The Role of Gut Microbiota and the Potential Effects of Probiotics in Heart Failure
Abstract
1. Introduction
2. Materials and Methods
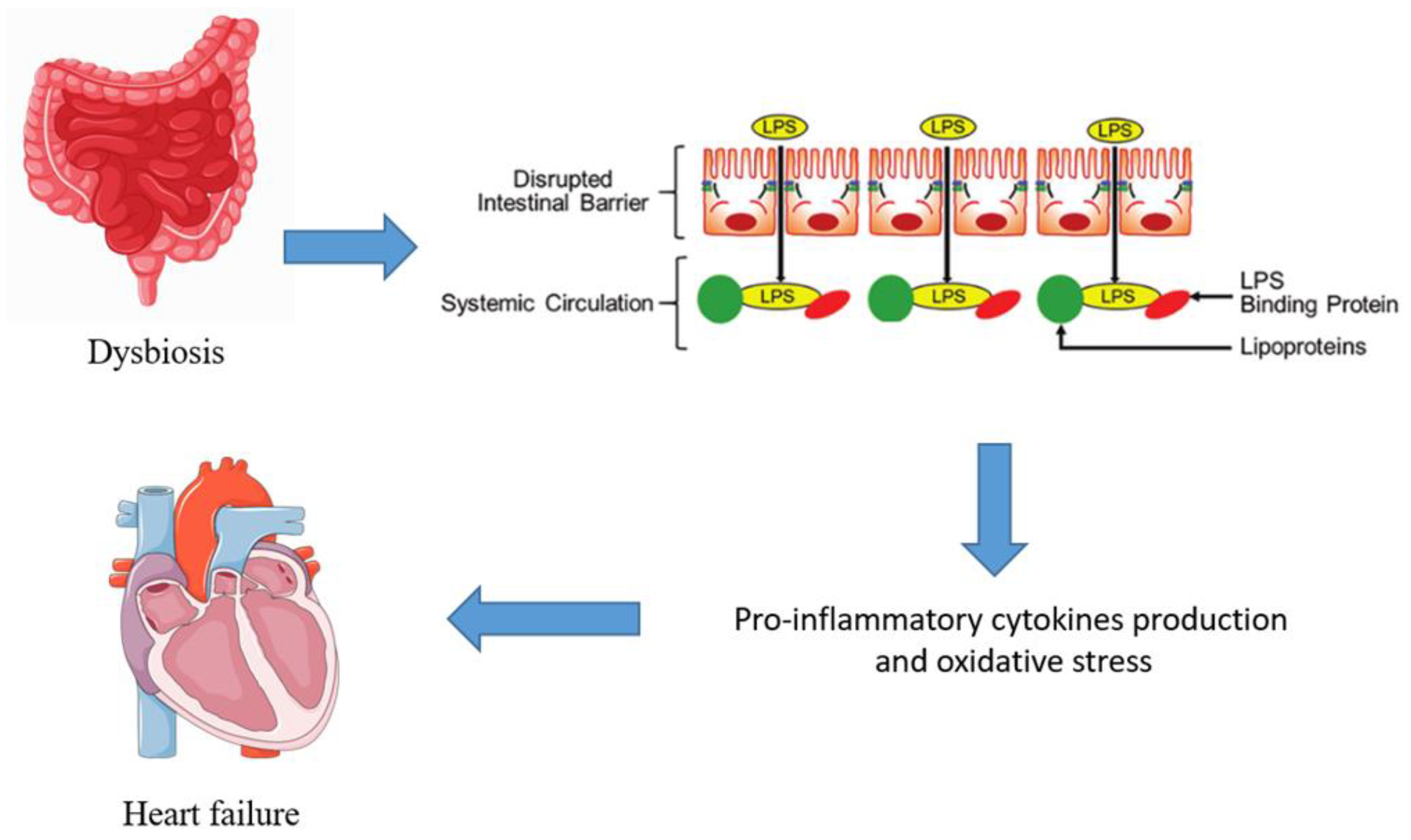
3. Gut Microbiota Dysbiosis in Heart Failure
4. Probiotics as Modulators of Gut Microbiota
5. Preclinical and Clinical Evidence
6. Challenges and Future Directions
7. Conclusions and Implication
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Card. Fail 2017, 23, 628–651. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Rev. Esp. Cardiol. 2016, 69, 1167. [Google Scholar] [CrossRef]
- Mann, D.L.; McMurray, J.J.; Packer, M.; Swedberg, K.; Borer, J.S.; Colucci, W.S.; Djian, J.; Drexler, H.; Feldman, A.; Kober, L.; et al. Targeted anticytokine therapy in patients with chronic heart failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004, 109, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Mahony, S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef]
- Tang, W.H.W. Dysregulated amino acid metabolism in heart failure: Role of gut microbiome. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 195–200. [Google Scholar] [CrossRef]
- Lam, V.; Su, J.; Hsu, A.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal Microbial Metabolites Are Linked to Severity of Myocardial Infarction in Rats. PLoS ONE 2016, 11, e0160840. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediators Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kummen, M.; Mayerhofer, C.C.K.; Vestad, B.; Broch, K.; Awoyemi, A.; Storm-Larsen, C.; Ueland, T.; Yndestad, A.; Hov, J.R.; Trøseid, M. Gut Microbiota Signature in Heart Failure Defined from Profiling of 2 Independent Cohorts. J. Am. Coll. Cardiol. 2018, 71, 1184–1186. [Google Scholar] [CrossRef]
- Sun, W.; Du, D.; Fu, T.; Han, Y.; Li, P.; Ju, H. Alterations of the Gut Microbiota in Patients with Severe Chronic Heart Failure. Front. Microbiol. 2022, 12, 813289. [Google Scholar] [CrossRef]
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC Heart Fail. 2016, 4, 220–227. [Google Scholar] [CrossRef]
- Mamic, P.; Heidenreich, P.A.; Hedlin, H.; Tennakoon, L.; Staudenmayer, K.L. Hospitalized Patients with Heart Failure and Common Bacterial Infections: A Nationwide Analysis of Concomitant Clostridium Difficile Infection Rates and In-Hospital Mortality. J. Card. Fail. 2016, 22, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Yuzefpolskaya, M.; Bohn, B.; Nasiri, M.; Zuver, A.M.; Onat, D.D.; Royzman, E.A.; Nwokocha, J.; Mabasa, M.; Pinsino, A.; Brunjes, D.; et al. Gut microbiota, endotoxemia, inflammation, and oxidative stress in patients with heart failure, left ventricular assist device, and transplant. J. Heart Lung Transplant. 2020, 39, 880–890. [Google Scholar] [CrossRef]
- Huang, Z.; Mei, X.; Jiang, Y.; Chen, T.; Zhou, Y. Gut Microbiota in Heart Failure Patients with Preserved Ejection Fraction (GUMPTION Study). Front. Cardiovasc. Med. 2022, 8, 803744. [Google Scholar] [CrossRef] [PubMed]
- Luedde, M.; Winkler, T.; Heinsen, F.A.; Rühlemann, M.C.; Spehlmann, M.E.; Bajrovic, A.; Lieb, W.; Franke, A.; Ott, S.J.; Frey, N. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail. 2017, 4, 282–290. [Google Scholar] [CrossRef]
- Modrego, J.; Ortega-Hernández, A.; Goirigolzarri, J.; Restrepo-Córdoba, M.A.; Bäuerl, C.; Cortés-Macías, E.; Sánchez-González, S.; Esteban-Fernández, A.; Pérez-Villacastín, J.; Collado, M.C.; et al. Gut Microbiota and Derived Short-Chain Fatty Acids Are Linked to Evolution of Heart Failure Patients. Int. J. Mol. Sci. 2023, 24, 13892. [Google Scholar] [CrossRef]
- Mann, D.L. Targeted anticytokine therapy and the failing heart. Am. J. Cardiol. 2005, 95, 9C–16C; discussion 38C–40C. [Google Scholar] [CrossRef]
- Polsinelli, V.B.; Sinha, A.; Shah, S.J. Visceral Congestion in Heart Failure: Right Ventricular Dysfunction, Splanchnic Hemodynamics, and the Intestinal Microenvironment. Curr. Heart Fail Rep. 2017, 14, 519–528. [Google Scholar] [CrossRef]
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic Gut Bacteria in Obesity: An Overview of the Metabolic Mechanisms and Therapeutic Perspectives of Next-Generation Probiotics. Microorganisms 2022, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Hazen, S.L. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 2015, 66, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Pabst, O. New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 2012, 12, 821–832. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Saini-Chohan, H.K.; Rodriguez-Leyva, D.; Elimban, V.; Dent, M.R.; Tappia, P.S. Subcellular remodelling may induce cardiac dysfunction in congestive heart failure. Cardiovasc. Res. 2009, 81, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Riehle, C.; Abel, E.D. Insulin Signaling and Heart Failure. Circ. Res. 2016, 118, 1151–1169. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. The gut microbiome as therapeutic target. Pharmacol. Ther. 2011, 130, 202–212. [Google Scholar] [CrossRef]
- Cani, P.D. Gut microbiota: Changes in gut microbes and host metabolism: Squaring the circle? Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 563–564. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.P.; Grisanti, L.A. The Dynamic Interplay Between Cardiac Inflammation and Fibrosis. Front. Physiol. 2020, 11, 529075. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef]
- Tang, W.H.; Hazen, S.L. The Gut Microbiome and Its Role in Cardiovascular Diseases. Circulation 2017, 135, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Andersen, G.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef]
- Karmazyn, M.; Gan, X.T. Probiotics as potential treatments to reduce myocardial remodelling and heart failure via the gut-heart axis: State-of-the-art review. Mol. Cell. Biochem. 2023, 478, 2539–2551. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Resta-Lenert, S.; Barrett, K.E. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 2003, 52, 988–997. [Google Scholar] [CrossRef]
- Gallo, A.; Macerola, N.; Favuzzi, A.M.; Nicolazzi, M.A.; Gasbarrini, A.; Montalto, M. The Gut in Heart Failure: Current Knowledge and Novel Frontiers. Med. Princ. Pract. 2022, 31, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Chatenoud, L.; Turati, F.; Galeone, C.; Moja, L.; Bach, J.F.; La Vecchia, C. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: A meta-analysis. Epidemiology 2012, 23, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, P.; Ferstl, R.; Ziegler, M.; Frei, R.; Nehrbass, D.; Lauener, R.P.; Akdis, C.A.; O’Mahony, L. Immunomodulation by Bifidobacterium infantis 35624 in the murine lamina propria requires retinoic acid-dependent and independent mechanisms. PLoS ONE 2013, 8, e62617. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Shin, H.S. Antimicrobial and Immunomodulatory Effects of. J. Microbiol. Biotechnol. 2020, 30, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, X.; Hu, M.; Zhang, X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018, 214, 153–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Ke, B.; Du, J. TMAO: How gut microbiota contributes to heart failure. Transl. Res. 2021, 228, 109–125. [Google Scholar] [CrossRef]
- Dhalla, A.K.; Hill, M.F.; Singal, P.K. Role of oxidative stress in transition of hypertrophy to heart failure. J. Am. Coll. Cardiol. 1996, 28, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G168–G175. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, L.J.; Leng, Y.Q.; Wang, Y.W.; Shi, T.; Wang, W.Z.; Sun, J.C. Inflammatory Response: A Crucial Way for Gut Microbes to Regulate Cardiovascular Diseases. Nutrients 2023, 15, 607. [Google Scholar] [CrossRef]
- Guan, X.; Sun, Z. The Role of Intestinal Flora and Its Metabolites in Heart Failure. Infect. Drug Resist. 2023, 16, 51–64. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef]
- Drapkina, O.M.; Yafarova, A.A.; Kaburova, A.N.; Kiselev, A.R. Targeting Gut Microbiota as a Novel Strategy for Prevention and Treatment of Hypertension, Atrial Fibrillation and Heart Failure: Current Knowledge and Future Perspectives. Biomedicines 2022, 10, 2019. [Google Scholar] [CrossRef]
- Awoyemi, A.; Mayerhofer, C.; Felix, A.S.; Hov, J.R.; Moscavitch, S.D.; Lappegård, K.T.; Hovland, A.; Halvorsen, S.; Halvorsen, B.; Gregersen, I.; et al. Rifaximin or Saccharomyces boulardii in heart failure with reduced ejection fraction: Results from the randomized GutHeart trial. EBioMedicine 2021, 70, 103511. [Google Scholar] [CrossRef]
- Moludi, J.; Saiedi, S.; Ebrahimi, B.; Alizadeh, M.; Khajebishak, Y.; Ghadimi, S.S. Probiotics Supplementation on Cardiac Remodeling Following Myocardial Infarction: A Single-Center Double-Blind Clinical Study. J. Cardiovasc. Transl. Res. 2021, 14, 299–307. [Google Scholar] [CrossRef]
- Pourrajab, B.; Naderi, N.; Janani, L.; Hajahmadi, M.; Mofid, V.; Dehnad, A.; Sohouli, M.H.; Hosseini, S.; Shidfar, F. The impact of probiotic yogurt versus ordinary yogurt on serum sTWEAK, sCD163, ADMA, LCAT and BUN in patients with chronic heart failure: A randomized, triple-blind, controlled trial. J Sci. Food Agric. 2022, 102, 6024–6035. [Google Scholar] [CrossRef]
- Karim, A.; Muhammad, T.; Shah, I.; Khan, J.; Qaisar, R. A multistrain probiotic reduces sarcopenia by modulating Wnt signaling biomarkers in patients with chronic heart failure. J. Cardiol. 2022, 80, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Costanza, A.C.; Moscavitch, S.D.; Faria Neto, H.C.; Mesquita, E.T. Probiotic therapy with Saccharomyces boulardii for heart failure patients: A randomized, double-blind, placebo-controlled pilot trial. Int. J. Cardiol. 2015, 179, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: A randomized, controlled trial. Mediators Inflamm. 2014, 2014, 348959. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. From yaks to yogurt: The history, development, and current use of probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. S2), S85–S90. [Google Scholar] [CrossRef] [PubMed]
- Mamic, P.; Chaikijurajai, T.; Tang, W.H.W. Gut microbiome—A potential mediator of pathogenesis in heart failure and its comorbidities: State-of-the-art review. J. Mol. Cell. Cardiol. 2021, 152, 105–117. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, Y.; Zhu, P.; Nasser, M.I.; Zhang, X.; Zhao, M. Implication of Gut Microbiota in Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 5394096. [Google Scholar] [CrossRef]
- Lewis, C.V.; Taylor, W.R. Intestinal barrier dysfunction as a therapeutic target for cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1227–H1233. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, G.; Gagliardi, L.; Egidi, G.; Leone, S.; Gasbarrini, A.; Miggiano, G.A.D.; Galiuto, L. Gut Microbiota and Cardiovascular Diseases: A Critical Review. Cardiol. Rev. 2021, 29, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Qian, Z.; Yin, J.; Xu, W.; Zhou, X. The role of intestinal microbiota in cardiovascular disease. J. Cell. Mol. Med. 2019, 23, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Katsimichas, T.; Antonopoulos, A.S.; Katsimichas, A.; Ohtani, T.; Sakata, Y.; Tousoulis, D. The intestinal microbiota and cardiovascular disease. Cardiovasc. Res. 2019, 115, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Povia, J.P.; Lwiindi, P.C.; Kirabo, A. Recent Advances in Microbiota-Associated Metabolites in Heart Failure. Biomedicines 2023, 11, 2313. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Type of article: in order of importance, we have considered clinical trials, observational studies, systematic reviews, and narrative reviews. | Topic: articles regarding only the topic “microbiota” or only the topic “heart failure”; articles treating other topics. |
| Language: only articles written in English. | |
| Year of publication: articles written in the last 25 years. |
| Authors, Year | Study Type | Probiotic Used | Conclusion of the Study |
|---|---|---|---|
| Awoyemi A et al., 2021 [52] | multicenter, prospective, randomized, open label | Saccharomyces boulardii | Improvement in left ventricular ejection fraction |
| Moludi J et al., 2020 [53] | single-center, double-blind placebo-controlled, randomized clinical study | Lactobacillus rhamnosus GG | Improvement in serum biomarkers, improvement in left ventricular ejection fraction |
| Pourrajab B et al., 2022 [54] | randomized, triple-blind clinical trial | Mix of probiotic yogurt | Improvement in serum biomarkers |
| Karim A et al., 2022 [55] | single-center, double-blind placebo-controlled, randomized clinical study | Mix of probiotics | Improvement in serum biomarkers |
| Costanza A et al., 2015 [56] | randomized, double-blind, placebo-controlled pilot trial | Saccharomyces boulardii | Improvement in serum biomarkers, improvement in left ventricular ejection fraction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruzziello, C.; Saviano, A.; Manetti, L.L.; Macerola, N.; Ojetti, V. The Role of Gut Microbiota and the Potential Effects of Probiotics in Heart Failure. Medicina 2024, 60, 271. https://doi.org/10.3390/medicina60020271
Petruzziello C, Saviano A, Manetti LL, Macerola N, Ojetti V. The Role of Gut Microbiota and the Potential Effects of Probiotics in Heart Failure. Medicina. 2024; 60(2):271. https://doi.org/10.3390/medicina60020271
Chicago/Turabian StylePetruzziello, Carmine, Angela Saviano, Luca Luigi Manetti, Noemi Macerola, and Veronica Ojetti. 2024. "The Role of Gut Microbiota and the Potential Effects of Probiotics in Heart Failure" Medicina 60, no. 2: 271. https://doi.org/10.3390/medicina60020271
APA StylePetruzziello, C., Saviano, A., Manetti, L. L., Macerola, N., & Ojetti, V. (2024). The Role of Gut Microbiota and the Potential Effects of Probiotics in Heart Failure. Medicina, 60(2), 271. https://doi.org/10.3390/medicina60020271







