Abstract
Coronary artery disease (CAD) presents a significant risk for patients with systemic vasculitides, a group of disorders characterized by the inflammation of blood vessels. In this review, we focus on the pathophysiological mechanisms, complications, and management strategies for CAD in systemic vasculitides. We highlight how the inflammatory processes inherent in vasculitis contribute to accelerated atherosclerosis and myocardial ischemia. Key strategies in managing CAD in this patient population include using medicine treatments to mitigate vascular inflammation while balancing the risk of promoting cardiovascular events and lifestyle modifications. Understanding the nuanced relationship between systemic vasculitides and CAD is crucial for improving patient outcomes and guiding therapeutic approaches.
1. Introduction
Systemic vasculitides represent a group of rare autoimmune disorders characterized by inflammation of the wall of blood vessels of various sizes, from small to large [1]. Cardiovascular involvement substantially contributes to the morbidity and mortality of those affected [1,2] and is often an underrecognized aspect of systemic vasculitides [2]. Although cardiac manifestations in these patients are relatively rare in clinical practice, affecting fewer than 10% of individuals [3], their presence is typically associated with a poorer prognosis. Among their multiple complications, coronary artery disease (CAD) emerges as a significant challenge even in the modern medical landscape. This underscores the critical importance of early detection and timely intervention [4]. Notably, Takayasu arteritis (TA), Kawasaki disease (KD) and eosinophilic granulomatosis with polyangiitis (EGPA) are the forms of systemic vasculitis most frequently linked to heart disease [3].
Furthermore, cardiac involvement is commonly observed in patients who are negative for anti-neutrophil cytoplasmic antibody (ANCA), highlighting the necessity of considering cardiac involvement even in the absence of conventional markers [2]. Research shows that primary systemic vasculitides are a steadily more prevalent contributor to cardiovascular burden among younger patients. Morbidity and mortality in this age group are progressively associated with accelerated atherosclerosis, ischemic heart disease (IHD), venous thromboembolism, and inflammatory/structural myo-pericardial changes [1]. The advent of modern diagnostic possibilities, such as transthoracic echocardiography, cardiac magnetic resonance imaging (MRI), and computed tomography (CT) coronary angiography, has significantly improved the accuracy of diagnosing these conditions. The advanced imaging modalities have not only enhanced diagnostic precision but have also improved the prognosis and informed the management strategies for patients with systemic vasculitides [5].
Understanding the nuanced relationship between systemic vasculitides and CAD is crucial for improving patient outcomes and guiding therapeutic approaches. In this review, we focus on the pathophysiological mechanisms of vascular involvement in specific systemic vasculitides while discussing their clinical manifestations and exploring the complications with which they are associated, thus emphasizing the importance of timely intervention. Moreover, by examining the existing management options, we will provide a clear overview of the current therapeutic approaches and the promising future advances. Advances in understanding disease mechanisms and treatment responses are paving the way for improved patient outcomes.
2. Search Strategy
The literature search for this review was conducted using the PubMed, Scopus, and Web of Science databases, covering articles published from January 1960 to July 2024. The search focused on peer-reviewed clinical studies/original articles, reviews, and guidelines for managing CAD in systemic vasculitides. Both Medical Subject Headings (MeSH) and relevant free-text terms were used: “systemic vasculitides”, “coronary artery disease”, “management,” “complications”, and “immunosuppressive therapy.” Boolean operators were used as follows: (“Coronary Artery Disease” OR “CAD” OR “coronary heart disease” OR “CHD”) AND (“Systemic Vasculitides” OR “vasculitis” OR “ANCA-associated vasculitis” OR “giant cell arteritis” OR “polyarteritis nodosa”) AND (“Management” OR “treatment” OR “therapy” OR “strategies”) AND (“Complications” OR “adverse outcomes” OR “risk factors”). We utilized MeSH terms for PubMed and “All Fields” for broader database searches.
A total of 245 papers were identified, of which 79 met the inclusion criteria, focusing on the clinical management and complications of CAD in patients with systemic vasculitides (Figure 1).
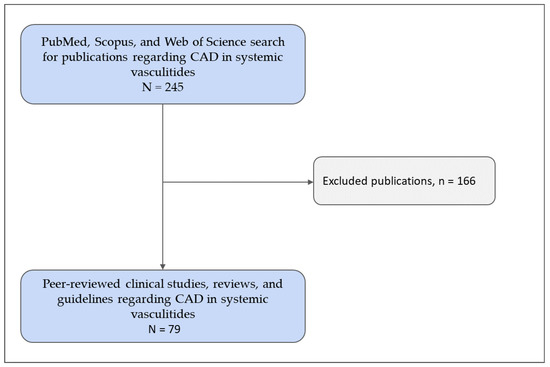
Figure 1.
Flowchart of the study inclusion process. CAD—coronary artery disease. Copyright: the authors.
3. Pathophysiology of Coronary Artery Diseases in Systemic Vasculitides
3.1. Mechanisms of Vascular Inflammation and Damage
Although systemic vasculitides represent a group of pathophysiologically heterogeneous entities, their common denominator is inflammation of the blood vessel wall [6]. Inflammation disrupts the vessel wall structure and its integrity, leading to consequences such as blood vessel stenosis, the formation of arterial aneurysms, or even pseudoaneurysms with vessel wall rupture, as well as in situ thrombosis and distal thromboembolism [6]. End-tissue ischemia is the main hemodynamic consequence related to the territory of the affected blood vessel [6,7]. However, hemorrhages due to vessel rupture or even organ rupture may be observed as well.
Coronary artery vasculitis may be especially challenging given the potentially detrimental consequences of ischemia of both epicardial and non-epicardial coronary arteries. Chronic inflammation may lead to necrosis and scarring of the myocardial tissue. Even in the absence of ongoing inflammation, previous coronary vasculitis is associated with accelerated atherosclerosis and an increased risk of “classical” atherosclerotic CAD [8].
3.2. Specific Vasculitides Associated with Coronary Artery Disease
Data on the coronary-specific aspects of the most common vasculitides affecting the coronary arteries are lacking. The knowledge of the pathophysiological aspects of systemic vasculitides affecting the coronary arteries stems mainly from studies assessing the non-coronary arteries.
3.2.1. Giant Cell Arteritis
The inflammatory process of Giant cell arteritis (GCA) seems to be initiated in the adventitia, where dendritic cells become activated by an unknown trigger in a genetically predisposed individual. This, in turn, leads to the activation of CD4+ lymphocytes and their polarization into Th1 and Th17 cells [9]. These cells produce interferon-gamma and interleukin-17, respectively, and are responsible for the migration and accumulation of other cell types in all vessel wall layers. These other cell types include CD8+ lymphocytes, monocytes differentiating into macrophages and giant multinuclear cells, and vascular smooth muscle cells that migrate to the intima, becoming myofibroblasts supporting further vascular stenosis [9].
Interleukin-6 (IL-6), one of the main proteins implicated in the acute phase reaction, has been recognized as one of the main therapeutic targets in GCA and large vessel vasculitis in general. In preclinical models, it has demonstrated its effect in promoting the differentiation of pathogenic Th17 cells from naive T cells. In contrast, IL-6 has been shown to inhibit the development of regulatory T cells [10].
Furthermore, IL-6 has been implicated as a molecule promoting the transendothelial migration of leukocytes via adhesion molecules such as VCAM-1 and ICAM-1 [11].
On the other hand, a recent study on 28 GCA patients has revealed a potential disconnection between the pronounced systemic inflammatory role of IL-6 in GCA and its potentially questionable role in the vessel wall. In this ex vivo study on temporal artery biopsies, intriguingly, IL-6 did not demonstrate an effect on cell migration or cytoskeletal rearrangement. This finding may explain ongoing arterial inflammation in a subset of GCA patients that have achieved reasonable control of the acute phase response using IL-6 inhibitory drugs [12].
3.2.2. Takayasu Arteritis
The prevailing pathogenetic concept of TA is that an unknown stimulus leads to the expression of heat shock protein-65 (HSP-65) in the vessel wall. HSP-65 induces the Major Histocompatibility Complex chain-related A, which is then recognized by T-lymphocytes. This, in turn, drives further inflammation, the migration of monocytes, macrophages, and smooth muscle cells, and intimal proliferation. B-cells also support the inflammatory process by producing anti-aorta, anti-endothelial, anti-cardiolipin, and anti-annexin-V antibodies [13,14].
The inflammatory process of TA seems to start in the inner layers of the blood vessel. In the early stages, inflammation resembles the granulomatous inflammation of GCA (with multinuclear giant cells), whereas histological features are less specific at its later stages [15]. The production of TNF-alpha and interferon-gamma by activated T-cells supports granuloma formation. However, NK and Th17 cells also contribute to the process. At an advanced disease stage, fibrosis is driven by matrix metalloproteinases and TNF-alpha. IL-6 also plays an essential role in supporting vessel wall inflammation and systemic inflammation [15].
3.2.3. Kawasaki Disease
The general understanding is that KD occurs after a child has been exposed to an infectious agent, after which a cascade of events occurs, leading to the hyperactivation of the innate and adaptive immune system. Damage-associated molecular patterns (DAMPs) produced as a consequence of cell death and oxidative stress affect various cells, including leukocytes, platelets, smooth muscle cells, and endothelial cells. Neutrophils and monocytes migrate to the vessel wall and become responsible for the development of coronary arterial vasculitis [16].
In addition to coronary artery vasculitis, the inflammatory cellular infiltrate may not be confined to the blood vessels, yet it can infiltrate the myocardium, causing myocarditis. Interestingly, myocarditis has been described even without the presence of vasculitis. Although myocarditis in the context of KD is usually deemed to be transient, in a subset of patients, it can lead to fatal arrhythmias and myocardial fibrosis [17].
3.2.4. ANCA-Associated Vasculitides
ANCA-associated vasculitides (AAV) are a group of small-vessel vasculitis that are not mediated by immune complexes. Three entities are included in the group: granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and EGPA [18]. AAV develops due to a loss of tolerance to the main proteins involved in the pathogenesis of the disease—myeloperoxidase (MPO) and proteinase 3 (PR3), both located in the neutrophils. ANCA plays a vital role by activating neutrophils and allowing the release of MPO/PR3 in the microvasculature to be presented by antigen-presenting cells to T-lymphocytes and effector T-cells that mediate further injury. Endothelial and tissue injury spreads beyond the microvasculature, leading to tissue destruction, irreversible damage, and fibrosis [18].
It is worth noting that, among the AAV, EGPA is more frequently associated with cardiac involvement. In addition to small-vessel vasculitis, direct eosinophilic infiltration of the heart tissues (myocardium, pericardium and the endocardium, including the valves) seems to play a role in the pathogenesis of heart disease in EGPA. Abundant eosinophil cells infiltrating the affected tissue exert a variety of cytotoxic and pro-inflammatory effects leading to endocarditis, myocardial dysfunction, and/or pericardial effusion with pericarditis [19]. Furthermore, approximately 50% of the deaths in EGPA are related to cardiac disease and occur within the first few months since diagnosis. However, Dalia et al. discussed that many EGPA patients may not even have cardiac signs or ECG abnormalities [20]. This contrasts with other AAV (ANCA-associated vasculitides) where cardiac involvement is less frequent.
4. Clinical Presentation and Diagnosis
4.1. Symptoms of Coronary Artery Involvement in Vasculitides
Vasculitides refer to a group of disorders characterized by the inflammation of blood vessels, which can lead to vessel wall damage and subsequent dysfunction of the affected organs. When vasculitis involves the coronary arteries, it can result in significant cardiovascular complications. As the coronary arteries supply blood to the heart, any compromise in their function can lead to severe cardiac conditions [21]. First, we will cover the general symptoms of coronary artery involvement. Coronary artery involvement in vasculitides can present with various symptoms, often mirroring those seen in other forms of CAD. The severity and nature of these symptoms can vary depending on the extent of inflammation and the specific type of vasculitis involved. Common symptoms include chest pain (angina), shortness of breath, fatigue, palpitations, and syncope [21].
KD primarily affects children and is characterized by inflammation of the coronary arteries. Symptoms include fever, rash, conjunctivitis, mucosal changes, and lymphadenopathy. Clinical manifestations may include myocarditis and arteritis, resulting in fibrinoid necrosis of the internal elastic lamina and the subsequent formation of coronary aneurysms in up to one-third of untreated patients [22]. Cerebral aneurysms are less frequent in 1–2% of patients. Monocytes, neutrophils, and macrophages appear to be involved in the pathogenesis of these vascular lesions. Resulting from these inflammatory processes, an inappropriate healing response may also cause coronary stenosis. Otherwise, typical complications associated with the presence of the aneurysms include thrombus formation, causing embolism and peripheral occlusion and rupture. In severe cases, coronary artery aneurysms can form in the coronary arteries, leading to the potential rupture or thrombosis, which can cause myocardial infarction [22].
TA primarily affects young women and involves large vessels, including the aorta and its major branches. Coronary artery involvement can lead to arm or leg claudication, diminished pulses, chest pain, and systemic symptoms: fever, night sweats, weight loss, and fatigue [23]. Angina pectoris may occur following coronary artery ostial stenosis from aortitis or coronary arteritis in 10–45% of the cases. They may have severe clinical sequelae, even though regression upon immunosuppression is possible.
Polyarteritis nodosa (PAN) involves medium-sized arteries and can affect various organs, including the heart. Symptoms related to coronary artery involvement include chest pain due to coronary artery inflammation, damage, or microaneurysms. Coronary involvement may manifest as stenosis, occlusion, aneurysm, or dissection [23]. In a recent review of cases, a total of 34 patients with an average age of 41 years were identified from 32 publications. The male sex is more frequent, and coronary disease was the first manifestation of PAN in ¾. The clinical course of the disease was, in general, very severe, with cases of death from cardiac arrest, pulmonary edema with alveolar hemorrhage, or multiple intracranial hemorrhages after thrombolytic therapy. The formation of immune complexes due to a virus infection (hepatitis B or C) and hairy cell leukemia is thought to mediate the inflammatory reaction, which most often leads to media thickening and stenosis rather than aneurysm formation [24].
Additionally, heart failure symptoms (i.e., dyspnoea, swelling in the legs, ankles, and feet, fatigue, weakness, reduced ability for exercise, pulmonary edema, chest pain, fainting) and arrhythmias (e.g., atrial fibrillation) result from myocardial damage. Hypertension due to renal arteritis and cardiac disease is present in 10–30% of cases [25]. New onset hypertension in a patient with systemic symptoms such as fever, weight loss, and joint pain is a clue of PAN. Other symptoms include deep skin inflammation or progression to infarction and gangrene (30–50%), neuropathy (mononeuritis multiplex in 20–50%), and mesenteric vasculitis.
GCA commonly affects older adults and involves large- and medium-sized arteries, including the aorta and branches. Symptoms related to coronary artery involvement can include jaw claudication, temporal headaches, scalp tenderness, vision problems, and chest pain in cases where the aorta and coronary arteries are involved [25].
MPA and GPA are small-vessel vasculitides that can also involve the coronary arteries, leading to chest pain, pulmonary symptoms (i.e., cough, hemoptysis, and shortness of breath), and renal symptoms (i.e., hematuria and proteinuria, indicating kidney involvement, which can have secondary effects on cardiovascular health) [26]. EGPA primarily affects small-to-medium-sized vessels and is associated with asthma and eosinophilia. Coronary artery involvement can present with chest pain, severe and often difficult-to-control asthma, congestive heart failure, and peripheral neuropathy (i.e., numbness, tingling, and weakness in the limbs [27].
In summary, coronary artery involvement can be identified due to coronary arteritis that causes thromboses, dissections, stenosis, and possible myocardial infarction/ischemia. Coronary involvement was documented in 50% of PAN. It is also widespread in KD (20% of untreated cases) and can be diagnosed via byechocardiography, cardiac computed tomography, cardiac magnetic resonance (CMR), or invasive coronary angiography [28,29].
In Figure 2, we present the overlapping clinical features of vasculitides regarding CAD and a stepwise clinical approach.
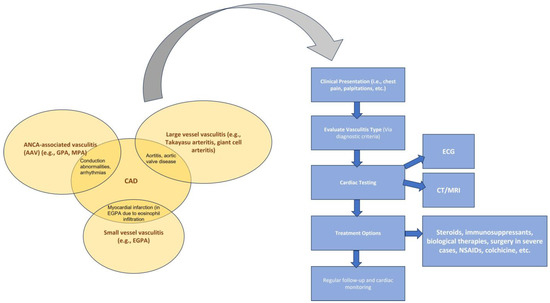
Figure 2.
Overlapping cardiac clinical signs of vasculitides and a stepwise clinical approach. Copyright: the authors.
4.2. Diagnostic Imaging and Tests
4.2.1. Transthoracic Echocardiography
Transthoracic echocardiography is usually the initial diagnostic method used to assess the heart and its associated structures in the context of suspected or confirmed vasculitis. The method allows for a comprehensive evaluation of myocardial function and left ventricular ejection fraction, and thus, it enables the detection of possible myocardial ischemia [30]. Due to its noninvasive nature, rapidity, affordability, and safety, echocardiography can be performed repeatedly throughout the course of the disease. However, this technique has several limitations, including poor visualization of the distal coronary artery segments and the physical characteristics of the patient and the necessity for an experienced operator [31].
4.2.2. Coronary Angiography
Invasive coronary angiography is the current gold standard for CAD evaluation. Furthermore, in addition to its diagnostic capabilities, this approach also offers potential for therapeutic intervention [31]. Also, intravascular imaging by means of intravascular ultrasound or optical cocherence tomography can be performed as a supplementary diagnostic tool, enabling optimal visualization and differentiation of the vessel wall layers [32]. The primary limitation of this method is its invasiveness, which may be associated with procedural complications. The method includes the application of X-ray and iodine contrast media, which may be associated with further patient risk.
The role of coronary computed tomographic angiography has been expanding in recent years, largely due to its numerous advantages over other diagnostic methods. CCTA can assess the dimensions and configuration of the arterial wall and lumen, enable plaque characterization and visualize calcifications and thrombi, and evaluate the presence, precise location, and characteristics of aneurysmal dilations or stenotic regions [33]. This method is noninvasive, which gives it a safer risk profile than conventional coronary angiography (CCA). Furthermore, CCTA can perform better in cases of aneurysms or thrombosis, where the size of the lumen may appear normal in other modalities [33]. The advent of the CT technology in recent years led to a reduction in the artifacts related to a high heart rate, thereby facilitating enhanced visualization of the distal branches of the coronary vessels and enabling the reconstruction of intricate anatomical structures in a three-dimensional model [31]. Some potential limitations of CCTA include administering contrast media, using ionizing radiation, and needing a well-trained imaging specialist [34].
4.2.3. Magnetic Resonance Angiography
Magnetic resonance angiography (MRA) is a noninvasive, non-ionizing method for assessing vascular structures, with specific value in examining children and pregnant women [31]. Some studies have reported that cardiac MRA has a lower sensitivity of 93% for detecting coronary vasculitis compared with CCTA, which has a sensitivity of nearly 100%. However, a whole heart cardiac MR can provide a comprehensive evaluation of the myocardial structure, which is crucial for the diagnosis, prognosis, and treatment of these patients [33]. Limitations of the method include its high cost, the need for specific software, technical equipment, and a well-trained and experienced imaging specialist, and contraindications such as claustrophobia and metal implants.
In the next figures, we present our experience in the diagnosis of these cardiovascular complications by means of imaging techniques (Figure 3, Figure 4, Figure 5 and Figure 6).
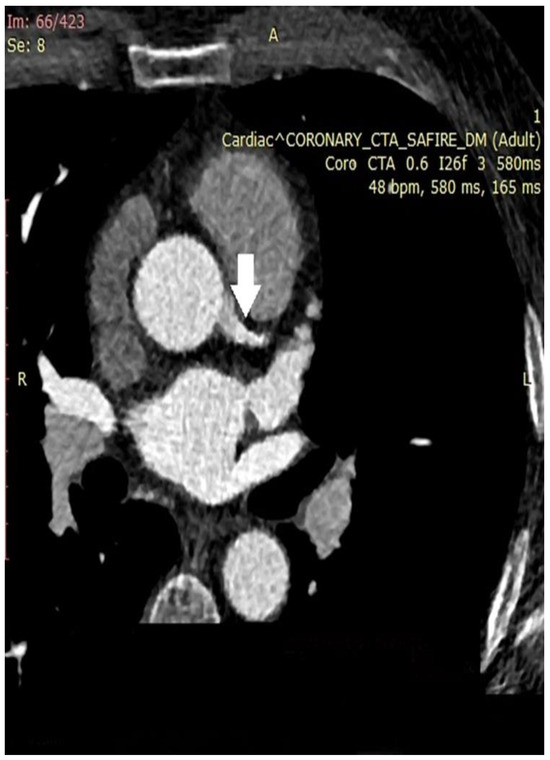
Figure 3.
Coronary computed tomographic angiography (CCTA), axial view, and maximum intensity projection (MIP) at the level of the ascending aorta and pulmonary veins. The place of origin of the left main coronary artery is presented (white arrow). A – orientation marker indicating the position of the abdomen; R – orientation marker indicating the right side of the patient. Copyright: the authors.
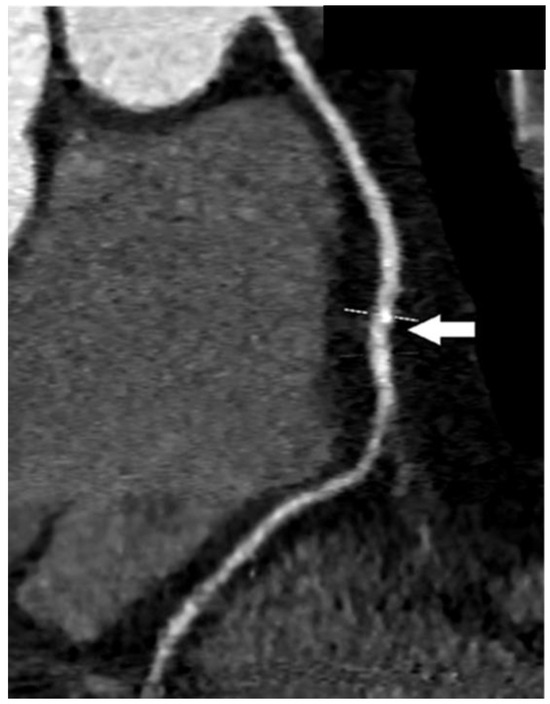
Figure 4.
Coronary computed tomographic angiography (CCTA), oblique view, maximum intensity projection (MIP). The right coronary artery is presented with the normal course and lumen width. The white arrow shows a non-obstructive calcification of the artery wall. Copyright: the authors.

Figure 5.
Coronary computed tomographic angiography (CCTA), oblique view, maximum intensity projection (MIP), and cross-sections at different levels of the right coronary artery (RCA). A) Cross-sectional view at the level of ostial RCA with visualization of fibro-lipid plaque and mild stenosis; B) Cross-sectional view at the level of proximal RCA—no evidence of plaque; C) Cross-sectional of the level of mid-RCA—presenting lipid low-attenuation atherosclerotic plaque, causing a significant 50–60% stenosis; D) Cross-sectional view at the level of mid-RCA—noevidence of plaque. Copyright: the authors.
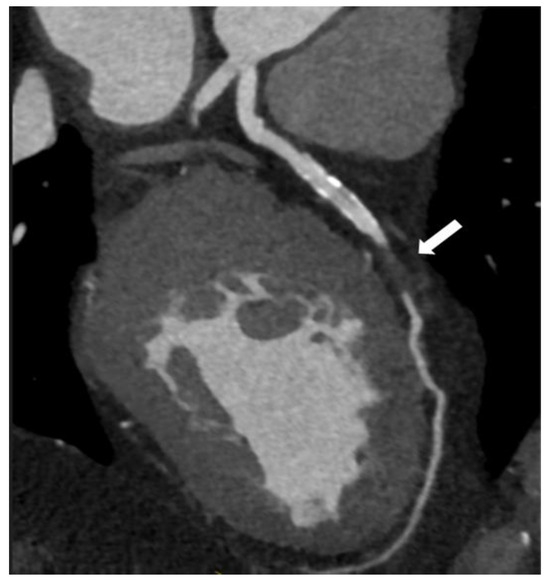
Figure 6.
Coronary computed tomographic angiography (CCTA), oblique view, and maximum intensity projection (MIP) present the left anterior descending artery with a total occlusion in the mid-segment of the vessel (white arrow). Copyright: the authors.
A summary of diagnostic tools for CAD in systemic vasculitides is presented in Table 1.

Table 1.
Diagnostic modalities for CAD and their characteristics: capabilities, advantages, and disadvantages.
4.3. Differential Diagnosis of CAD in Systemic Vasculitides
The differential diagnoses include other causes of large vessel vasculitis such as inflammatory aortitis (e.g., syphilis, tuberculosis, chronic periaortitis, lupus, rheumatoid arthritis, spondyloarthropathies), developmental abnormalities (coarctation of the aorta and Marfan syndrome), and other aortic pathologies such as ergotism and neurofibromatosis.
Differential diagnoses that can be considered coronary artery affection in systemic vasculitis can include heart palpitations, heart arrhythmias, myocarditis, endocarditis, pericarditis, valvular manifestations, heart failure, aortic dissection, deep vein thrombosis, embolism, hypertension, cerebral stroke, transient ischemic attack, kidney disease (cardiorenal syndrome), peripheral artery disease, antiphospholipid syndrome, lung issues such as shortness of breath, bleeding, coughing, gastrointestinal tract manifestations such as diarrhea, vomiting, hematemesis, stomach pain, chest pain, and neurological conditions presenting with fainting/syncope, dizziness, weakness, and tingling.
We also propose a table that outlines how different manifestations impact the risk of CAD in systemic vasculitides [35] (Table 2).

Table 2.
The association between different disease manifestations and CAD risk in systemic vasculitides.
5. Complications of Coronary Artery Diseases in Systemic Vasculitides
Cardiac involvement in systemic vasculitides may be associated with adverse effects such as arrhythmias, myocardial infarction, and even sudden cardiac death [36]. Arrhythmias and conduction abnormalities are essential manifestations of cardiac involvement in systemic vasculitides and can have a severe impact on morbidity and mortality in these patients. The most common arrhythmias include but are not limited to premature supra- and ventricular beats, tachyarrhythmias, and atrial fibrillation [37].
In addition, conduction disturbances such as AV block are another common manifestation of systematic vasculitides. The underlying pathophysiological mechanisms of arrhythmias are complex and multifactorial. However, myocardial fibrosis is considered to be the main mechanism of cardiac involvement, usually as a consequence of an inflammatory process or obstructive CAD [38].
Several possible mechanisms for the association between vasculitis and arrhythmias have been suggested. Firstly, vessel wall inflammation may increase arterial stiffening, impairing the peripheral blood flow and leading to end-organ ischemia. On the other hand, coronary artery damage in systemic vasculitides may be associated with myocardial ischemia and may, therefore, be complicated by myocardial infarction and even sudden cardiac death [39].
There are several cases in the literature describing patients with KD complicated by acute myocardial infarction. Even though data on histopathology reports fail to prove a process of accelerated atherosclerosis in this group of patients, cases of acute myocardial infarction have been published not only in adults but also in children [40,41].
Although much rarer, TA is another systemic vasculitis that an acute myocardial infarction can complicate due to the process of accelerated atherosclerosis [42]. Microvascular dysfunction is another possible pathophysiological mechanism that has been recognized as a potential cause of myocardial ischemia in patients with vasculitis [43].
Coronary microvascular dysfunction may be endothelial-dependent and endothelial-independent based on the specific subset of abnormalities in the microcirculation. The alteration of microvascular function is a pathology that requires specific diagnostic methods and may be easily overlooked. Hence, specific attention should be paid to patients with symptoms of myocardial ischemia and non-obstructive coronary arteries [44].
Many cardiac manifestations may not be clinically evident, especially in the early stages of involvement (Table 3). Therefore, cardiologists should systematically assess cardiac function by applying a multi-modality imaging approach.

Table 3.
Cardiovascular manifestations and complications in different types of systemic vasculitides.
We propose an algorithm for diagnosing systemic vasculitides presenting with CAD involvement based on laboratory, clinical, and imaging data (Figure 7).
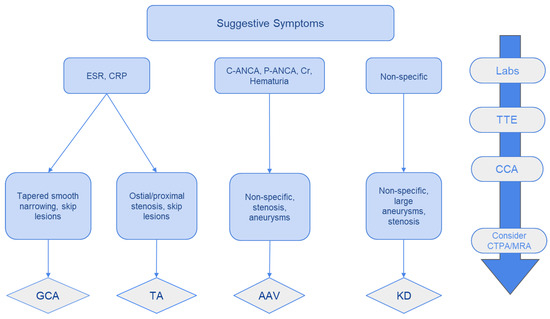
Figure 7.
A schematic approach in the diagnosis of coronary vasculitides with CAD. TTE: Transthoracic echocardiography; CCA: Conventional coronary angiography +/− therapeutic intervention; CCTA: coronary computed tomographic angiography; MRA: magnetic resonance angiography. Copyright: the authors.
6. Management Strategies for CAD in Systemic Vasculitides
6.1. Medical Management
Managing cardiovascular manifestations in systemic vasculitides is complex and usually includes glucocorticosteroids (GCS) and immunosuppressants. While GCS are mainly used in the active phase to suppress the disease activity, immunosuppressants are used as steroid-sparing drugs for remission induction and the suppression of disease progression. Novel biological treatment includes the use of TNF-alfa inhibitors, IL-6 inhibitors, and monoclonal antibodies against CD20 [52].
GCS is the first-line treatment in GCA and TA with any manifestations, including CAD. Their usage in a dose ranging from 0.5 to 1 mg/kg is the initial treatment choice for controlling the inflammation. Together with immunosuppressants and heart-failure treatment, GCS improve heart function [53]. In GCA, the use of GCS is crucial for the induction of remission and the prevention of blindness in this subset of patients.
Depending on the severity of clinical symptoms, cardiac involvement in systemic necrotizing vasculitides (SNV), including PAN, GPA, EGPA, and MPA, could be treated symptomatically or by immunosuppression with GCS or cyclophosphamide. GCS are combined with immunosuppressive agents like cyclophosphamide for remission induction in cases of severe, life-threatening manifestations. In the case of GPA, Rituximab could be used both for induction as well as for maintaining remission [54,55,56].
In Behcet’s disease, the use of GCS is controversial as it has been shown that GCS increase the risk of CV mortality [57]. Cyclophosphamide is used as a first-line treatment for remission induction for severe vascular involvement, and azathioprine or mycophenolate mofetil are recommended for maintaining remission in these patients [58,59].
KD is primarily treated with acetylsalicylic acid (ASA) and intravenous immunoglobulin (IVIG). The use of GCS in the acute phase of KD, especially in high-risk patients, leads to improved CAD, clinical symptoms, and laboratory markers for inflammation. According to the literature, using GCS in combination with the standard treatment of KD should be considered in all children, including IVIG resistant- and high-risk patients [60].
Similarly, multiple GWAS aim to reveal KD patients with an increased risk of coronary involvement and IVIG resistance. The results of such studies involving different susceptibility genes have demonstrated a role for calcineurin inhibitors in treating acute KD [61].
Combination therapy with IVIG and corticosteroids in KD patients demonstrated superior efficacy in limiting the occurrence of coronary complications. The response to treatment was found to be directly related to the initial TTE evaluation and the levels of albumin, sodium, hemoglobin, and procalcitonin [62].
6.2. Interventional and Surgical Approaches for CAD in Systemic Vasculitides
Percutaneous coronary intervention (PCI) is the predominant method of revascularization in patients with obstructive CAD. However, when it comes to patients with systemic vasculitides undergoing coronary revascularization, most of the reported data come from small studies and case reports [63]. Although some data have been published on the outcomes of PCI in patients with systemic vasculitides, these are from single-center studies with relatively small sample sizes and are usually underpowered to detect statistically significant results [64].
A retrospective study evaluating patients with AAV in the Danish population shows that this subset of patients is at increased risk for coronary revascularization and has a higher associated risk of adverse cardiovascular events [65]. However, the percutaneous revascularization led to a reduction in cardiovascular events over a 3-year follow-up period. Although patients with systemic vasculitides represent a specific subset of patients with advanced progression of CAD, the published data suggest a favorable effect of coronary revascularization. Even after successful PCI or surgical revascularization, vasculitis patients may remain at an increased risk for future adverse ischemic events and the progression of CAD, which is often more advanced than in patients without vasculitis [66]. Currently, there are no officially accepted recommendations regarding revascularization strategies for obstructive coronary disease in these patients. An observational study assessing ninety patients with TA and coronary stenosis reports that in 39 patients, a conservative approach was applied, whereas 51 patients underwent revascularization (28 patients with PCI and 23 with surgical revascularization). The results showed that there was no significant difference in cardiovascular death between the groups with conservative treatment and revascularization. Moreover, cardiovascular mortality was similar in the coronary artery bypass grafting (CABG) and PCI groups, but the restenosis rate was higher in the percutaneous intervention group [67]. In the specific subset of patients with CAD and systemic vasculitis, the decision on the treatment strategy and the revascularization approach should be made after careful consideration and on an individual basis based on the patient’s specific clinical characteristics, the type of vasculitis, and the severity of CAD.
7. Emerging Therapies and Future Directions
7.1. Advances in Targeted Biological Therapies
For TA, tocilizumab, an anti-IL6 targeting molecule, when used in patients with coronary involvement, reduced the number of active coronary lesions and vessel wall thickening, restoring the function of the coronary artery while simultaneously improving the levels of circulating inflammatory markers (CRP, ESR) and the Kerr score [31].
Tocilizumab in GCA demonstrated decreased dosage requirements for steroids, extended the interval between flare-ups, and reduced the overall frequency of disease flare-ups [68]. A potential pitfall of IL-6 inhibition is the blockade of CRP synthesis. This could potentially mean that ongoing inflammation is concealed by tocilizumab, thus making CRP an ineffective marker for disease monitoring.
Another novel therapeutic approach involves blocking IL-12 and IL-23 pathways by directing a monoclonal antibody against their shared P40 subunit. In this way, ustekinumab inhibits the polarization of Th1 cells towards IFN-γ production while suppressing IL-17 secretion by Th17 cells. Ustekinumab achieved a notable reduction in steroid requirements (from 20 mg to 5 mg) and facilitated complete steroid cessation in ¼ of the patients after approximately one year of treatment for systemic vasculitides [69].
Rituximab is directed against the CD20 molecule on the surface of B cells, leading to their depletion. Standard therapy for AAV includes the combination of a steroid with CYC, AZP, MTX, MMF, or rituximab. However, the significant adverse effects of CYC call for a more precise and less deleterious therapeutic effect. Regarding this aspect, multiple trials (e.g., RITUXVAS) have concluded that rituximab is at least not inferior to cyclophosphamide when used for induction and/or remission for AAV [70].
7.2. Role of Personalized Medicine
Personalized medicine attempts to use targeted therapies curated specifically for each patient who has failed to respond to conventional therapeutic options.
As suggested by Yap et al. [71], an example of personalized medicine in the treatment of AAV would entail treating patients according to the type of ANCA present and would abolish the standard classification of EGPA, GPA, or MPA. Another proposed mechanism involves cytotoxic T cells coupled to an autoantibody receptor that binds antibodies, targeting and thus killing PR3- or MPO-expressing B cells in AAV patients.
7.3. Potential for Novel Treatment Modalities
Despite the beneficial effect of rituximab in the treatment of GCA and TA being undeniable, it is also accompanied by significant disadvantages, including hypogammaglobulinemia, latent hepatitis B infection reactivation, neutropenia, and immunogenic reactions (due to its chimeric structure). A potential solution to these challenges comes from the emergence of obinutuzumab and ofatumumab, fully humanized antibodies targeting the CD-20 molecule [69].
A very interesting finding in biopsies of the temporal artery from GCA patients revealed the elevated expression of endothelin-1 [72,73]. Therefore, it appears likely that ET-1 inhibition through the employment of existing receptor antagonists could have a central role in the therapy of GCA.
T cell dysregulation has been suspected to contribute to the pathogenesis of AAV following the granulomatous lesions found on kidney and lung biopsies of AAV patients. Therefore, future interventions have shifted towards blocking antigen-presenting B cells from activating T cells using abatacept. The degree to which abatacept can accomplish this should be determined by the ongoing multicenter, randomized, placebo-controlled ABROGATE trial (Abatacept for the Treatment of Relapsing, Non-Severe, Granulomatosis With Polyangiitis) [74].
Alemtuzumab, an anti-CD52 monoclonal antibody, is also being studied for AAV due to its depleting effect on T and B cell populations [75,76,77]. Another novel targeting strategy includes using belimumab to inhibit the maturation of B cells by interfering with their activating factor (BAFF). The BREVAS study (Belimumab in Remission of Vasculitis) aimed to compare belimumab with azathioprine for the maintenance of remission in GPA and microscopic polyangiitis [78].
Further research into the pathogenesis of AAV implicates the inappropriate activation of the alternative complement pathway as a potential target. Avacopan, a C5a receptor inhibitor, is currently considered for its capacity to replace glucocorticoids in the induction and maintenance therapeutic regimens of AAV [79]. A combination of low-dose glucocorticoids and avacopan, avacopan with cyclophosphamide or rituximab induction, or high-dose glucocorticoids was administered to sixty-seven patients with ANCA-associated vasculitis in a randomized trial. At 12 weeks, 86% of the avacopan/glucocorticoid patients and 81% of the avacopan-alone group achieved the primary end-point of the treatment response, which is a 50% reduction from baseline in the Birmingham Vasculitis Activity Score. In contrast, 70% of the glucocorticoid group achieved this goal. All groups had decreases in markers of inflammation and kidney damage, although avacopan caused a faster and more significant reduction. In the high-glucocorticoid group, serious side effects such psychiatric disorders and newly diagnosed diabetes mellitus were more prevalent [79]. These encouraging findings imply that new targeted therapeutics may be able to induce glucocorticoid-free remission in ANCA-associated vasculitis.
This will be investigated further in the bigger, longer-running phase 3 ADVOCATE experiment (A Phase 3 Clinical experiment of CCX168 [Avacopan] in Patients With ANCA-Associated Vasculitis) [80].
Advancements have also been made in treating EGPA, an AAV phenotype characterized by profound eosinophilia. It has been hypothesized that suppressing IL-5 levels with mepolizumab can lead to remission, deplete eosinophils, and allow for steroid dose reduction. In total, 136 patients with refractory or relapsing EGPA were randomly assigned to receive monthly mepolizumab or a placebo in a recent randomized, double-blinded trial [81].
The patients in the mepolizumab arm remained in remission for much longer after 52 weeks; 28% of them remained in remission for more than 24 weeks, while only 3% of patients in the placebo group did the same [81]. The placebo group experienced more serious side effects, while mepolizumab was well tolerated.
8. Conclusions
The management of CAD in patients with systemic vasculitides requires a comprehensive approach that addresses both the underlying inflammatory disease and traditional cardiovascular risk factors. The early detection and management of these complications in systemic vasculitides is of utmost importance. A nuanced understanding of the pathogenesis of CAD in this context can inform treatment strategies, optimize outcomes, and minimize complications. Future research should continue to explore targeted therapies and personalized treatment plans to enhance care for these complex patients.
Author Contributions
Conceptualization, R.S.; N.M. and T.V.; methodology, I.P.; validation, P.S.; L.C.; K.B.; I.E.K. and D.M.; formal analysis, I.P.; investigation, P.S.; resources, D.V.; data curation, D.V.; writing—original draft preparation, R.S.; N.M.; I.P.; P.S.; K.B. and I.E.K.; writing—review and editing, T.V.; visualization, L.C.; A.V.; N.M. and K.B.; supervision, T.V.; project administration, T.V.; funding acquisition, T.V. All authors have read and agreed to the published version of the manuscript.
Funding
This study is financed by the European Union-NextGenerationEU through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No. BG-RRP-2.004-0008.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vats, V.; Patel, K.; Sharma, D.D.; Almansouri, N.E.; Makkapati, N.S.R.; Nimal, S.; Ramteke, P.; Mohammed Arifuddin, B.; Jagarlamudi, N.S.; Narain, A.; et al. Exploring Cardiovascular Manifestations in Vasculitides: An In-Depth Review. Cureus 2023, 15, e44417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ali, A.M.; Yakupoglu, H.Y.; Fuchs, T.A.; Larsen, T.H.; Aukrust, P.; Gunnarsson, R.; Saeed, S. Cardiac Involvement in Systemic and Local Vasculitides: The Value of Noninvasive Multimodality Imaging. Curr. Probl. Cardiol. 2023, 48, 101718. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.H. Cardiovascular Manifestations of Systemic Vasculitides. Curr. Rheumatol. Rep. 2020, 22, 72. [Google Scholar] [CrossRef]
- Weber, B.; Wallace, Z.S.; Parks, S.; Cook, C.; Huck, D.M.; Garshick, M.; Brown, J.M.; Divakaran, S.; Hainer, J.; Dorbala, S.; et al. Association between Systemic Vasculitis and Coronary Microvascular Dysfunction in the Absence of Obstructive Coronary Artery Disease. Circ. Cardiovasc. Imaging 2023, 16, e014940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jeon, C.H.; Kim, Y.K.; Chun, E.J.; Kim, J.A.; Yong, H.S.; Doo, K.W.; Choi, S.I. Coronary artery vasculitis: Assessment with cardiac multi-detector computed tomography. Int. J. Cardiovasc. Imaging 2015, 31 (Suppl. 1), 59–67. [Google Scholar] [CrossRef]
- Warrington, K.J.; Matteson, E.L. A primer on vasculitis. Minn. Med. 2013, 96, 36–39. [Google Scholar] [PubMed]
- Hankard, A.; Puéchal, X.; Martin Silva, N.; Deshayes, S.; Lorcy, N.; Le Gallou, T.; Carron, P.L.; Daugas, E.; Kaplanski, G.; Boutemy, J.; et al. Characteristics of ANCA-associated vasculitis with aneurysms: Case series and review of the literature. Autoimmun. Rev. 2023, 22, 103293. [Google Scholar] [CrossRef] [PubMed]
- Gori, T. Coronary Vasculitis. Biomedicines 2021, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- Greigert, H.; Genet, C.; Ramon, A.; Bonnotte, B.; Samson, M. New Insights into the Pathogenesis of Giant Cell Arteritis: Mechanisms Involved in Maintaining Vascular Inflammation. J. Clin. Med. 2022, 11, 2905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Espígol-Frigolé, G.; Planas-Rigol, E.; Ohnuki, H.; Salvucci, O.; Kwak, H.; Ravichandran, S.; Luke, B.; Cid, M.C.; Tosato, G. Identification of IL-23p19 as an endothelial proinflammatory peptide that promotes gp130-STAT3 signaling. Sci. Signal. 2016, 9, ra28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Neill, L.; McCormick, J.; Gao, W.; Veale, D.J.; McCarthy, G.M.; Murphy, C.C.; Fearon, U.; Molloy, E.S. Interleukin-6 does not upregulate pro-inflammatory cytokine expression in an ex vivo model of giant cell arteritis. Rheumatol. Adv. Pract. 2019, 3, rkz011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arnaud, L.; Haroche, J.; Mathian, A.; Gorochov, G.; Amoura, Z. Pathogenesis of Takayasu’s arteritis: A 2011 update. Autoimmun. Rev. 2011, 11, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Vaideeswar, P.; Deshpande, J.R. Pathology of Takayasu arteritis: A brief review. Ann. Pediatr. Cardiol. 2013, 6, 52–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Espinoza, J.L.; Ai, S.; Matsumura, I. New Insights on the Pathogenesis of Takayasu Arteritis: Revisiting the Microbial Theory. Pathogens 2018, 7, 73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hara, T.; Yamamura, K.; Sakai, Y. The up-to-date pathophysiology of Kawasaki disease. Clin. Transl. Immunol. 2021, 10, e1284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noval Rivas, M.; Arditi, M. Kawasaki disease: Pathophysiology and insights from mouse models. Nat. Rev. Rheumatol. 2020, 16, 391–405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kitching, A.R.; Anders, H.J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.S.; et al. ANCA-associated vasculitis. Nat. Rev. Dis. Primers 2020, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Pakbaz, M.; Pakbaz, M. Cardiac Involvement in Eosinophilic Granulomatosis with Polyangiitis: A Meta-Analysis of 62 Case Reports. J. Tehran Heart Cent. 2020, 15, 18–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dalia, T.; Parashar, S.; Patel, N.V.; Gautam, A.; Dai, H.; Bormann, S. Eosinophilic Myocarditis Demonstrated Using Cardiac Magnetic Resonance Imaging in a Patient with Eosinophilic Granulomatosis with Polyangiitis (Churg-Strauss Disease). Cureus 2018, 10, e2792. [Google Scholar] [CrossRef]
- Misra, D.P.; Shenoy, S.N. Cardiac involvement in primary systemic vasculitis and potential drug therapies to reduce cardiovascular risk. Rheumatol. Int. 2017, 37, 151–167. [Google Scholar] [CrossRef] [PubMed]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Johnston, S.L.; Lock, R.J.; Gompels, M.M. Takayasu arteritis: A review. J. Clin. Pathol. 2002, 55, 481–486. [Google Scholar] [CrossRef]
- Guillevin, L.; Mahr, A.; Callard, P.; Godmer, P.; Pagnoux, C.; Leray, E.; Cohen, P.; French Vasculitis Study Group. Hepatitis B virus–associated polyarteritis nodosa: Clinical characteristics, outcome, and impact of treatment in 115 patients. Medicine 2005, 84, 313–322. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kronbichler, A.; Bajema, I.M.; Bruchfeld, A.; Mastroianni Kirsztajn, G.; Stone, J.H. Diagnosis and management of ANCA-associated vasculitis. Lancet 2024, 403, 683–698. [Google Scholar] [CrossRef]
- Groh, M.; Pagnoux, C.; Baldini, C.; Bel, E.; Bottero, P.; Cottin, V.; Dalhoff, K.; Dunogué, B.; Gross, W.; Holle, J.; et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): Evolution of disease classifications and treatments over time. J. Autoimmun. 2015, 65, 40–47. [Google Scholar]
- Mavrogeni, S.; Papadopoulos, G.; Douskou, M.; Kaklis, S.; Seimenis, I.; Baras, P.; Nikolaidou, P.; Bakoula, C.; Karanasios, E.; Manginas, A.; et al. Magnetic resonance angiography is equivalent to X-ray coronary angiography for the evaluation of coronary arteries in Kawasaki disease. J. Am. Coll. Cardiol. 2004, 43, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.; Papadopoulos, G.; Douskou, M.; Kaklis, S.; Seimenis, I.; Varlamis, G.; Karanasios, E.; Krikos, X.; Giannoulia, A.; Cokkinos, D.V. Magnetic resonance angiography, function and viability evaluation in patients with Kawasaki disease. J. Cardiovasc. Magn. Reson. 2006, 8, 493–498. [Google Scholar] [CrossRef]
- Singh, S.; Jindal, A.K.; Pilania, R.K. Diagnosis of Kawasaki disease. Int. J. Rheum. Dis. 2018, 21, 36–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khanna, S.; Garikapati, K.; Goh, D.S.L.; Cho, K.; Lo, P.; Bhojaraja, M.V.; Tarafdar, S. Coronary artery vasculitis: A review of current literature. BMC Cardiovasc. Disord. 2021, 21, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimizu, T.; Sato, A.; Sakamoto, K.; Seino, Y.; Kijima, M.; Matsumoto, T.; Takeishi, Y. Intravascular ultrasound imaging of isolated and non aorto-ostial coronary Takayasu arteritis: A case report. BMC Cardiovasc. Disord. 2020, 20, 260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thangathurai, J.; Kalashnikova, M.; Takahashi, M.; Shinbane, J.S. Coronary Artery Aneurysm in Kawasaki Disease: Coronary CT Angiography through the Lens of Pathophysiology and Differential Diagnosis. Radiol. Cardiothorac. Imaging 2021, 3, e200550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andreini, D.; Belmonte, M.; Penicka, M.; Van Hoe, L.; Mileva, N.; Paolisso, P.; Nagumo, S.; Nørgaard, B.L.; Ko, B.; Otake, H.; et al. Impact of coronary CT image quality on the accuracy of the FFRCT Planner. Eur. Radiol. 2024, 34, 2677–2688. [Google Scholar] [CrossRef] [PubMed]
- Misra, D.P.; Sharma, A.; Karpouzas, G.A.; Kitas, G.D. Cardiovascular risk in vasculitis. Best Pract. Res. Clin. Rheumatol. 2023, 37, 101831. [Google Scholar] [CrossRef]
- Pagnoux, C.; Guillevin, L. Cardiac involvement in small and medium-sized vessel vasculitides. Lupus 2005, 14, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Plastiras, S.C.; Moutsopoulos, H.M. Arrhythmias and Conduction Disturbances in Autoimmune Rheumatic Disorders. Arrhythm. Electrophysiol. Rev. 2021, 10, 17–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soulaidopoulos, S.; Madenidou, A.V.; Daoussis, D.; Melissaropoulos, K.; Mavrogeni, S.; Kitas, G.; Dimitroulas, T. Cardiovascular Disease in the Systemic Vasculitides. Curr. Vasc. Pharmacol. 2020, 18, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Cohen Tervaert, J.W. Cardiovascular disease due to accelerated atherosclerosis in systemic vasculitides. Best Pract. Res. Clin. Rheumatol. 2013, 27, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Anzai, F.; Yoshihisa, A.; Takeishi, R.; Hotsuki, Y.; Sato, Y.; Sumita, Y.; Nakai, M.; Misaka, T.; Takeishi, Y. Acute myocardial infarction caused by Kawasaki disease requires more intensive therapy: Insights from the Japanese registry of All Cardiac and Vascular Diseases-Diagnosis Procedure combination. Catheter. Cardiovasc. Interv. 2022, 100, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Serpytis, P.; Petrulioniene, Z.; Gargalskaite, U.; Gedminaite, A.; Panaviene, V. Myocardial infarction associated with Kawasaki disease in adult man: Case report and review of literature. Am. J. Med. 2015, 128, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Peng, B.; Tu, X.; Zhang, S.; Zhong, S.; Cao, W. Acute myocardial infarction as the first manifestation of Takayasu arteritis: A case report. Medicine 2019, 98, e15143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zanatta, E.; Colombo, C.; D’Amico, G.; d’Humières, T.; Dal Lin, C.; Tona, F. Inflammation and Coronary Microvascular Dysfunction in Autoimmune Rheumatic Diseases. Int. J. Mol. Sci. 2019, 20, 5563. [Google Scholar] [CrossRef]
- Vandeloo, B.; Andreini, D.; Brouwers, S.; Mizukami, T.; Monizzi, G.; Lochy, S.; Mileva, N.; Argacha, J.F.; De Boulle, M.; Muyldermans, P.; et al. Diagnostic performance of exercise stress tests for detection of epicardial and microvascular coronary artery disease: The UZ Clear study. EuroIntervention 2023, 18, e1090–e1098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernández-Rodríguez, J.; Murgia, G.; Villar, I.; Campo, E.; Mackie, S.L.; Chakrabarty, A.; Hensor, E.M.A.; Morgan, A.W.; Font, C.; Prieto-González, S.; et al. Description and Validation of Histological Patterns and Proposal of a Dynamic Model of Inflammatory Infiltration in Giant-cell Arteritis. Medicine 2016, 95, e2368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takahashi, K.; Oharaseki, T.; Yokouchi, Y. Histopathological aspects of cardiovascular lesions in Kawasaki disease. Int. J. Rheum. Dis. 2018, 21, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.; Tiwari, V. Polyarteritis Nodosa. [Updated 2023 Feb 22]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482157/ (accessed on 19 September 2024).
- Masiak, A.; Zdrojewski, Z.; Pęksa, R.; Smoleńska, Ż.; Czuszyńska, Z.; Siemińska, A.; Kowalska, B.; Stankiewicz, C.; Rutkowski, B.; Bułło-Piontecka, B. The usefulness of histopathological examinations of non-renal biopsies in diagnosing granulomatosis with polyangiitis. Reumatologia 2017, 55, 230–236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gioffredi, A.; Maritati, F.; Oliva, E.; Buzio, C. Eosinophilic granulomatosis with polyangiitis: An overview. Front. Immunol. 2014, 5, 549. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, H.J.; Jung, S.M.; Song, J.J.; Park, Y.B.; Song, J.S.; Lee, S.W. Comparison of Radiological and Histological Findings of Lung Parenchyma in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Yonsei Med. J. 2019, 60, 454–460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demirkesen, C.; Öz, B.; Göksel, S. Behçet’s Disease: Pathology. In Behçet’s Syndrome; Yazıcı, Y., Yazıcı, H., Eds.; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Araújo Correia, J.; Crespo, J.; Alves, G.; Salvador, F.; Matos-Costa, J.; Alves, J.D.; Fortuna, J.; Almeida, I.; Campar, A.; Brandão, M.; et al. Biologic therapy in large and small vessels vasculitis, and Behçet’s disease: Evidence- and practice-based guidance. Autoimmun. Rev. 2023, 22, 103362. [Google Scholar] [CrossRef] [PubMed]
- Kotake, T.; Sueyoshi, E.; Sakamoto, I.; Izumida, S. Myocarditis associated with Takayasu arteritis. Eur. Heart J. 2015, 36, 2564. [Google Scholar] [CrossRef]
- Merkel, P.A.; Niles, J.L.; Mertz, L.E.; Lehane, P.B.; Pordeli, P.; Erblang, F. Long-Term Safety of Rituximab in Granulomatosis With Polyangiitis and in Microscopic Polyangiitis. Arthritis Care Res. 2021, 73, 1372–1378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geetha, D.; Kallenberg, C.; Stone, J.H.; Salama, A.D.; Appel, G.B.; Duna, G.; Brunetta, P.; Jayne, D. Current therapy of granulomatosis with polyangiitis and microscopic polyangiitis: The role of rituximab. J. Nephrol. 2015, 28, 17–27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puéchal, X. Granulomatosis with polyangiitis (Wegener’s). Jt. Bone Spine 2020, 87, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, H.; Huang, Q.; Zheng, Y. Optimizing glucocorticoid therapy for Behçet’s uveitis: Efficacy, adverse effects, and advances in combination approaches. Int. Ophthalmol. 2023, 43, 4373–4381. [Google Scholar] [CrossRef]
- Alpsoy, E.; Leccese, P.; Emmi, G.; Ohno, S. Treatment of Behçet’s Disease: An Algorithmic Multidisciplinary Approach. Front. Med. 2021, 8, 624795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demirelli, S.; Degirmenci, H.; Inci, S.; Arisoy, A. Cardiac manifestations in Behcet’s disease. Intractable Rare Dis. Res. 2015, 4, 70–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wardle, A.J.; Connolly, G.M.; Seager, M.J.; Tulloh, R.M. Corticosteroids for the treatment of Kawasaki disease in children. Cochrane Database Syst. Rev. 2017, 1, CD011188, Update in: Cochrane Database Syst. Rev. 2022, 5, CD011188. https://doi.org/10.1002/14651858.CD011188.pub3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ozen, S.; Batu, E.D. Vasculitis Pathogenesis: Can We Talk about Precision Medicine? Front. Immunol. 2018, 9, 1892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, B.Y.; Kim, D.; Kim, Y.H.; Ryoo, E.; Sun, Y.H.; Jeon, I.S.; Jung, M.J.; Cho, H.K.; Tchah, H.; Choi, D.Y.; et al. Non-Responders to Intravenous Immunoglobulin and Coronary Artery Dilatation in Kawasaki Disease: Predictive Parameters in Korean Children. Korean Circ. J. 2016, 46, 542–549. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kikuchi, S.; Okada, K.; Hibi, K.; Maejima, N.; Yabu, N.; Uchida, K.; Tamura, K.; Kimura, K. Coronary arteritis: A case series. Eur. Heart J. Case Rep. 2020, 4, 1–6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mourguet, M.; Chauveau, D.; Faguer, S.; Ruidavets, J.B.; Béjot, Y.; Ribes, D.; Huart, A.; Alric, L.; Balardy, L.; Astudillo, L.; et al. Increased ischemic stroke, acute coronary artery disease and mortality in patients with granulomatosis with polyangiitis and microscopic polyangiitis. J. Autoimmun. 2019, 96, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, L.; Polcwiartek, C.; Nelveg-Kristensen, K.E.; Carlson, N.; Kristensen, S.; Torp-Pedersen, C.; Gregersen, J.W. Long-term cardiovascular outcomes and temporal trends in patients diagnosed with ANCA-associated vasculitis: A Danish nationwide registry study. Rheumatology 2023, 62, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Wallace, Z.S.; Fu, X.; Harkness, T.; Stone, J.H.; Zhang, Y.; Choi, H. All-cause and cause-specific mortality in ANCA-associated vasculitis: Overall and according to ANCA type. Rheumatology 2020, 59, 2308–2315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Z.; Zhang, H.; Wang, M.; Yang, W.; Qiao, S.; Hu, F. Revascularization Versus Medical Therapy in Takayasu’s Arteritis Patients with Coronary Artery Involvement. Rheumatol. Therapy 2021, 8, 119–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Regola, F.; Cerudelli, E.; Bosio, G.; Andreoli, L.; Tincani, A.; Franceschini, F.; Toniati, P. Long-term treatment with tocilizumab in giant cell arteritis: Efficacy and safety in a monocentric cohort of patients. Rheumatol. Adv. Pract. 2020, 4, rkaa017. [Google Scholar] [CrossRef]
- Farrah, T.E.; Basu, N.; Dweck, M.; Calcagno, C.; Fayad, Z.A.; Dhaun, N. Advances in Therapies and Imaging for Systemic Vasculitis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1520–1541. [Google Scholar] [CrossRef] [PubMed]
- Poledniczek, M.H. Coronary Artery Disease in Granulomatosis with Polyangiitis: A Review. SN Compr. Clin. Med. 2022, 4, 75. [Google Scholar] [CrossRef]
- Yap, B.J.M.; Lai-Foenander, A.S.; Goh, B.H.; Ong, Y.S.; Duangjai, A.; Saokaew, S.; Chua, C.L.L.; Phisalprapa, P.; Yap, W.H. Unraveling the Immunopathogenesis and Genetic Variants in Vasculitis toward Development of Personalized Medicine. Front. Cardiovasc. Med. 2021, 8, 732369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lozano, E.; Segarra, M.; Corbera-Bellalta, M.; García-Martínez, A.; Espígol-Frigolé, G.; Plà-Campo, A.; Hernández-Rodríguez, J.; Cid, M.C. Increased expression of the endothelin system in arterial lesions from patients with giant-cell arteritis: Association between elevated plasma endothelin levels and the development of ischaemic events. Ann. Rheum. Dis. 2010, 69, 434–442. [Google Scholar] [CrossRef]
- Planas-Rigol, E.; Terrades-Garcia, N.; Corbera-Bellalta, M.; Lozano, E.; Alba, M.A.; Segarra, M.; Espígol-Frigolé, G.; Prieto-González, S.; Hernández-Rodríguez, J.; Preciado, S.; et al. Endothelin-1 promotes vascular smooth muscle cell migration across the artery wall: A mechanism contributing to vascular remodelling and intimal hyperplasia in giant-cell arteritis. Ann. Rheum. Dis. 2017, 76, 1624–1634. [Google Scholar] [CrossRef]
- US National Library of Medicine. Abatacept for the Treatment of Relapsing, Non-Severe, Granulomatosis with Polyangiitis (Wegener’s). Available online: https://clinicaltrials.gov/ct2/show/NCT02108860 (accessed on 19 September 2024).
- Hu, Y.; Turner, M.J.; Shields, J.; Gale, M.S.; Hutto, E.; Roberts, B.L.; Siders, W.M.; Kaplan, J.M. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology 2009, 128, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Chaudhry, A.; Jayne, D. Long-term follow-up of relapsing/refractory anti-neutrophil cytoplasm antibody associated vasculitis treated with the lymphocyte depleting antibody alemtuzumab (CAMPATH-1H). Ann. Rheum. Dis. 2008, 67, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Gopaluni, S.; Smith, R.; Goymer, D.; Cahill, H.; Broadhurst, E.; Wallin, E.; McClure, M.; Chaudhry, A.; Jayne, D. Alemtuzumab for refractory primary systemic vasculitis-a randomised controlled dose ranging clinical trial of efficacy and safety (ALEVIATE). Arthritis Rheum. Therapy 2022, 24, 81. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Petri, M.; Zamani, O.; Cervera, R.; Wallace, D.J.; Tegzová, D.; Sanchez-Guerrero, J.; Schwarting, A.; Merrill, J.T.; Chatham, W.W.; et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 3918–3930. [Google Scholar] [CrossRef]
- Jayne, D.R.W.; Bruchfeld, A.N.; Harper, L.; Schaier, M.; Venning, M.C.; Hamilton, P.; Burst, V.; Grundmann, F.; Jadoul, M.; Szombati, I.; et al. Randomized Trial of C5a receptor inhibitor avacopan in ANCA-Associated vasculitis. J. Am. Soc. Nephrol. 2017, 28, 2756–2767. [Google Scholar] [CrossRef]
- US National Library of Medicine. A Phase 3 Clinical Trial of CCX168 (Avacopan) in Patients with ANCA-Associated Vasculitis (ADVOCATE). Available online: https://clinicaltrials.gov/ct2/show/NCT02994927 (accessed on 19 September 2024).
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.; Klion, A.; Langford, C.A.; Merkel, P.A.; Moosig, F.; Specks, U.; Cid, M.C.; et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).