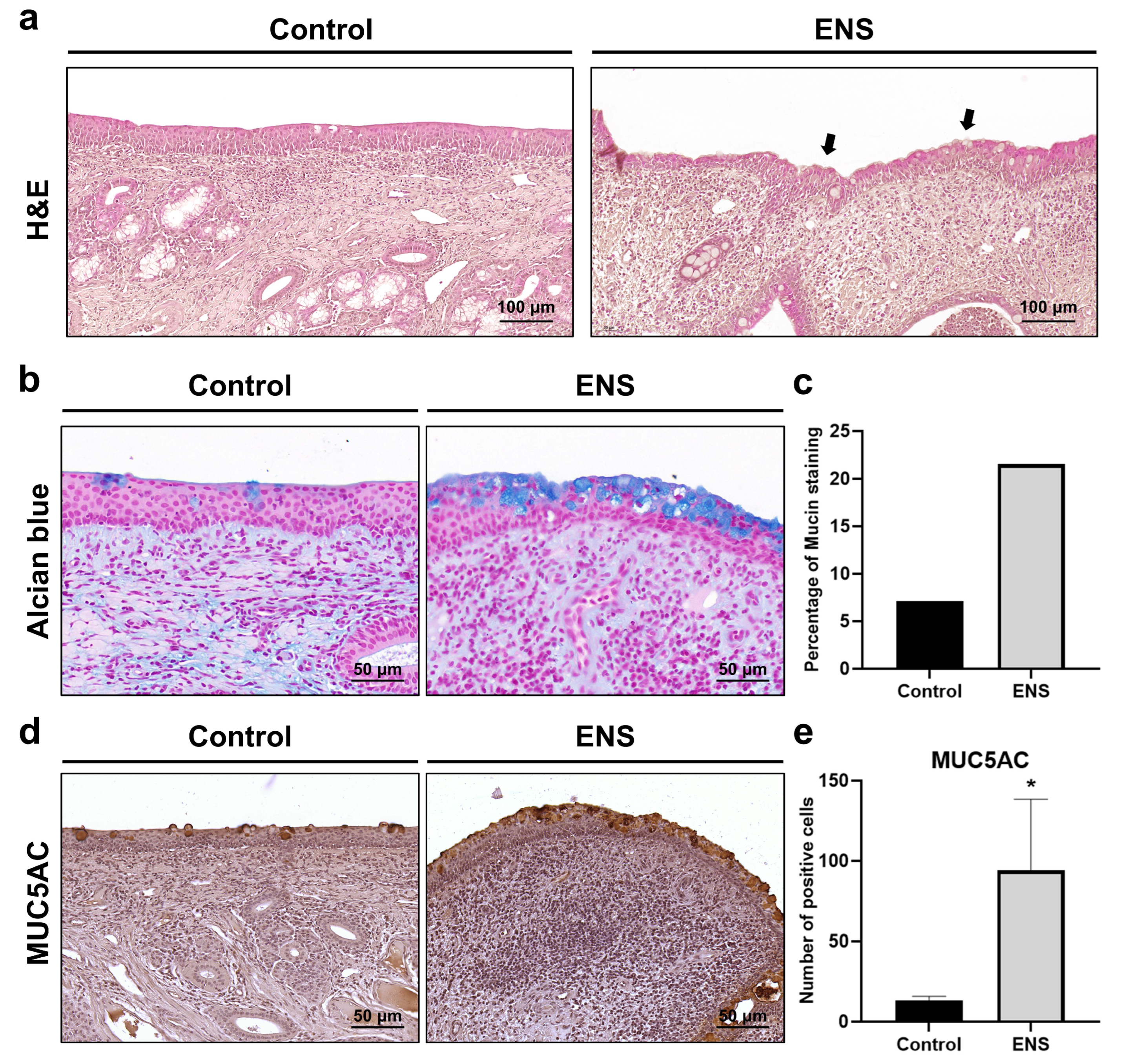
3.2. Histological Changes in the Mucosal Epithelium in the ENS Model
3.3. Histological Modifications in Submucosal Structure in the ENS Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Moore, E.J.; Kern, E.B. Atrophic rhinitis: A review of 242 cases. Am. J. Rhinol. 2001, 15, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, N.; Houser, S.M. The diagnosis and management of empty nose syndrome. Otolaryngol. Clin. North Am. 2009, 42, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Guarderas, J.; Krishnaswamy, G. Empty nose syndrome and atrophic rhinitis. Ann. Allergy Asthma Immunol. 2016, 117, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, D.A.; Thamboo, A.V. Empty Nose Syndrome as an Iatrogenic Condition from Surgery. Curr. Otorhinolaryngol. Rep. 2023, 11, 458–465. [Google Scholar] [CrossRef]
- Fu, C.H.; Chen, C.C.; Huang, C.C.; Chang, P.H.; Chen, Y.W.; Tang, Y.C.; Lee, T.J. Morphology, Not Only Volume: A Study on Empty Nose Syndrome and Inferior Turbinates. Laryngoscope 2024, 134, 3060–3066. [Google Scholar] [CrossRef]
- Kuan, E.C.; Suh, J.D.; Wang, M.B. Empty nose syndrome. Curr. Allergy Asthma Rep. 2015, 15, 493. [Google Scholar] [CrossRef]
- Talmadge, J.; Nayak, J.V.; Yao, W.; Citardi, M.J. Management of Postsurgical Empty Nose Syndrome. Facial Plast. Surg. Clin. North Am. 2019, 27, 465–475. [Google Scholar] [CrossRef]
- Leong, S.C. The clinical efficacy of surgical interventions for empty nose syndrome: A systematic review. Laryngoscope 2015, 125, 1557–1562. [Google Scholar] [CrossRef]
- Życzko, K.; Sielawa, H.; Łaszyn, M. Serum C-reactive protein (CRP) levels and the values of blood hematological indices in piglets. Sci. Ann. Pol. Soc. Anim. Prod. 2012, 8, 63–71. [Google Scholar]
- Yeom, S.C.; Cho, S.Y.; Park, C.G.; Lee, W.J. Analysis of reference interval and age-related changes in serum biochemistry and hematology in the specific pathogen free miniature pig. Lab. Anim. Res. 2012, 28, 245–253. [Google Scholar] [CrossRef]
- Hamilton, T.D.; Roe, J.M.; Webster, A.J. Synergistic role of gaseous ammonia in etiology of Pasteurella multocida-induced atrophic rhinitis in swine. J. Clin. Microbiol. 1996, 34, 2185–2190. [Google Scholar] [CrossRef] [PubMed]
- Sumaily, I.A.; Hakami, N.A.; Almutairi, A.D.; Alsudays, A.A.; Abulqusim, E.M.; Abualgasem, M.M.; Alghulikah, A.A.; Alserhani, A.A. An Updated Review on Atrophic Rhinitis and Empty Nose Syndrome. Ear Nose Throat J. 2023, 14, 1455613231185022. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, M.O. Surgery of the turbinates and “empty nose” syndrome. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2011, 9, 98–126. [Google Scholar] [CrossRef]
- Law, R.H.; Ahmed, A.M.; Van Harn, M.; Craig, J.R. Middle turbinate resection is unlikely to cause empty nose syndrome in first year postoperatively. Am. J. Otolaryngol. 2021, 42, 102931. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, Y. Swine atrophic rhinitis caused by pasteurella multocida toxin and bordetella dermonecrotic toxin. Curr. Top. Microbiol. Immunol. 2012, 361, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Balur, M.B.; Kocak, H.E.; Altinay, S.; Ozdamar, K.; Taskin, U.; Oktay, M.F. Is submucosal fat injection effective in atrophic rhinitis? An experimental animal study. Eur. Arch. Otorhinolaryngol. 2017, 274, 3637–3642. [Google Scholar] [CrossRef]
- Hamilton, T.D.C.; Roe, J.M.; Hayes, C.M.; Jones, P.; Pearson, G.R.; Webster, A.J.F. Contributory and Exacerbating Roles of Gaseous Ammonia and Organic Dust in the Etiology of Atrophic Rhinitis. Clin. Diagn. Lab. Immunol. 1999, 6, 199–203. [Google Scholar] [CrossRef]
- Magyar, T.; Donkó, T.; Repa, I.; Kovács, M. Regeneration of toxigenic Pasteurella multocida induced severe turbinate atrophy in pigs detected by computed tomography. BMC Vet. Res. 2013, 9, 222. [Google Scholar] [CrossRef]
- Allam, T.S.; Said, L.; Elsayed, M.S.A.E.; Saleh, N. Clinical Investigation of the Pathogenicity of Pasteurella multocida Isolated from Cattle in Egypt Regarding its Effect on Hematological, Biochemical, and Oxidant-Antioxidant Biomarkers as well as Proinflammatory Cytokines and Acute Phase Proteins. Adv. Anim. Vet. Sci. 2021, 9, 792–801. [Google Scholar] [CrossRef]
- Thakor, J.C.; Kumar, P.; Dinesh, M.; Vishwa, K.V.; Qureshi, S.; Singh, K.P.; Sahoo, M. Sequential development of pathology and pathogenesis of capsular serotype D of Pasteurella multocida of porcine origin in mouse model. Indian J. Vet. Pathol. 2020, 44, 69–80. [Google Scholar] [CrossRef]
- Canonne, A.M.; Menard, M.; Maurey, C.; Benchrekroun, G.; Fernandes Rodrigues, N.; Billen, F.; Clercx, C. Comparison of C-reactive protein concentrations in dogs with Bordetella bronchiseptica infection and aspiration bronchopneumonia. J. Vet. Intern. Med. 2021, 35, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Gordiienko, I.M.; Gubar, O.S.; Sulik, R.; Kunakh, T.; Zlatskiy, I.; Zlatska, A. Empty nose syndrome pathogenesis and cell-based biotechnology products as a new option for treatment. World J. Stem. Cells 2021, 13, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Dzhenkov, D.L.; Stoyanov, G.S.; Georgiev, R.; Sapundzhiev, N. Histopathological Findings in an Unclassifiable Case of Empty Nose Syndrome with Long-term Follow-up. Cureus 2018, 10, e2655. [Google Scholar] [CrossRef] [PubMed]
- Stavniichuk, R.; DeLaForest, A.; Thompson, C.A.; Miller, J.; Souza, R.F.; Battle, M.A. GATA4 blocks squamous epithelial cell gene expression in human esophageal squamous cells. Sci. Rep. 2021, 11, 3206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Y.; Guo, L.; Qian, J.; Ge, J.; Sinner, D.; Ding, H.; Califano, A.; Cardoso, W.V. Airway basal cells show regionally distinct potential to undergo metaplastic differentiation. eLife 2022, 11, e80083. [Google Scholar] [CrossRef]
- Mitani, Y.; Li, J.; Weber, R.S.; Lippman, S.L.; Flores, E.R.; Caulin, C.; El-Naggar, A.K. Expression and regulation of the DeltaN and TAp63 isoforms in salivary gland tumorigenesis clinical and experimental findings. Am. J. Pathol. 2011, 179, 391–399. [Google Scholar] [CrossRef]
- Samanta, A.; Saha, P.; Johnson, O.; Bishayee, A.; Sinha, D. Dysregulation of delta Np63 alpha in squamous cell carcinoma and its therapeutic targeting. Biochim. Biophy.s Acta Rev. Cancer 2024, 1879, 189034. [Google Scholar] [CrossRef]
- Moses, M.A.; George, A.L.; Sakakibara, N.; Mahmood, K.; Ponnamperuma, R.M.; King, K.E.; Weinberg, W.C. Molecular Mechanisms of p63-Mediated Squamous Cancer Pathogenesis. Int. J. Mol. Sci. 2019, 20, 3590. [Google Scholar] [CrossRef]
- Gatti, V.; Fierro, C.; Annicchiarico-Petruzzelli, M.; Melino, G.; Peschiaroli, A. ΔNp63 in squamous cell carcinoma: Defining the oncogenic routes affecting epigenetic landscape and tumour microenvironment. Mol. Oncol. 2019, 13, 981–1001. [Google Scholar] [CrossRef]
- McCravy, M.S.; Ingram, J.L. A Hint from Wnt: Squamous Cell Differentiation in the Airways. Am. J. Respir. Cell. Mol. Biol. 2023, 68, 601–602. [Google Scholar] [CrossRef]
- Zhang, Y.; Black, K.E.; Phung, T.N.; Thundivalappil, S.R.; Lin, T.; Wang, W.; Xu, J.; Zhang, C.; Hariri, L.P.; Lapey, A.; et al. Human Airway Basal Cells Undergo Reversible Squamous Differentiation and Reshape Innate Immunity. Am. J. Respir. Cell. Mol. Biol. 2023, 68, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, X.; Xiong, Q.; Han, J.; Zhu, Q. The dual role of p63 in cancer. Front. Oncol. 2023, 13, 1116061. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Giovannini, S.; Wang, T.; Fang, J.; Li, P.; Shao, C.; Wang, Y.; Shi, Y.; Candi, E.; TOR centre; et al. p63: A crucial player in epithelial stemness regulation. Oncogene 2023, 42, 3371–3384. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Ma, Q.; Peng, S.; Adelmant, G.; Swain, D.; Song, W.; Fox, C.; Francis, J.M.; Pedamallu, C.S.; DeLuca, D.S.; et al. SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. J. Clin. Investig. 2014, 124, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Fu, C.H.; Lee, T.J. Distinct Histopathology Characteristics in Empty Nose Syndrome. Laryngoscope 2021, 131, E14–E18. [Google Scholar] [CrossRef]
- Salazar, I.; Lombardero, M.; Cifuentes, J.M.; Sanchez Quinteiro, P.; Aleman, N. Morphogenesis and growth of the soft tissue and cartilage of the vomeronasal organ in pigs. J. Anat. 2003, 202, 503–514. [Google Scholar] [CrossRef]
- Beard, S. Rhinitis. Prim. Care 2014, 41, 33–46. [Google Scholar] [CrossRef]
- Orhan, M.; Govsa, F.; Saylam, C. A surgical view of the superior nasal turbinate: Anatomical study. Eur. Arch. Otorhinolaryngol. 2010, 267, 909–916. [Google Scholar] [CrossRef]
- Jing, J.Y.; Lei, D.; Qing, H.Y.; Qian, Y. Histological and anatomical structure of the nasal cavity of Bama minipigs. PLoS ONE 2017, 12, e0173902. [Google Scholar] [CrossRef]
- Marks, T.N.; Maddux, S.D.; Butaric, L.N.; Franciscus, R.G. Climatic adaptation in human inferior nasal turbinate morphology: Evidence from Arctic and equatorial populations. Am. J. Phys. Anthropol. 2019, 169, 498–512. [Google Scholar] [CrossRef]
- Yuk, J.; Akash, M.M.H.; Chakraborty, A.; Basu, S.; Chamorro, L.P.; Jung, S. Morphology of pig nasal structure and modulation of airflow and basic thermal conditioning. Integr. Comp. Biol. 2023, 63, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, N.; Thamboo, A.; Habib, A.R.; Huang, Z.; Nayak, J.V. The Empty Nose Syndrome 6-Item Questionnaire (ENS6Q): A validated 6-item questionnaire as a diagnostic aid for empty nose syndrome patients. Int. Forum Allergy Rhinol. 2017, 7, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Wu, P.W.; Lee, C.C.; Huang, C.C.; Fu, C.H.; Chang, P.H.; Lee, T.J. Comparison of SNOT-25 and ENS6Q in evaluating patients with empty nose syndrome. Laryngoscope Investig. Otolaryngol. 2022, 7, 342–348. [Google Scholar] [CrossRef] [PubMed]



| Variables | Unit | Criterion Range | 0 Day | 56 Days |
|---|---|---|---|---|
| CRP | μg/mL | 1.5~25.0 | 2 | 3 |
| BUN | mg/dL | 5.59~18.87 | 14 | 6 |
| AST | IU/L | 16.4~504 | 53 | 29 |
| ALT | IU/L | 13.2~53.2 | 50 | 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.B.; Jang, D.W.; Kim, D.H.; Kim, S.W. Development of a New Swine Model Resembling Human Empty Nose Syndrome. Medicina 2024, 60, 1559. https://doi.org/10.3390/medicina60101559
Park DB, Jang DW, Kim DH, Kim SW. Development of a New Swine Model Resembling Human Empty Nose Syndrome. Medicina. 2024; 60(10):1559. https://doi.org/10.3390/medicina60101559
Chicago/Turabian StylePark, Dan Bi, David W. Jang, Do Hyun Kim, and Sung Won Kim. 2024. "Development of a New Swine Model Resembling Human Empty Nose Syndrome" Medicina 60, no. 10: 1559. https://doi.org/10.3390/medicina60101559







