Saccharomyces boulardii Mitigates Fructose-Induced Non-Alcoholic Fatty Liver in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocol
2.3. Plasma Lipid Peroxidation Analysis
2.4. Blood Biochemistry
2.5. Liver Biochemical Analysis
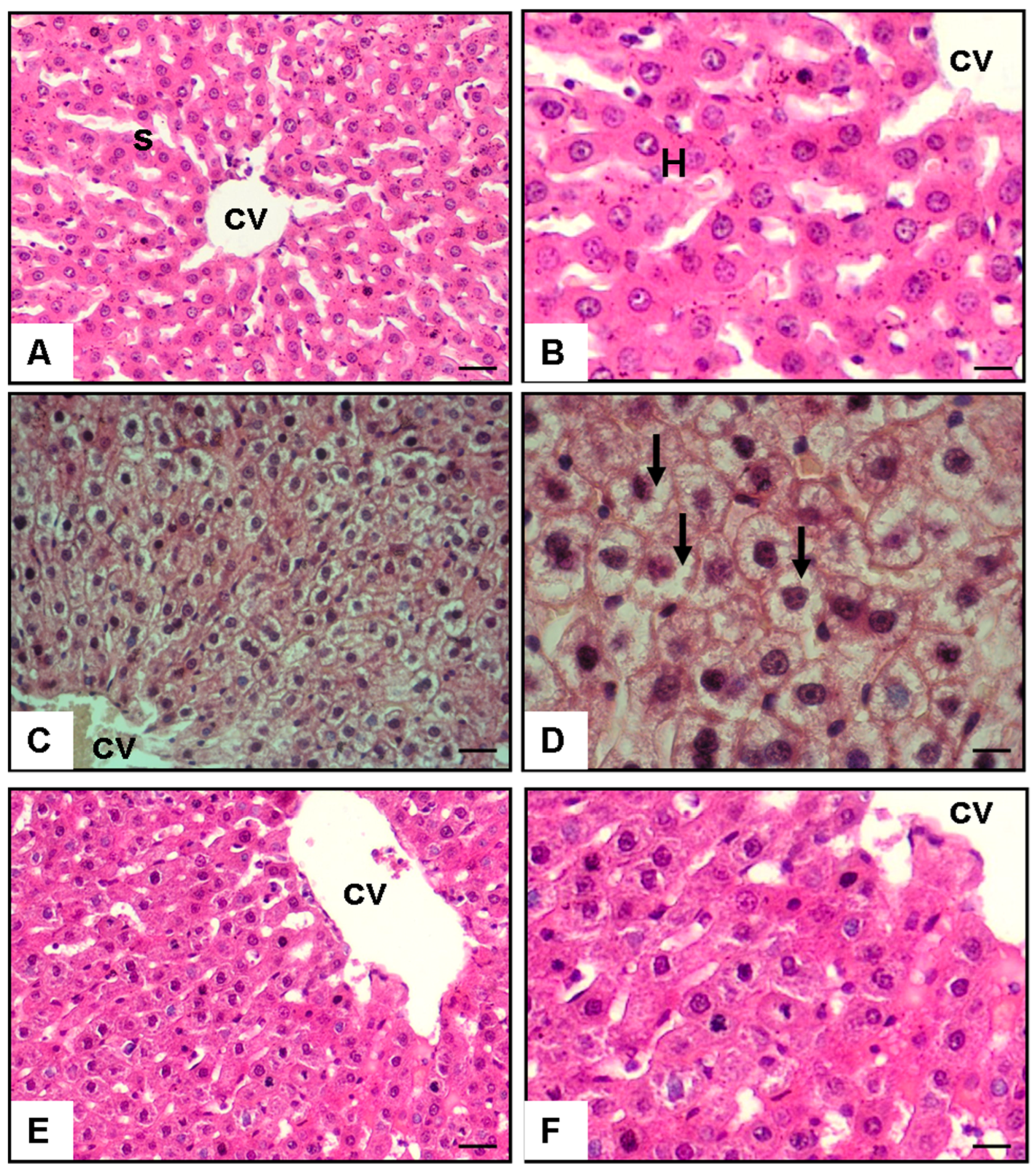
2.6. Histopathological Assessment of the Liver
2.7. Statistical Analysis
3. Results
3.1. Effects of S. boulardii on Body Weight, Food Consumption, and Tissue Weights
3.2. Histopathological and Quantitative Analyses of Lipid Accumulation
3.3. Effects of S. boulardii on Biochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doulberis, M.; Kotronis, G.; Gialamprinou, D.; Kountouras, J.; Katsinelos, P. Non-alcoholic fatty liver disease: An update with special focus on the role of gut microbiota. Metabolism 2017, 71, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Finelli, C.; Tarantino, G. Is there any consensus as to what diet or lifestyle approach is the right one for NAFLD patients. J. Gastrointestin. Liver Dis. 2012, 21, 293–302. [Google Scholar] [PubMed]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; DeFronzo, R.A.; Bugianesi, E.; Gastaldelli, A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013, 5, 1544–1560. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Zou, J.; Ran, W.; Qi, X.; Chen, Y.; Cui, H.; Guo, J. Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD). Front. Endocrinol. 2023, 13, 1087260. [Google Scholar] [CrossRef]
- Szóstak, N.; Figlerowicz, M.; Philips, A. The emerging role of the gut mycobiome in liver diseases. Gut Microbes 2023, 15, 2211922. [Google Scholar] [CrossRef]
- Al-Muzafar, H.M.; Amin, K.A. Probiotic mixture improves fatty liver disease by virtue of its action on lipid profiles, leptin, and inflammatory biomarkers. BMC Complement. Altern. Med. 2017, 17, 43. [Google Scholar] [CrossRef]
- Gholam, P.M.; Flancbaum, L.; Machan, J.T.; Charney, D.A.; Kotler, D.P. Nonalcoholic fatty liver disease in severely obese subjects. Am. J. Gastroenterol. 2007, 102, 399–408. [Google Scholar] [CrossRef]
- Nabavi, S.; Rafraf, M.; Somi, M.H.; Homayouni-Rad, A.; Asghari-Jafarabadi, M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J. Dairy Sci. 2014, 97, 7386–7393. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef]
- Carreras, F.I.; Gradilone, S.A.; Mazzone, A.; García, F.; Huang, B.Q.; Ochoa, J.E.; Marinelli, R.A. Rat hepatocyte aquaporin-8 water channels are down-regulated in extrahepatic cholestasis. Hepatology 2003, 37, 1026–1033. [Google Scholar] [CrossRef]
- Xiang, M.; Qian, X.; Han, L.; Wang, H.; Wang, J.; Liu, W.; Gu, Y.; Yao, S.; Yang, J.; Zhang, Y.; et al. Aquaporin-8 ameliorates hepatic steatosis through farnesoid X receptor in obese mice. iScience 2023, 26, 104552. [Google Scholar] [CrossRef]
- Huebert, R.C.; Splinter, P.L.; Garcia, F.; Marinelli, R.A.; LaRusso, N.F. Expression and localization of aquaporin water channels in rat hepatocytes: Evidence for a role in canalicular bile secretion. J. Biol. Chem. 2002, 277, 22710–22717. [Google Scholar] [CrossRef]
- Lang, S.; Duan, Y.; Liu, J.; Torralba, M.G.; Kuelbs, C.; Ventura-Cots, M.; Abraldes, J.G.; Bosques-Padilla, F.; Verna, E.C.; Brown, R.S.; et al. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology 2020, 71, 522–538. [Google Scholar] [CrossRef]
- Scarpellini, E.; Campanale, M.; Leone, D.; Purchiaroni, F.; Vitale, G.; Lauritano, E.C.; Gasbarrini, A. Gut microbiota and obesity. Intern. Emerg. Med. 2010, 5, 53–56. [Google Scholar] [CrossRef]
- Mar Rodríguez, M.; Pérez, D.; Javier Chaves, F.; Esteve, E.; Marin-Garcia, P.; Xifra, G.; Vendrell, J.; Jove, M.; Pamplona, R.; Ricart, W.; et al. Obesity changes the human gut mycobiome. Sci. Rep. 2015, 5, 14600. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Liu, E.J.; Kheradman, R.; Fagan, A.; Heuman, D.M.; White, M.; Gavis, E.A.; Hylemon, P.; Sikaroodi, M.; Gillevet, P.M. Fungal dysbiosis in cirrhosis. Gut 2018, 67, 1146–1154. [Google Scholar] [CrossRef]
- Lemoinne, S.; Kemgang, A.; Belkacem, K.B.; Straube, M.; Jegou, S.; Corpechot, C.; Network, S.I.; Chazouilleres, O.; Housset, C.; Sokol, H. Fungi participate in the dysbiosis of gut microbiota in patients with primary sclerosing cholangitis. Gut 2020, 69, 92–102. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Li, C.; Lei, G.; Zhou, L.; Chen, X.; Jia, X.; Lu, Y. Intestinal Candida albicans promotes hepatocarcinogenesis by up-regulating NLRP6. Front. Microbiol. 2022, 13, 812771. [Google Scholar] [CrossRef]
- You, N.; Xu, J.; Wang, L.; Zhuo, L.; Zhou, J.; Song, Y.; Ali, A.; Luo, Y.; Yang, J.; Yang, W.; et al. Fecal fungi dysbiosis in nonalcoholic fatty liver disease. Obesity 2021, 29, 350–358. [Google Scholar] [CrossRef]
- Meng, X.; Li, S.; Li, Y.; Gan, R.Y.; Li, H.B. Gut microbiota’s relationship with liver disease and role in hepatoprotection by dietary natural products and probiotics. Nutrients 2018, 10, 1457. [Google Scholar] [CrossRef]
- Aller, R.; De Luis, D.A.; Izaola, O.; Conde, R.; Gonzalez Sagrado, M.; Primo, D.; Gonzalez, J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: A double blind randomized clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1090–1095. [Google Scholar]
- Duman, D.G.; Kumral, Z.N.O.; Ercan, F.; Deniz, M.; Can, G.; Yegen, B.C. Saccharomyces boulardii ameliorates clarithromycin-and methotrexate-induced intestinal and hepatic injury in rats. Br. J. Nutr. 2013, 110, 493–499. [Google Scholar] [CrossRef]
- Everard, A.; Matamoros, S.; Geurts, L.; Delzenne, N.M.; Cani, P.D. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. MBio 2014, 5, e010112. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, X.K.; Cheng, M.L.; Yang, G.Z.; Wang, B.; Liu, H.J.; Mu, M. Saccharomyces boulardii administration changes gut microbiota and attenuates D-galactosamine-induced liver injury. Sci. Rep. 2017, 7, 1359. [Google Scholar] [CrossRef]
- Liu, Y.T.; Li, Y.Q.; Wang, Y.Z. Protective effect of Saccharomyces boulardii against intestinal mucosal barrier injury in rats with nonalcoholic fatty liver disease. Chin. J. Hepatol. 2016, 24, 921–926. [Google Scholar]
- Chatuphonprasert, W.; Udomsuk, L.; Monthakantirat, O.; Churikhit, Y.; Putalun, W.; Jarukamjorn, K. Effects of Pueraria mirifica and miroestrol on the antioxidation-related enzymes in ovariectomized mice. J. Pharm. Pharmacol. 2013, 65, 447–456. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, R.; Yang, X.; Dai, J.; Huang, M.; Ji, X.; Sun, C. Yogurt improves insulin resistance and liver fat in obese women with nonalcoholic fatty liver disease and metabolic syndrome: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1611–1619. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Amaretti, A.; Di Nunzio, M.; Pompei, A.; Raimondi, S.; Rossi, M.; Bordoni, A. Antioxidant properties of potentially probiotic bacteria: In vitro and in vivo activities. Appl. Microbiol. Biotechnol. 2013, 97, 809–817. [Google Scholar] [CrossRef]
- Yaghchi, S.S.; Fatemi, M.; Ghandehari, F. Comparing the effects of Saccharomyces boulardii and selenium-enriched S. boulardii on hematological parameters and total antioxidant capacity in aluminum induced toxicity in rats. J. Kermanshah Univ. Med. Sci. 2018, 22, 3. [Google Scholar] [CrossRef]
- Datta, S.; Timson, D.J.; Annapure, U.S. Antioxidant properties and global metabolite screening of the probiotic yeast Saccharomyces cerevisiae var. boulardii. J. Sci. Food Agric. 2017, 97, 3039–3049. [Google Scholar] [CrossRef]
- Fakruddin, M.D.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017, 17, 64. [Google Scholar] [CrossRef]
- Barssotti, L.; Abreu, I.C.; Brandão, A.B.P.; Albuquerque, R.C.; Ferreira, F.G.; Salgado, M.A.; Cunha, T.S. Saccharomyces boulardii modulates oxidative stress and renin angiotensin system attenuating diabetes-induced liver injury in mice. Sci. Rep. 2021, 11, 9189. [Google Scholar] [CrossRef]
- Erbas, O.; Solmaz, V.; Aksoy, D.; Yavasoglu, A.; Sagcan, M.; Taskiran, D. Cholecalciferol (vitamin D3) improves cognitive dysfunction and reduces inflammation in a rat fatty liver model of metabolic syndrome. Life Sci. 2014, 103, 68–72. [Google Scholar] [CrossRef]
- Fawzy, M.H.; Saeed, N.M.; El-Sherbiny, D.A.; El-Demerdash, E. Eugenol modulates insulin sensitivity by upregulating insulin receptor substrate-2 in non-alcoholic fatty liver disease in rats. J. Pharm. Pharmacol. 2021, 73, 846–854. [Google Scholar] [CrossRef]
- Pala, E.E.; Pala, H.G.; Ekmekci, S.; Erbas, O. Vitamin C (Ascorbic acid) protects from neuropathy caused by cisplatin, through enhanced heat shock protein-70 and reduced oxidant effect. Rev. Assoc. Med. Bras. 2022, 68, 1017–1022. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cotter, T.G.; Rinella, M. Nonalcoholic fatty liver disease 2020: The state of the disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef]
- Albuquerque, R.C.M.F.; Brandão, A.B.P.; De Abreu, I.C.M.E.; Ferreira, F.G.; Santos, L.B.; Moreira, L.N.; Cunha, T.S. Saccharomyces boulardii Tht 500101 changes gut microbiota and ameliorates hyperglycaemia, dyslipidaemia, and liver inflammation in streptozotocin-diabetic mice. Benef. Microbes 2019, 10, 901–912. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Xie, A.; Yuan, J. Oral administration of Saccharomyces boulardii ameliorates carbon tetrachloride-induced liver fibrosis in rats via reducing intestinal permeability and modulating gut microbial composition. Inflammation 2015, 38, 170–179. [Google Scholar] [CrossRef]
- Yesilova, Z.; Yaman, H.; Oktenli, C.; Ozcan, A.; Uygun, A.; Cakir, E.; Dagalp, K. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2005, 100, 850–855. [Google Scholar] [CrossRef]
- Morita, M.; Ishida, N.; Uchiyama, K.; Yamaguchi, K.; Itoh, Y.; Shichiri, M.; Niki, E. Fatty liver induced by free radicals and lipid peroxidation. Free Radic. Res. 2012, 46, 758–765. [Google Scholar] [CrossRef]
- Begriche, K.; Massart, J.; Robin, M.A.; Bonnet, F.; Fromenty, B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef]
- Mansouri, A.; Gattolliat, C.H.; Asselah, T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef]
- Hoffmann, A.; Kleniewska, P.; Pawliczak, R. Antioxidative activity of probiotics. Arch. Med. Sci. 2021, 17, 792–804. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, Y.; Wu, J.; Zhou, H.; Yang, C. Efficacy of probiotics, prebiotics, and synbiotics on liver enzymes, lipid profiles, and inflammation in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. BMC Gastroenterol. 2024, 24, 283. [Google Scholar] [CrossRef]
- Hizo, G.H.; Rampelotto, P.H. The Impact of Probiotic Bifidobacterium on Liver Diseases and the Microbiota. Life 2024, 14, 239. [Google Scholar] [CrossRef]
- Ding, R.B.; Bao, J.; Deng, C.X. Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef]
- Deng, X.Q.; Chen, L.L.; Li, N.X. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007, 27, 708–715. [Google Scholar] [CrossRef]
- Xu, R.Y.; Wan, Y.P.; Fang, Q.Y.; Lu, W.; Cai, W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J. Clin. Biochem. Nutr. 2011, 50, 72–77. [Google Scholar] [CrossRef]
- Yang, A.M.; Lin, C.Y.; Liu, S.H.; Syu, G.D.; Sun, H.J.; Lee, K.C.; Lin, H.C.; Hou, M.C. Saccharomyces boulardii ameliorates non-alcoholic steatohepatitis in mice induced by a methionine-choline-deficient diet through Gut-liver axis. Front. Microbiol. 2022, 13, 887728. [Google Scholar] [CrossRef] [PubMed]
| Initial Body Weight (g) | Final Body Weight (g) | Food Consumption (g/day) | Liver Weight (g) | Adipose Tissue Weight (g) | |
|---|---|---|---|---|---|
| Control Group | 200.2 ± 2.1 | 250.3 ± 5.4 | 25.4 ± 0.3 | 8.2 ± 0.5 | 6.4 ± 0.4 |
| High-Fructose Group | 199.8 ± 2.3 | 300.4 ± 6.2 | 25.6 ± 0.4 | 12.5 ± 1.0 | 10.8 ± 0.8 |
| Treatment Group | 200.5 ± 2.0 | 260.1 ± 4.9 | 25.5 ± 0.3 | 9.1 ± 0.6 | 7.0 ± 0.5 |
| Control Group | High-Fructose Group | Treatment Group | |
|---|---|---|---|
| Aminotransferase (U/L) | 41.7 ± 2.06 | 59.4 ± 4.6 * | 45.9 ± 3.2 # |
| Plasma Malondialdehyde Level (nmol/mg protein) | 32.8 ± 1.2 | 51.7 ± 2.2 * | 33.5 ± 1.7 # |
| Plasma Triglyceride (mg/dL) | 32.3 ± 1.9 | 56.4 ± 5.1 * | 43.4 ± 1.8 # |
| Plasma Total Cholesterol (mg/dL) | 50.5 ± 7.1 | 126.8 ± 9.3 ** | 101.3 ± 6.07 # |
| Liver Aquaporin-8 Level (pg/mg protein) | 3.6 ± 0.1 | 2.4 ± 0.1 * | 3.5 ± 0.2 ## |
| Liver Sirtuin-1 Level (pg/g protein) | 4.06 ± 0.3 | 3.1 ± 0.08 * | 3.8 ± 0.09 # |
| Fatty Infiltration Cell (percent) | 1.3 ± 0.2 | 85.4 ± 5.7 ** | 11.8 ± 3.5 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulusan, M.; Erdogan, M.A.; Simsek, O.; Gunes, V.; Erbas, O. Saccharomyces boulardii Mitigates Fructose-Induced Non-Alcoholic Fatty Liver in Rats. Medicina 2024, 60, 1713. https://doi.org/10.3390/medicina60101713
Ulusan M, Erdogan MA, Simsek O, Gunes V, Erbas O. Saccharomyces boulardii Mitigates Fructose-Induced Non-Alcoholic Fatty Liver in Rats. Medicina. 2024; 60(10):1713. https://doi.org/10.3390/medicina60101713
Chicago/Turabian StyleUlusan, Mehmet, Mumin Alper Erdogan, Ozkan Simsek, Vehbi Gunes, and Oytun Erbas. 2024. "Saccharomyces boulardii Mitigates Fructose-Induced Non-Alcoholic Fatty Liver in Rats" Medicina 60, no. 10: 1713. https://doi.org/10.3390/medicina60101713
APA StyleUlusan, M., Erdogan, M. A., Simsek, O., Gunes, V., & Erbas, O. (2024). Saccharomyces boulardii Mitigates Fructose-Induced Non-Alcoholic Fatty Liver in Rats. Medicina, 60(10), 1713. https://doi.org/10.3390/medicina60101713







