Perceived Health Status Predicts Resilience after Hip Fracture in Older People
Abstract
:1. Introduction
2. Methods
2.1. Data Source
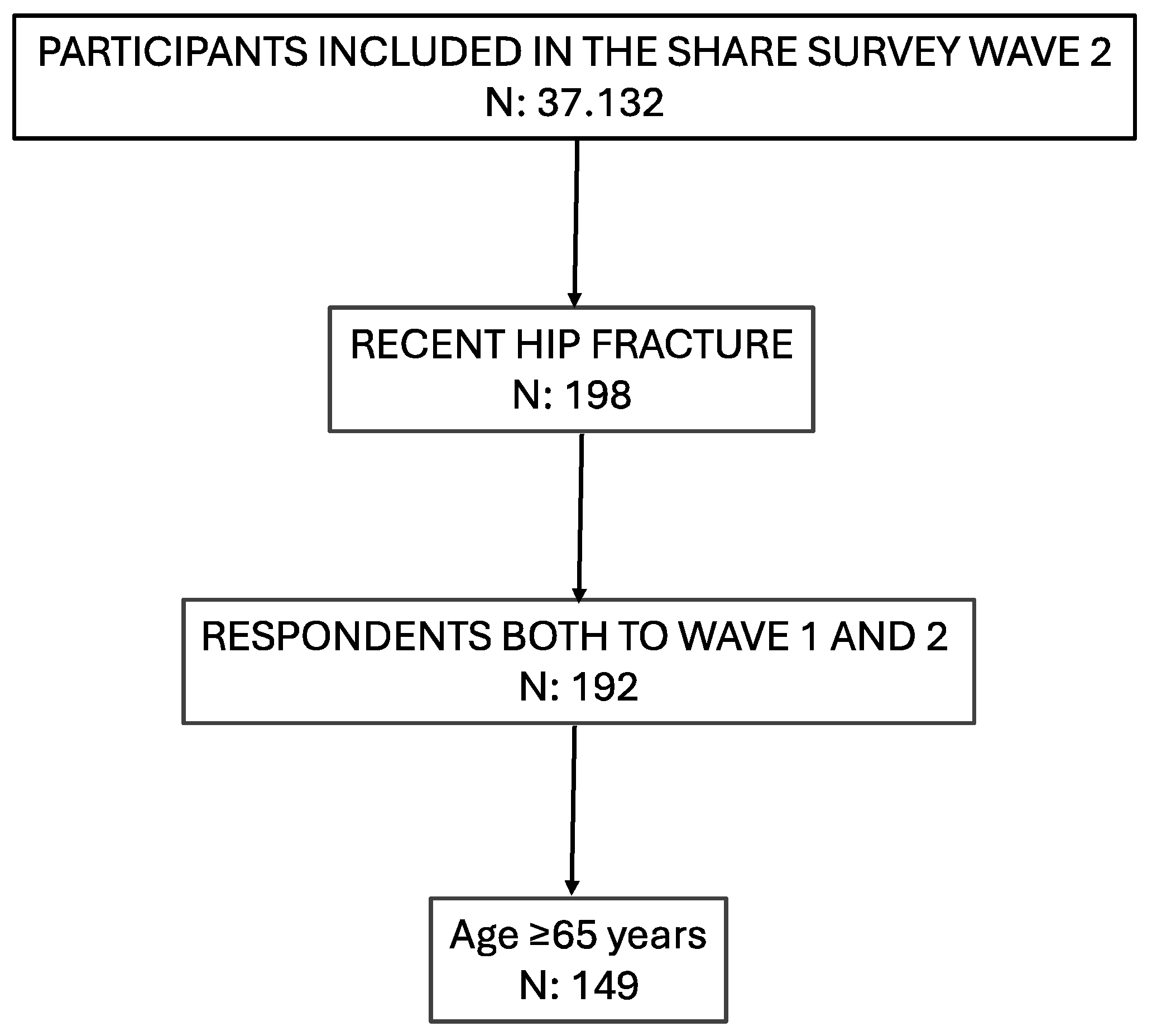
2.2. Sample Selection
2.3. Measure of Exposure
2.4. Study Outcomes
2.5. Study Variables
2.6. Analytic Approach
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lima, G.S.; Figueira, A.L.G.; de Carvalho, E.C.; Kusumota, L.; Caldeira, S. Resilience in Older People: A Concept Analysis. Healthcare 2023, 11, 2491. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.G.; Carr, D. Psychological Resilience and Health among Older Adults: A Comparison of Personal Resources. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 76, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Resnick, B.; Galik, E.; Dorsey, S.; Scheve, A.; Gutkin, S. Reliability and validity testing of the physical resilience measure. Gerontologist 2011, 51, 643–652. [Google Scholar] [CrossRef]
- Park, E.R.; Luberto, C.M.; Chad-Friedman, E.; Traeger, L.; Hall, D.; Perez, G.; Goshe, B.; Vranceanu, A.M.; Baim, M.; PhD, J.; et al. A Comprehensive Resiliency Framework: Theoretical Model, Treatment, and Evaluation. Glob. Adv. Health Med. 2021, 10, 21649561211000306. [Google Scholar] [CrossRef]
- Traeger, L.; Styklunas, G.M.; Park, E.Y.; Lee, M.T.; Fricchione, G.; Park, E.R. Promoting Resilience and Flourishing Among Older Adult Residents in Community Living: A Feasibility Study. Gerontologist 2022, 62, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Whitson, H.E.; Duan-Porter, W.; Schmader, K.E.; Morey, M.C.; Cohen, H.J.; Colón-Emeric, C.S. Physical resilience in older adults: Systematic review and development of an emerging construct. J. Gerontol. Ser. A. Biol. Sci. Med. Sci. 2016, 71, 489–495. [Google Scholar] [CrossRef]
- Rousari, M.S.; Payab, M.; Shahrestanaki, S.K.; Ebrahimpur, M.; Mehrdad, N.; Alhosseini, S.; Bidmeshgipour, F.; Adibi, H.; Astaraei, A.; Hosseini, R.; et al. Self-perceived health and functional status of older people: Telephone-based lifestyle survey of older adults in Tehran province. Health. Promot. Perspect. 2022, 12, 37–44. [Google Scholar] [CrossRef]
- Dramé, M.; Cantegrit, E.; Godaert, L. Self-Rated Health as a Predictor of Mortality in Older Adults: A Systematic Review. Int. J. Environ. Res. Public. Health 2023, 20, 3813. [Google Scholar] [CrossRef]
- Szybalska, A.; Broczek, K.; Puzianowska-Kuznicka, M.; Slusarczyk, P.; Chudek, J.; Skalska, A.; Mossakowska, M. Self-rated health and its association with all-cause mortality of older adults in Poland: The PolSenior project. Arch. Gerontol. Geriatr. 2018, 79, 13–20. [Google Scholar] [CrossRef]
- Mu, S.Z.; Hicks, C.W.; Daya, N.R.; Foraker, R.; Kucharska-Newton, A.; Lutsey, P.; Coresh, J.; Selvin, E. Self-Rated Health in Middle Age and Risk of Hospitalizations and Death: Recurrent Event Analysis of the ARIC Study. J. Gen. Intern. Med. 2024. ahead of print. [Google Scholar] [CrossRef]
- Pedone, C.; Costanzo, L.; Finamore, P.; Bandinelli, S.; Ferrucci, L.; Antonelli Incalzi, R. Defining Resilience in Older People: Does a Subjective Definition of Stressor Work? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Sing, C.W.; Lin, T.C.; Bartholomew, S.; Bell, J.; Bennet, C.; Beyene, K.; Bosco, P.; Chan, A.; Chandran, M.; Cheung, C.L.; et al. Global epidemiology of hip fractures: A study protocol using a common analytical platform among multiple countries. BMJ Open 2021, 11, e047258. [Google Scholar] [CrossRef]
- Cooper, C. The Crippling Consequences of Fractures and Their Impact on Quality of Life. Am. J. Med. 1997, 103, 12S–17S. [Google Scholar] [CrossRef] [PubMed]
- Dyer, S.M.; Crotty, M.; Fairhall, N.; Magaziner, J.; Beaupre, L.A.; Cameron, I.D.; Sherrington, C.; for the Fragility Fracture Network (FFN) Rehabilitation Research Special Interest Group. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016, 16, 158. [Google Scholar] [CrossRef]
- SHARE. Available online: http://www.share-project.org/organisation/share-eric.html (accessed on 19 May 2024).
- Börsch-Supan, A.; Brandt, M.; Hunkler, C.; Kneip, T.; Korbmacher, J.; Malter, F.; Schaan, B.; Stuck, S.; Zuber, S.; on behalf of the SHARE Central Coordination Team. Data resource profile: The survey of health, ageing and retirement in Europe (share). Int. J. Epidemiol. 2013, 42, 992–1001. [Google Scholar] [CrossRef]
- Borsch-Supan, A.; Jurges, H. (Eds.) The Survey of Health, Ageing and Retirement in Europe—Methodology; Mannheim Research Institute for the Economics of Aging (MEA): Mannheim, Germany, 2005. [Google Scholar]
- Prince, M.J.; Reischies, F.; Beekman, A.T.F.; Fuhrer, R.; Jonker, C.; Kivelä, S.L.; Lawlor, B.; Lobo, A.; Magnusson, A.; MM, F.; et al. Development of the EURO-D scale—A European Union initiative to compare symptoms of depression in 14 European centres. Br. J. Psychiatry. 1999, 174, 330–338. [Google Scholar] [CrossRef]
- Guerra, M.; Ferri, C.; Llibre, J.; Prina, A.M.; Prince, M. Psychometric properties of EURO-D, a geriatric depression scale: A cross-cultural validation study. BMC Psychiatry 2015, 15, 12. [Google Scholar] [CrossRef]
- Tomioka, K.; Kurumatani, N.; Hosoi, H. Self-rated health predicts decline in instrumental activities of daily living among high-functioning community-dwelling older people. Age Ageing 2017, 46, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Hirosaki, M.; Okumiya, K.; Wada, T.; Ishine, M.; Sakamoto, R.; Ishimoto, Y.; Kasahara, Y.; Kimura, Y.; Fukutomi, E.; Chen, W.; et al. Self-rated health is associated with subsequent functional decline among older adults in Japan. Int. Psychogeriatr. 2017, 29, 1475–1483. [Google Scholar] [CrossRef]
- Mennig, E.F.; Schäfer, S.K.; Eschweiler, G.W.; Rapp, M.A.; Thomas, C.; Wurm, S. The relationship between pre-surgery self-rated health and changes in functional and mental health in older adults: Insights from a prospective observational study. BMC Geriatr. 2023, 23, 203. [Google Scholar] [CrossRef]
- Srivastava, M.; Deal, C. Osteoporosis in elderly: Prevention and treatment. Clin. Geriatr. Med. 2002, 18, 529–555. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chhetri, J.K.; Ma, L. Physical resilience in older adults: Potential use in promoting healthy aging. Ageing Res. Rev. 2022, 81, 101701. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.; Fortinsky, R.H.; Mangione, K.; Beamer, B.; Magder, L.; Binder, E.; Craik, R.; Gruber-Baldini, A.; Orwig, D.; Resnick, B.; et al. Impact of psychological resilience on walking capacity in older adults following hip fracture. J. Am. Geriatr. Soc. 2022, 70, 3087–3095. [Google Scholar] [CrossRef] [PubMed]
- Fieo, R.A.; Austin, E.J.; Starr, J.M.; Deary, I.J. Calibrating ADL-IADL scales to improve measurement accuracy and to extend the disability construct into the preclinical range: A systematic review. BMC Geriatr. 2011, 11, 42. [Google Scholar] [CrossRef]
| Good n: 60 Mean (SD) or % | Poor n: 89 Mean (SD) or % | |
|---|---|---|
| Age | 76.30 (7.3) | 75.28 (6.0) |
| Sex (female) | 63.3 | 68.5 |
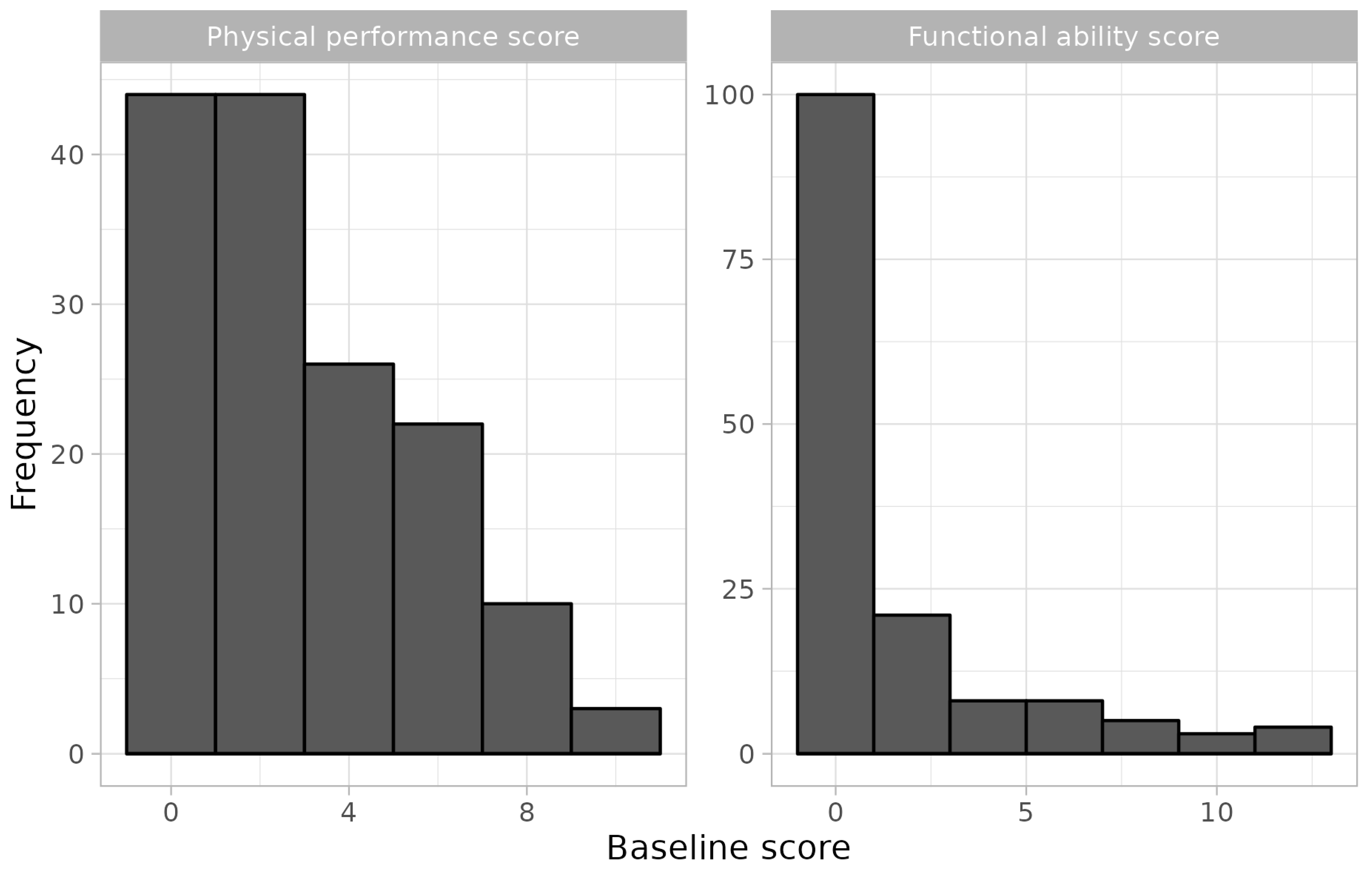
| Functional ability score | 0.73 (1.8) | 2.70 (3.5) |
| Physical function performance score | 1.80 (1.8) | 4.33 (2.7) |
| EUROD score | 2.10 (1.9) | 4.80 (2.6) |
| Number of diseases | 2.18 (1.3) | 3.13 (1.8) |
| Number of diseases pharmacologically treated | 1.62 (1.2) | 3.31 (2.0) |
| Social support | ||
| No need | 71.7 | 39.3 |
| Help | 20 | 49.4 |
| No help | 8.3 | 11.2 |
| Marital status (married or partnership) | 45 | 44.9 |
| Smoking | ||
| Former smoker | 26.7 | 20.2 |
| Smoker | 18.3 | 13.5 |
| Education (high school) | 31.7 | 24.1 |
| Net income | 16,112.74 (14,513.9) | 10,363.45 (7989.3) |
| Good PHS n: 60 | Poor PHS n: 89 | p-Value | |
|---|---|---|---|
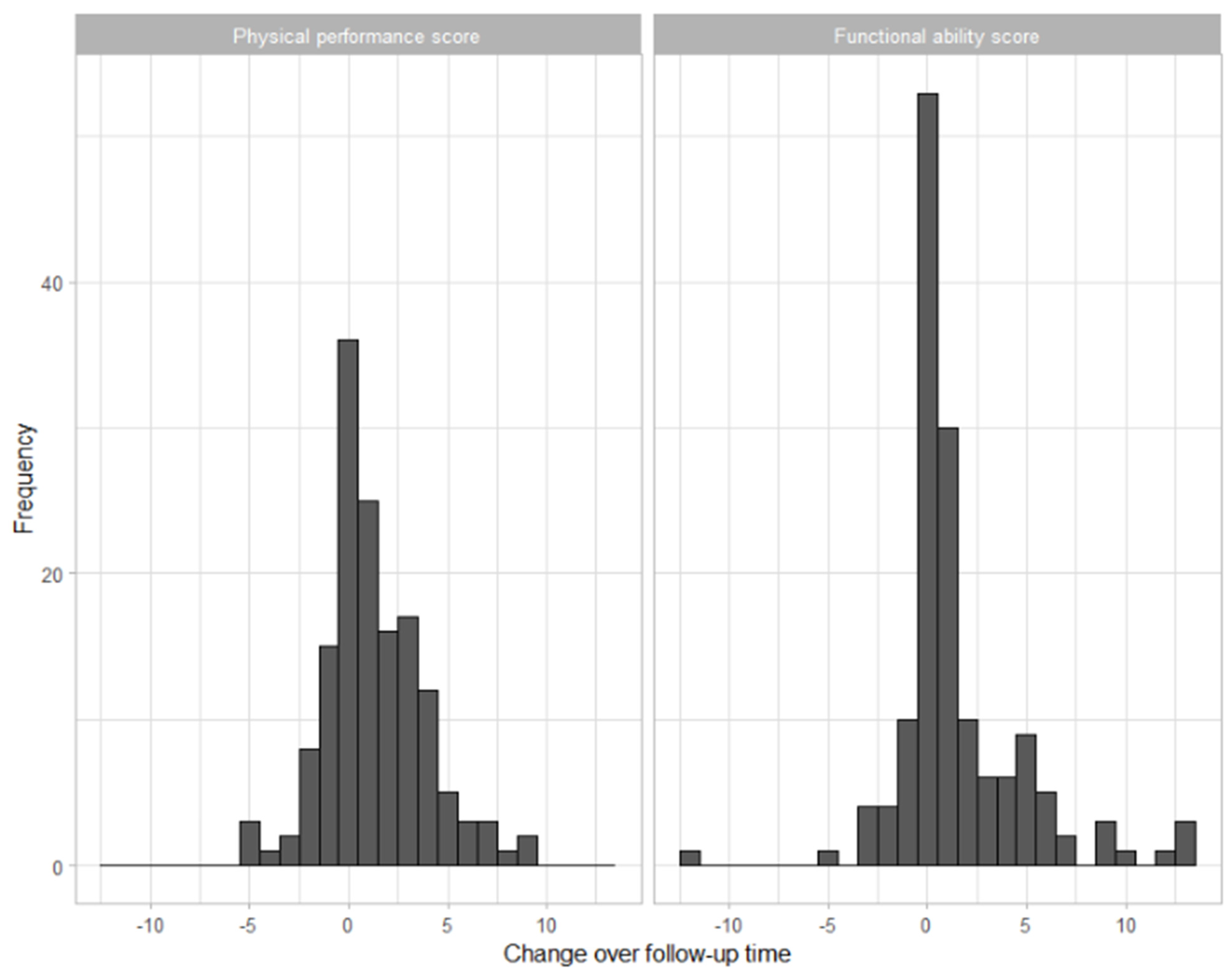
| Change in physical performance score (mean [SD]) | 1.20 (2.48) | 1.33 (2.59) | 0.398 |
| Change in functional ability score (mean [SD]) | 1.32 (2.99) | 1.61 (3.51) | 0.217 |
| Crude | Adjusted | |
|---|---|---|
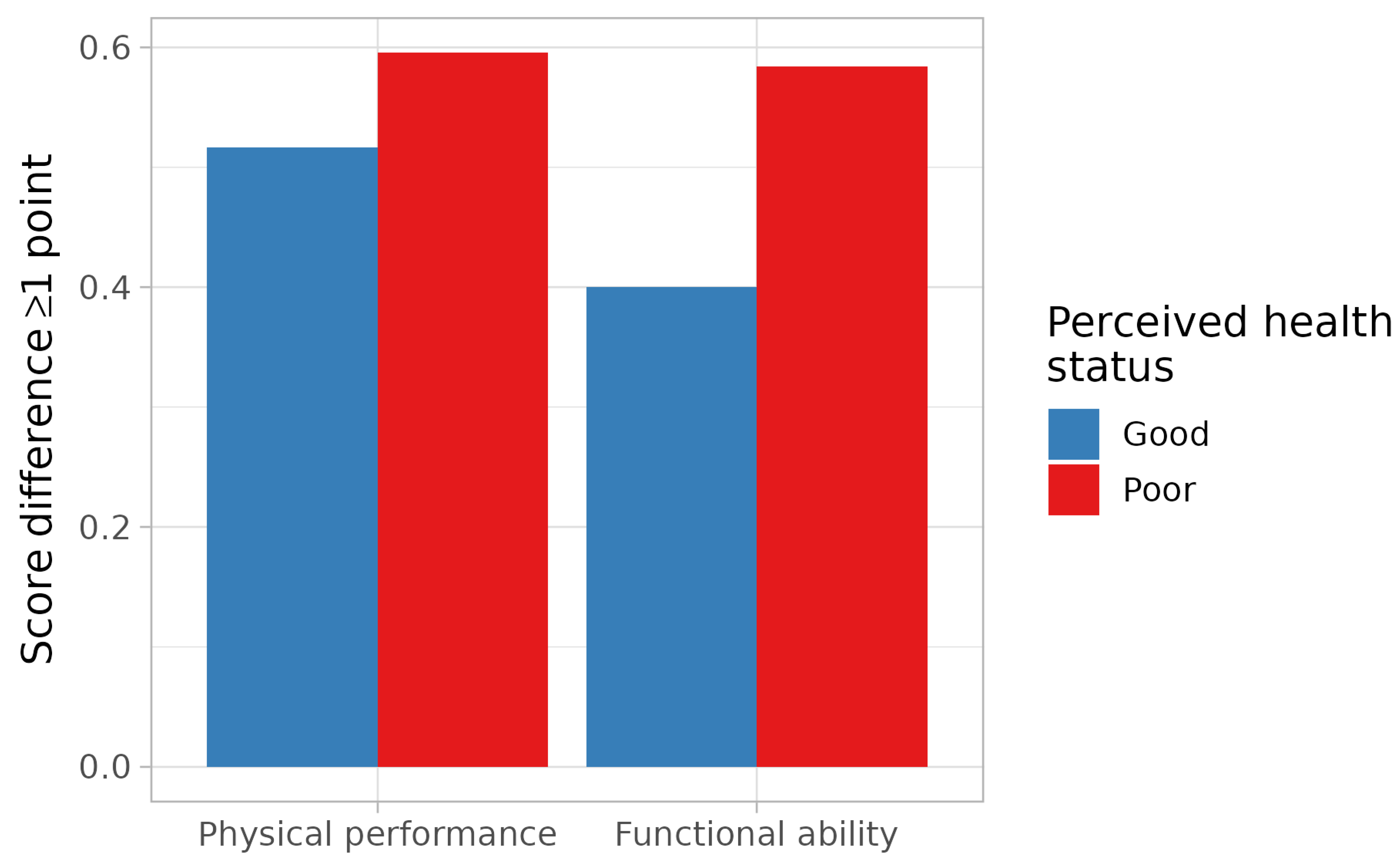
| Physical performance score | 0.87 (0.64–1.17) | 0.66 (0.41–1.06) * |
| Functional ability score | 0.68 (0.48–0.98) | 0.64 (0.40–1.04) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lelli, D.; Iuorio, M.S.; Antonelli Incalzi, R.; Pedone, C. Perceived Health Status Predicts Resilience after Hip Fracture in Older People. Medicina 2024, 60, 1621. https://doi.org/10.3390/medicina60101621
Lelli D, Iuorio MS, Antonelli Incalzi R, Pedone C. Perceived Health Status Predicts Resilience after Hip Fracture in Older People. Medicina. 2024; 60(10):1621. https://doi.org/10.3390/medicina60101621
Chicago/Turabian StyleLelli, Diana, Maria Serena Iuorio, Raffaele Antonelli Incalzi, and Claudio Pedone. 2024. "Perceived Health Status Predicts Resilience after Hip Fracture in Older People" Medicina 60, no. 10: 1621. https://doi.org/10.3390/medicina60101621






