Unraveling the Complex Web of Fibromyalgia: A Narrative Review
Abstract
1. Introduction
2. Clinical Features and Diagnosis
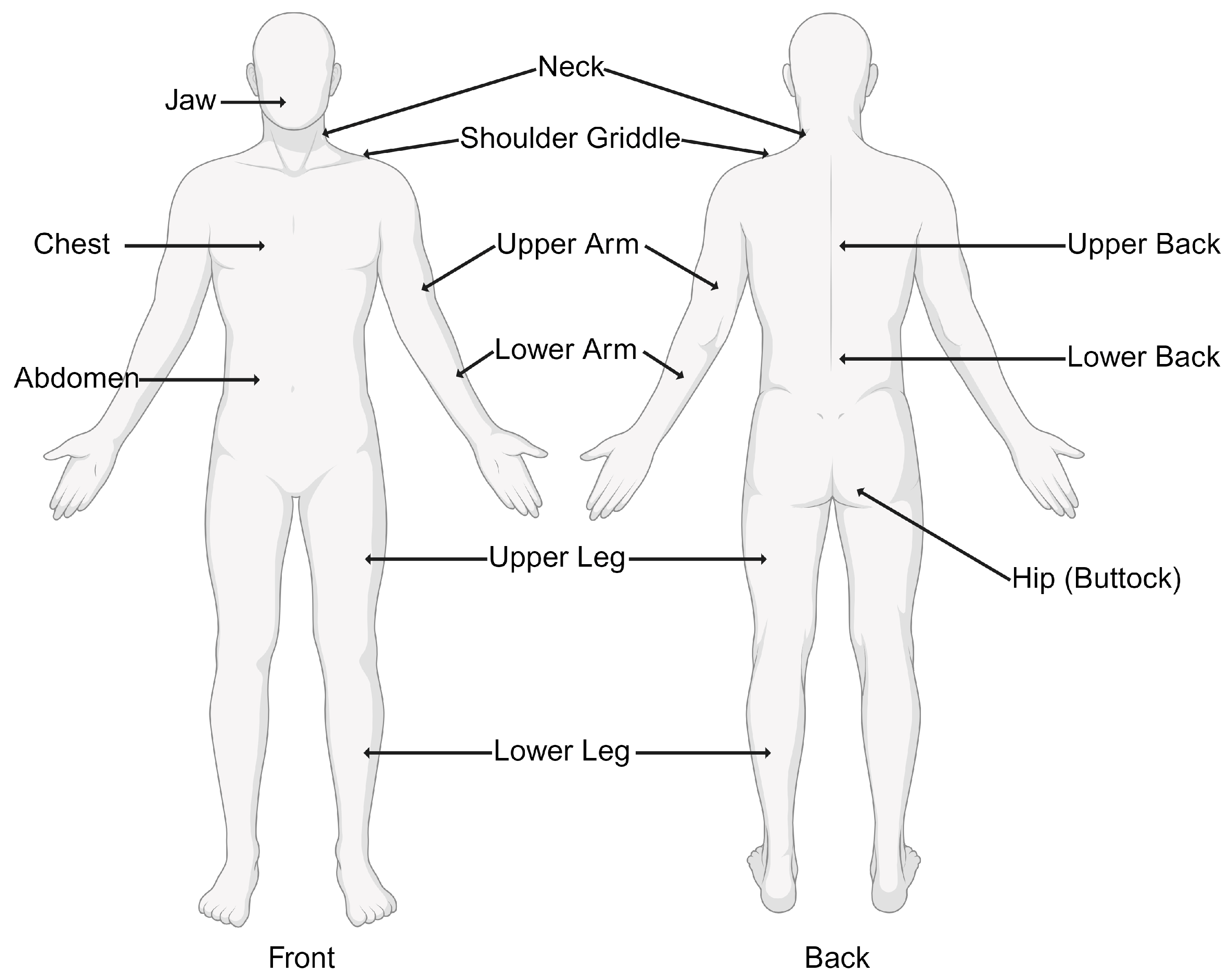
- Widespread Pain Index (WPI): This involves assessing pain in 19 specified body areas over the past week. The areas include the neck, shoulders, chest, arms, lower back, hips, and legs. Figure 1 demonstrates the 19 specific tender points used in the diagnosis of fibromyalgia.
- 2.
- Symptom severity (SS) score: In addition to the WPI, the SS score considers the severity of other symptoms such as fatigue, sleep disturbances, and cognitive difficulties. Table 1 demonstrates SS score calculation variables.
3. Differential Diagnosis
4. Etiology and Pathophysiology
4.1. Genetic Factors
4.2. Neurotransmitter Dysregulation
4.3. Central Sensitization
4.4. Immune System Involvement
4.5. Oxidative Stress
5. Risk Factors
5.1. Gender and Age
5.2. Family History
5.3. Comorbid Conditions
6. Impact on Quality of Life
7. Management Approaches
7.1. Pharmacological Interventions
7.2. Non-Pharmacological Therapies
7.3. Lifestyle Modifications
7.4. Alternative and Complementary Therapies
8. Emerging Treatments and Research
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inanici, F.; Yunus, M.B. History of fibromyalgia: Past to present. Curr. Pain Headache Rep. 2004, 8, 369–378. [Google Scholar] [CrossRef]
- Goldenberg, D.L.; Bradley, L.A.; Arnold, L.M.; Glass, J.M.; Clauw, D.J. Understanding fibromyalgia and its related disorders. Prim. Care Companion J. Clin. Psychiatry 2008, 10, 133–144. [Google Scholar] [CrossRef]
- Wood, P.B. Fibromyalgia. In Encyclopedia of Stress, 2nd ed.; Fink, G., Ed.; Academic Press: New York, NY, USA, 2007; pp. 56–62. [Google Scholar]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Sánchez, C.M.; Duschek, S.; Reyes Del Paso, G.A. Psychological impact of fibromyalgia: Current perspectives. Psychol. Res. Behav. Manag. 2019, 12, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Rasker, J.J. The Evolution of Fibromyalgia, Its Concepts, and Criteria. Cureus 2021, 13, e20010. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Bidari, A.; Ghavidel Parsa, B.; Ghalehbaghi, B. Challenges in fibromyalgia diagnosis: From meaning of symptoms to fibromyalgia labeling. Korean J. Pain 2018, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Aggarwal, A.; Lawrence, A. Performance of the American College of Rheumatology 2016 criteria for fibromyalgia in a referral care setting. Rheumatol. Int. 2019, 39, 1397–1403. [Google Scholar] [CrossRef]
- Häuser, W.; Burgmer, M.; Köllner, V.; Schaefert, R.; Eich, W.; Hausteiner-Wiehle, C.; Henningsen, P. Fibromyalgia syndrome as a psychosomatic disorder—Diagnosis and therapy according to current evidence-based guidelines. Z. Psychosom. Med. Psychother. 2013, 59, 132–152. [Google Scholar] [CrossRef]
- Dizner-Golab, A.; Lisowska, B.; Kosson, D. Fibromyalgia—Etiology, diagnosis and treatment including perioperative management in patients with fibromyalgia. Reumatologia 2023, 61, 137–148. [Google Scholar] [CrossRef]
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, B.; Amital, H. Diagnostic and therapeutic challenge-fibromyalgia. Reumatologia 2018, 56, 273–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wolfe, F.; Walitt, B.; Perrot, S.; Rasker, J.J.; Häuser, W. Fibromyalgia diagnosis and biased assessment: Sex, prevalence and bias. PLoS ONE 2018, 13, e0203755. [Google Scholar] [CrossRef] [PubMed]
- Neumann, L.; Buskila, D. Epidemiology of fibromyalgia. Curr. Pain Headache Rep. 2003, 7, 362–368. [Google Scholar] [CrossRef]
- Arout, C.A.; Sofuoglu, M.; Bastian, L.A.; Rosenheck, R.A. Gender Differences in the Prevalence of Fibromyalgia and in Concomitant Medical and Psychiatric Disorders: A National Veterans Health Administration Study. J. Womens Health 2018, 27, 1035–1044. [Google Scholar] [CrossRef]
- Taylor, S.; Furness, P.; Ashe, S.; Haywood-Small, S.; Lawson, K. Comorbid Conditions, Mental Health and Cognitive Functions in Adults with Fibromyalgia. West J. Nurs. Res. 2021, 43, 115–122. [Google Scholar] [CrossRef]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Varrassi, G.; Rekatsina, M.; Perrot, S.; Bouajina, E.; Paladini, A.; Coaccioli, S.; Narvaez Tamayo, M.A.; Sarzi Puttini, P. Is Fibromyalgia a Fashionable Diagnosis or a Medical Mystery? Cureus 2023, 15, e44852. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Giorgi, V.; Atzeni, F.; Gorla, R.; Kosek, E.; Choy, E.H.; Bazzichi, L.; Häuser, W.; Ablin, J.N.; Aloush, V.; et al. Diagnostic and therapeutic care pathway for fibromyalgia. Clin. Exp. Rheumatol. 2021, 39 (Suppl. S130), 120–127. [Google Scholar] [CrossRef]
- Perrot, S. Fibromyalgia syndrome: A relevant recent construction of an ancient condition? Curr. Opin. Support. Palliat. Care 2008, 2, 122–127. [Google Scholar] [CrossRef]
- Culpepper, L. Evaluating the patient with fibromyalgia. J. Clin. Psychiatry 2010, 71, e25. [Google Scholar] [CrossRef]
- Climent-Sanz, C.; Morera-Amenós, G.; Bellon, F.; Pastells-Peiró, R.; Blanco-Blanco, J.; Valenzuela-Pascual, F.; Gea-Sánchez, M. Poor Sleep Quality Experience and Self-Management Strategies in Fibromyalgia: A Qualitative Metasynthesis. J. Clin. Med. 2020, 9, 4000. [Google Scholar] [CrossRef]
- Bigatti, S.M.; Hernandez, A.M.; Cronan, T.A.; Rand, K.L. Sleep disturbances in fibromyalgia syndrome: Relationship to pain and depression. Arthritis Rheum. 2008, 59, 961–967. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Reyes Del Paso, G.A.; Duschek, S. Cognitive Impairments in Fibromyalgia Syndrome: Associations with Positive and Negative Affect, Alexithymia, Pain Catastrophizing and Self-Esteem. Front. Psychol. 2018, 9, 377. [Google Scholar] [CrossRef]
- Thierheimer, M.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Ostrom, Q.T.; Barnholtz-Sloan, J.S. Mortality trends in primary malignant brain and central nervous system tumors vary by histopathology, age, race, and sex. J. Neurooncol. 2023, 162, 167–177. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Yunus, M.B. The clinical concept of fibromyalgia as a changing paradigm in the past 20 years. Pain Res. Treat. 2012, 2012, 184835. [Google Scholar] [CrossRef][Green Version]
- Kim, S.M.; Lee, S.H.; Kim, H.R. Applying the ACR Preliminary Diagnostic Criteria in the Diagnosis and Assessment of Fibromyalgia. Korean J. Pain 2012, 25, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Cristiani, C.M.; Ilari, S.; Passacatini, L.C.; Malafoglia, V.; Viglietto, G.; Maiuolo, J.; Oppedisano, F.; Palma, E.; Tomino, C.; et al. Fibromyalgia and Irritable Bowel Syndrome Interaction: A Possible Role for Gut Microbiota and Gut-Brain Axis. Biomedicines 2023, 11, 1701. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.S.; Pimentel, M.J.; Rizzatti-Barbosa, C.M. Temporomandibular disorders in fibromyalgia syndrome: A short-communication. Rev. Bras. Reumatol. 2015, 55, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Cetingok, S.; Seker, O.; Cetingok, H. The relationship between fibromyalgia and depression, anxiety, anxiety sensitivity, fear avoidance beliefs, and quality of life in female patients. Medicine 2022, 101, e30868. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.G.; Jha, S.K.; Iskander, J.; Avanthika, C.; Jhaveri, S.; Patel, V.H.; Rasagna Potini, B.; Talha Azam, A. Diagnostic Challenges and Management of Fibromyalgia. Cureus 2021, 13, e18692. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Sic, R.A.; Vega-Morales, D.; Santoyo-Fexas, L.; Garza-Elizondo, M.A.; Mendiola-Jiménez, A.; González Marquez, K.I.; Carrillo-Haro, B. Are the cut-offs of the rheumatoid factor and anti-cyclic citrullinated peptide antibody different to distinguish rheumatoid arthritis from their primary differential diagnoses? Int. J. Immunogenet. 2023, 51, 1–9. [Google Scholar] [CrossRef]
- Stussman, B.; Williams, A.; Snow, J.; Gavin, A.; Scott, R.; Nath, A.; Walitt, B. Characterization of Post-exertional Malaise in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Neurol. 2020, 11, 1025. [Google Scholar] [CrossRef]
- Fischer, S.; Markert, C.; Strahler, J.; Doerr, J.M.; Skoluda, N.; Kappert, M.; Nater, U.M. Thyroid Functioning and Fatigue in Women with Functional Somatic Syndromes—Role of Early Life Adversity. Front. Physiol. 2018, 9, 564. [Google Scholar] [CrossRef]
- McManimen, S.L.; Jason, L.A. Post-Exertional Malaise in Patients with ME and CFS with Comorbid Fibromyalgia. SRL Neurol. Neurosurg. 2017, 3, 22–27. [Google Scholar] [PubMed]
- Hackshaw, K. Assessing our approach to diagnosing Fibromyalgia. Expert Rev. Mol. Diagn. 2020, 20, 1171–1181. [Google Scholar] [CrossRef]
- Schmidt-Wilcke, T.; Diers, M. New Insights into the Pathophysiology and Treatment of Fibromyalgia. Biomedicines 2017, 5, 22. [Google Scholar] [CrossRef]
- Park, D.J.; Lee, S.S. New insights into the genetics of fibromyalgia. Korean J. Intern. Med. 2017, 32, 984–995. [Google Scholar] [CrossRef]
- Shukla, H.; Mason, J.L.; Sabyah, A. Identifying genetic markers associated with susceptibility to cardiovascular diseases. Future Sci. OA 2019, 5, Fso350. [Google Scholar] [CrossRef]
- Dutta, D.; Brummett, C.M.; Moser, S.E.; Fritsche, L.G.; Tsodikov, A.; Lee, S.; Clauw, D.J.; Scott, L.J. Heritability of the Fibromyalgia Phenotype Varies by Age. Arthritis Rheumatol. 2020, 72, 815–823. [Google Scholar] [CrossRef] [PubMed]
- van Reij, R.R.I.; Joosten, E.A.J.; van den Hoogen, N.J. Dopaminergic neurotransmission and genetic variation in chronification of post-surgical pain. Br. J. Anaesth. 2019, 123, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Bradley, L.A. Pathophysiology of fibromyalgia. Am. J. Med. 2009, 122, S22–S30. [Google Scholar] [CrossRef] [PubMed]
- D’Agnelli, S.; Arendt-Nielsen, L.; Gerra, M.C.; Zatorri, K.; Boggiani, L.; Baciarello, M.; Bignami, E. Fibromyalgia: Genetics and epigenetics insights may provide the basis for the development of diagnostic biomarkers. Mol. Pain 2019, 15, 1744806918819944. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Schweinhardt, P. Dysfunctional neurotransmitter systems in fibromyalgia, their role in central stress circuitry and pharmacological actions on these systems. Pain Res. Treat. 2012, 2012, 741746. [Google Scholar] [CrossRef] [PubMed]
- Al-Nimer, M.S.M.; Mohammad, T.A.M.; Alsakeni, R.A. Serum levels of serotonin as a biomarker of newly diagnosed fibromyalgia in women: Its relation to the platelet indices. J. Res. Med. Sci. 2018, 23, 71. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Raja, S.N.; Wesselmann, U.; Fuchs, P.N.; Meyer, R.A.; Campbell, J.N. Intradermal injection of norepinephrine evokes pain in patients with sympathetically maintained pain. Pain 2000, 88, 161–168. [Google Scholar] [CrossRef]
- Martinez-Lavin, M.; Vidal, M.; Barbosa, R.E.; Pineda, C.; Casanova, J.M.; Nava, A. Norepinephrine-evoked pain in fibromyalgia. A randomized pilot study [ISRCTN70707830]. BMC Musculoskelet. Disord. 2002, 3, 2. [Google Scholar] [CrossRef]
- Albrecht, D.S.; MacKie, P.J.; Kareken, D.A.; Hutchins, G.D.; Chumin, E.J.; Christian, B.T.; Yoder, K.K. Differential dopamine function in fibromyalgia. Brain Imaging Behav. 2016, 10, 829–839. [Google Scholar] [CrossRef]
- Wood, P.B.; Schweinhardt, P.; Jaeger, E.; Dagher, A.; Hakyemez, H.; Rabiner, E.A.; Bushnell, M.C.; Chizh, B.A. Fibromyalgia patients show an abnormal dopamine response to pain. Eur. J. Neurosci. 2007, 25, 3576–3582. [Google Scholar] [CrossRef]
- Smeets, Y.; Soer, R.; Chatziantoniou, E.; Preuper, R.; Reneman, M.F.; Wolff, A.P.; Timmerman, H. Role of non-invasive objective markers for the rehabilitative diagnosis of central sensitization in patients with fibromyalgia: A systematic review. J. Back Musculoskelet. Rehabil. 2023. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Li, Y.L.; Fang, Z.H.; Liao, H.L.; Zhang, Y.Y.; Lin, J.; Liu, F.; Shen, J.F. NMDARs mediate peripheral and central sensitization contributing to chronic orofacial pain. Front. Cell. Neurosci. 2022, 16, 999509. [Google Scholar] [CrossRef] [PubMed]
- Björkander, S.; Ernberg, M.; Bileviciute-Ljungar, I. Reduced immune system responsiveness in fibromyalgia—A pilot study. Clin. Immunol. Commun. 2022, 2, 46–53. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; de Miguel, M.; Carmona-López, I.; Bonal, P.; Campa, F.; Moreno-Fernández, A.M. Oxidative stress and mitochondrial dysfunction in fibromyalgia. Neuro Endocrinol. Lett. 2010, 31, 169–173. [Google Scholar]
- Assavarittirong, C.; Samborski, W.; Grygiel-Górniak, B. Oxidative Stress in Fibromyalgia: From Pathology to Treatment. Oxid. Med. Cell. Longev. 2022, 2022, 1582432. [Google Scholar] [CrossRef]
- Coppens, E.; Kempke, S.; Van Wambeke, P.; Claes, S.; Morlion, B.; Luyten, P.; Van Oudenhove, L. Cortisol and Subjective Stress Responses to Acute Psychosocial Stress in Fibromyalgia Patients and Control Participants. Psychosom. Med. 2018, 80, 317–326. [Google Scholar] [CrossRef]
- Yunus, M.B. Gender differences in fibromyalgia and other related syndromes. J. Gend. Specif. Med. 2002, 5, 42–47. [Google Scholar] [PubMed]
- Yunus, M.B. The role of gender in fibromyalgia syndrome. Curr. Rheumatol. Rep. 2001, 3, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Schertzinger, M.; Wesson-Sides, K.; Parkitny, L.; Younger, J. Daily Fluctuations of Progesterone and Testosterone Are Associated with Fibromyalgia Pain Severity. J. Pain 2018, 19, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Fitzcharles, M.A. Facts and myths pertaining to fibromyalgia. Dialogues Clin. Neurosci. 2018, 20, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Gota, C.E.; Kaouk, S.; Wilke, W.S. Fibromyalgia and Obesity: The Association between Body Mass Index and Disability, Depression, History of Abuse, Medications, and Comorbidities. J. Clin. Rheumatol. 2015, 21, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Brill, S.; Ablin, J.N.; Goor-Aryeh, I.; Hyat, K.; Slefer, A.; Buskila, D. Prevalence of fibromyalgia syndrome in patients referred to a tertiary pain clinic. J. Investig. Med. 2012, 60, 685–688. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Abbasciano, V.; Soliman, A.T.; Soliman, N.; Di Maio, S.; Fiscina, B.; Kattamis, C. The juvenile fibromyalgia syndrome (JFMS): A poorly defined disorder. Acta Biomed. 2019, 90, 134–148. [Google Scholar] [CrossRef]
- Mogil, J.S. Pain genetics: Past, present and future. Trends Genet. 2012, 28, 258–266. [Google Scholar] [CrossRef]
- Arnold, L.M.; Fan, J.; Russell, I.J.; Yunus, M.B.; Khan, M.A.; Kushner, I.; Olson, J.M.; Iyengar, S.K. The fibromyalgia family study: A genome-wide linkage scan study. Arthritis Rheum. 2013, 65, 1122–1128. [Google Scholar] [CrossRef]
- Hudson, J.I.; Goldenberg, D.L.; Pope, H.G., Jr.; Keck, P.E., Jr.; Schlesinger, L. Comorbidity of fibromyalgia with medical and psychiatric disorders. Am. J. Med. 1992, 92, 363–367. [Google Scholar] [CrossRef]
- Okifuji, A.; Donaldson, G.W.; Barck, L.; Fine, P.G. Relationship between fibromyalgia and obesity in pain, function, mood, and sleep. J. Pain 2010, 11, 1329–1337. [Google Scholar] [CrossRef]
- Okifuji, A.; Bradshaw, D.H.; Olson, C. Evaluating obesity in fibromyalgia: Neuroendocrine biomarkers, symptoms, and functions. Clin. Rheumatol. 2009, 28, 475–478. [Google Scholar] [CrossRef]
- Mannerkorpi, K.; Gard, G. Hinders for continued work among persons with fibromyalgia. BMC Musculoskelet. Disord. 2012, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The association of sleep and pain: An update and a path forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Baroni, M.; Ranchelli, A.; Lauretani, F.; Maggio, M.; Mecocci, P.; Ruggiero, C. Interaction between bone and muscle in older persons with mobility limitations. Curr. Pharm. Des. 2014, 20, 3178–3197. [Google Scholar] [CrossRef] [PubMed]
- Farin, E.; Ullrich, A.; Hauer, J. Participation and social functioning in patients with fibromyalgia: Development and testing of a new questionnaire. Health Qual. Life Outcomes 2013, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Yilmaz, S.D.; Polat, H.A.; Salli, A.; Erkin, G.; Ugurlu, H. The effects of fibromyalgia syndrome on female sexuality: A controlled study. J. Sex. Med. 2012, 9, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, H.M.; Katz, R.S. Fibrofog and fibromyalgia: A narrative review and implications for clinical practice. Rheumatol. Int. 2015, 35, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Laroche, F.; Azoulay, D.; Trouvin, A.P.; Coste, J.; Perrot, S. Fibromyalgia in the workplace: Risk factors for sick leave are related to professional context rather than fibromyalgia characteristics- a French national survey of 955 patients. BMC Rheumatol. 2019, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Ashe, S.C.; Furness, P.J.; Taylor, S.J.; Haywood-Small, S.; Lawson, K. A qualitative exploration of the experiences of living with and being treated for fibromyalgia. Health Psychol. Open 2017, 4, 2055102917724336. [Google Scholar] [CrossRef]
- Derry, S.; Wiffen, P.J.; Häuser, W.; Mücke, M.; Tölle, T.R.; Bell, R.F.; Moore, R.A. Oral nonsteroidal anti-inflammatory drugs for fibromyalgia in adults. Cochrane Database Syst. Rev. 2017, 3, Cd012332. [Google Scholar] [CrossRef]
- Moret, C.; Briley, M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr. Dis. Treat. 2006, 2, 537–548. [Google Scholar] [CrossRef]
- Tzellos, T.G.; Toulis, K.A.; Goulis, D.G.; Papazisis, G.; Zampeli, V.A.; Vakfari, A.; Kouvelas, D. Gabapentin and pregabalin in the treatment of fibromyalgia: A systematic review and a meta-analysis. J. Clin. Pharm. Ther. 2010, 35, 639–656. [Google Scholar] [CrossRef]
- Leite, F.M.; Atallah, A.N.; El Dib, R.; Grossmann, E.; Januzzi, E.; Andriolo, R.B.; da Silva, E.M. Cyclobenzaprine for the treatment of myofascial pain in adults. Cochrane Database Syst. Rev. 2009, 2009, Cd006830. [Google Scholar] [CrossRef]
- Gilron, I.; Chaparro, L.E.; Tu, D.; Holden, R.R.; Milev, R.; Towheed, T.; DuMerton-Shore, D.; Walker, S. Combination of pregabalin with duloxetine for fibromyalgia: A randomized controlled trial. Pain 2016, 157, 1532–1540. [Google Scholar] [CrossRef]
- Kim, S.Y.; Busch, A.J.; Overend, T.J.; Schachter, C.L.; van der Spuy, I.; Boden, C.; Góes, S.M.; Foulds, H.J.; Bidonde, J. Flexibility exercise training for adults with fibromyalgia. Cochrane Database Syst. Rev. 2019, 9, Cd013419. [Google Scholar] [CrossRef] [PubMed]
- Bidonde, J.; Busch, A.J.; Schachter, C.L.; Webber, S.C.; Musselman, K.E.; Overend, T.J.; Góes, S.M.; Dal Bello-Haas, V.; Boden, C. Mixed exercise training for adults with fibromyalgia. Cochrane Database Syst. Rev. 2019, 5, Cd013340. [Google Scholar] [CrossRef] [PubMed]
- Lagueux, É.; Dépelteau, A.; Masse, J. Occupational Therapy’s Unique Contribution to Chronic Pain Management: A Scoping Review. Pain Res. Manag. 2018, 2018, 5378451. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Li, Q.; Zhang, X.; Varrassi, G.; Wang, H. Effectiveness of Hyperbaric Oxygen for Fibromyalgia: A Meta-Analysis of Randomized Controlled Trials. Clin. Pract. 2023, 13, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Shirotsuki, K.; Sugaya, N. Cognitive-behavioral therapy for management of mental health and stress-related disorders: Recent advances in techniques and technologies. Biopsychosoc. Med. 2021, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.A.; Liu, Y.; Nguyen, J.; Spraggins, R.; Reed, D.S.; Lee, C.; Hasoon, J.; Kaye, A.D. Efficacy of acupuncture in the treatment of fibromyalgia. Orthop. Rev. 2021, 13, 25085. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, N.M.; Hilal, F.M.; Bashawyah, A.; Dammas, F.A.; Yamak Altinpulluk, E.; Hou, J.-D.; Lin, J.-A.; Varrassi, G.; Chang, K.-V.; Allam, A.E. Efficacy of Acupuncture, Intravenous Lidocaine, and Diet in the Management of Patients with Fibromyalgia: A Systematic Review and Network Meta-Analysis. Healthcare 2022, 10, 1176. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, W. Multimodal non-invasive non-pharmacological therapies for chronic pain: Mechanisms and progress. BMC Med. 2023, 21, 372. [Google Scholar] [CrossRef]
- Glombiewski, J.A.; Bernardy, K.; Häuser, W. Efficacy of EMG- and EEG-Biofeedback in Fibromyalgia Syndrome: A Meta-Analysis and a Systematic Review of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2013, 2013, 962741. [Google Scholar] [CrossRef]
- Alnawwar, M.A.; Alraddadi, M.I.; Algethmi, R.A.; Salem, G.A.; Salem, M.A.; Alharbi, A.A. The Effect of Physical Activity on Sleep Quality and Sleep Disorder: A Systematic Review. Cureus 2023, 15, e43595. [Google Scholar] [CrossRef]
- Bidonde, J.; Busch, A.J.; Schachter, C.L.; Overend, T.J.; Kim, S.Y.; Góes, S.M.; Boden, C.; Foulds, H.J. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst. Rev. 2017, 6, Cd012700. [Google Scholar] [CrossRef]
- Vambheim, S.M.; Kyllo, T.M.; Hegland, S.; Bystad, M. Relaxation techniques as an intervention for chronic pain: A systematic review of randomized controlled trials. Heliyon 2021, 7, e07837. [Google Scholar] [CrossRef]
- Firth, J.; Gangwisch, J.E.; Borisini, A.; Wootton, R.E.; Mayer, E.A. Food and mood: How do diet and nutrition affect mental wellbeing? BMJ 2020, 369, m2382. [Google Scholar] [CrossRef]
- Lowry, E.; Marley, J.; McVeigh, J.G.; McSorley, E.; Allsopp, P.; Kerr, D. Dietary Interventions in the Management of Fibromyalgia: A Systematic Review and Best-Evidence Synthesis. Nutrients 2020, 12, 2664. [Google Scholar] [CrossRef]
- Zhou, X.; Afzal, S.; Wohlmuth, H.; Münch, G.; Leach, D.; Low, M.; Li, C.G. Synergistic Anti-Inflammatory Activity of Ginger and Turmeric Extracts in Inhibiting Lipopolysaccharide and Interferon-γ-Induced Proinflammatory Mediators. Molecules 2022, 27, 3877. [Google Scholar] [CrossRef]
- Pfalzgraf, A.R.; Lobo, C.P.; Giannetti, V.; Jones, K.D. Use of Complementary and Alternative Medicine in Fibromyalgia: Results of an Online Survey. Pain Manag. Nurs. 2020, 21, 516–522. [Google Scholar] [CrossRef]
- Hawk, C.; Whalen, W.; Farabaugh, R.J.; Daniels, C.J.; Minkalis, A.L.; Taylor, D.N.; Anderson, D.; Anderson, K.; Crivelli, L.S.; Cark, M.; et al. Best Practices for Chiropractic Management of Patients with Chronic Musculoskeletal Pain: A Clinical Practice Guideline. J. Altern. Complement. Med. 2020, 26, 884–901. [Google Scholar] [CrossRef]
- Borsook, D.; Sava, S.; Becerra, L. The pain imaging revolution: Advancing pain into the 21st century. Neuroscientist 2010, 16, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, K.V. The Search for Biomarkers in Fibromyalgia. Diagnostics 2021, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, G.; Guymer, E. Modulation of NMDA Receptor Activity in Fibromyalgia. Biomedicines 2017, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Elikkottil, J.; Gupta, P.; Gupta, K. The analgesic potential of cannabinoids. J. Opioid Manag. 2009, 5, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Strand, N.H.; Maloney, J.; Kraus, M.; Wie, C.; Turkiewicz, M.; Gomez, D.A.; Adeleye, O.; Harbell, M.W. Cannabis for the Treatment of Fibromyalgia: A Systematic Review. Biomedicines 2023, 11, 1621. [Google Scholar] [CrossRef]
- Erdrich, S.; Hawrelak, J.A.; Myers, S.P.; Harnett, J.E. Determining the association between fibromyalgia, the gut microbiome and its biomarkers: A systematic review. BMC Musculoskelet. Disord. 2020, 21, 181. [Google Scholar] [CrossRef]
- Quaranta, G.; Guarnaccia, A.; Fancello, G.; Agrillo, C.; Iannarelli, F.; Sanguinetti, M.; Masucci, L. Fecal Microbiota Transplantation and Other Gut Microbiota Manipulation Strategies. Microorganisms 2022, 10, 2424. [Google Scholar] [CrossRef]
- Leroux, A.; Rzasa-Lynn, R.; Crainiceanu, C.; Sharma, T. Wearable Devices: Current Status and Opportunities in Pain Assessment and Management. Digit. Biomark. 2021, 5, 89–102. [Google Scholar] [CrossRef] [PubMed]
| No Problem | Mild | Moderate | Severe | |
|---|---|---|---|---|
| Fatigue | 0 | 1 | 2 | 3 |
| Trouble thinking or remembering | 0 | 1 | 2 | 3 |
| Waking up tired (unrefreshed) | 0 | 1 | 2 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Sharie, S.; Varga, S.J.; Al-Husinat, L.; Sarzi-Puttini, P.; Araydah, M.; Bal’awi, B.R.; Varrassi, G. Unraveling the Complex Web of Fibromyalgia: A Narrative Review. Medicina 2024, 60, 272. https://doi.org/10.3390/medicina60020272
Al Sharie S, Varga SJ, Al-Husinat L, Sarzi-Puttini P, Araydah M, Bal’awi BR, Varrassi G. Unraveling the Complex Web of Fibromyalgia: A Narrative Review. Medicina. 2024; 60(2):272. https://doi.org/10.3390/medicina60020272
Chicago/Turabian StyleAl Sharie, Sarah, Scott J. Varga, Lou’i Al-Husinat, Piercarlo Sarzi-Puttini, Mohammad Araydah, Batool Riyad Bal’awi, and Giustino Varrassi. 2024. "Unraveling the Complex Web of Fibromyalgia: A Narrative Review" Medicina 60, no. 2: 272. https://doi.org/10.3390/medicina60020272
APA StyleAl Sharie, S., Varga, S. J., Al-Husinat, L., Sarzi-Puttini, P., Araydah, M., Bal’awi, B. R., & Varrassi, G. (2024). Unraveling the Complex Web of Fibromyalgia: A Narrative Review. Medicina, 60(2), 272. https://doi.org/10.3390/medicina60020272








