Complications Subsequent to Urinary Tract Stent Placement: An Overview Focusing on the Imaging of Cancer Patients
Abstract
1. Introduction
2. Generalities on Urinary Devices
3. Indications in Cancer Patients
4. Imaging Findings
5. Complications
- Stent discomfort: Patients with urinary stents frequently experience pain in the suprapubic and flank regions, with an incidence rate of up to 80%. Several factors can contribute to this pain, including vesicoureteral reflux causing an upward surge in intra-ureteral pressure, primarily spasms in the distal ureter, and irritation of the bladder mucosa due to the presence of a foreign body in the bladder [1,2]. It is crucial to emphasize that the root cause of this pain remains unknown to date. Topographically, it corresponds to two distinct regions where patients report discomfort. Approximately 60–77% of patients describe the onset of lateral pain, primarily but not exclusively associated with micturition and vesicoureteral reflux induced by the stent. The occurrence of suprapubic pain, reaching up to 38%, is linked to adverse effects at this level related to the bladder pigtail and the irritation of the bladder trigone [19,20]. Despite the correct placement of contemporary stents, irritative symptoms in the lower urinary tract may manifest in 80–90% of patients. Occasionally, these symptoms can be so unbearable that they necessitate the early removal of the stent. These symptoms, categorized as filling symptoms, emptying symptoms, and post-mictional symptoms, are unequivocally attributed to the irritation of the bladder urothelium by a vesical stent end by causing inflammation and increased activity of the bladder detrusor [21]. Indeed, despite advancements in using biocompatible materials for constructing stents, the epithelium of the renal collecting system, ureter, and bladder reacts to the presence of the foreign entity [2].
- Vesicoureteral reflux (VUR): Anatomically, the ureterovesical junction (UVJ) stands as a crucial structure safeguarding the upper urinary tract from intermittent high pressures in the bladder. Functionally, the UVJ, through its momentary opening, facilitates the passage of urine into the bladder while preventing a retrograde flow into the kidneys during micturition. Several factors contribute to the effective operation of this anti-reflux mechanism: an appropriate length of the intravesical ureter, an oblique angle of ureter insertion into the bladder, and the proper development of the smooth muscle and extracellular matrix capable of compressing the ureteral orifice. Any deviation in these features results in the retrograde flow of urine or VUR. This is inevitable in the presence of an unobstructed stent. Typically, this occurs during the voiding phase when the bladder pressure rises, and the stent, maintaining an open communication between the bladder and the ureter, induces the retrograde flow of urine. In a voiding cystourethrography analysis conducted on patients with stents, reflux during the voiding stage was observed in 80% of cases, likely contributing to the flank pain experienced during voiding by these patients [22].
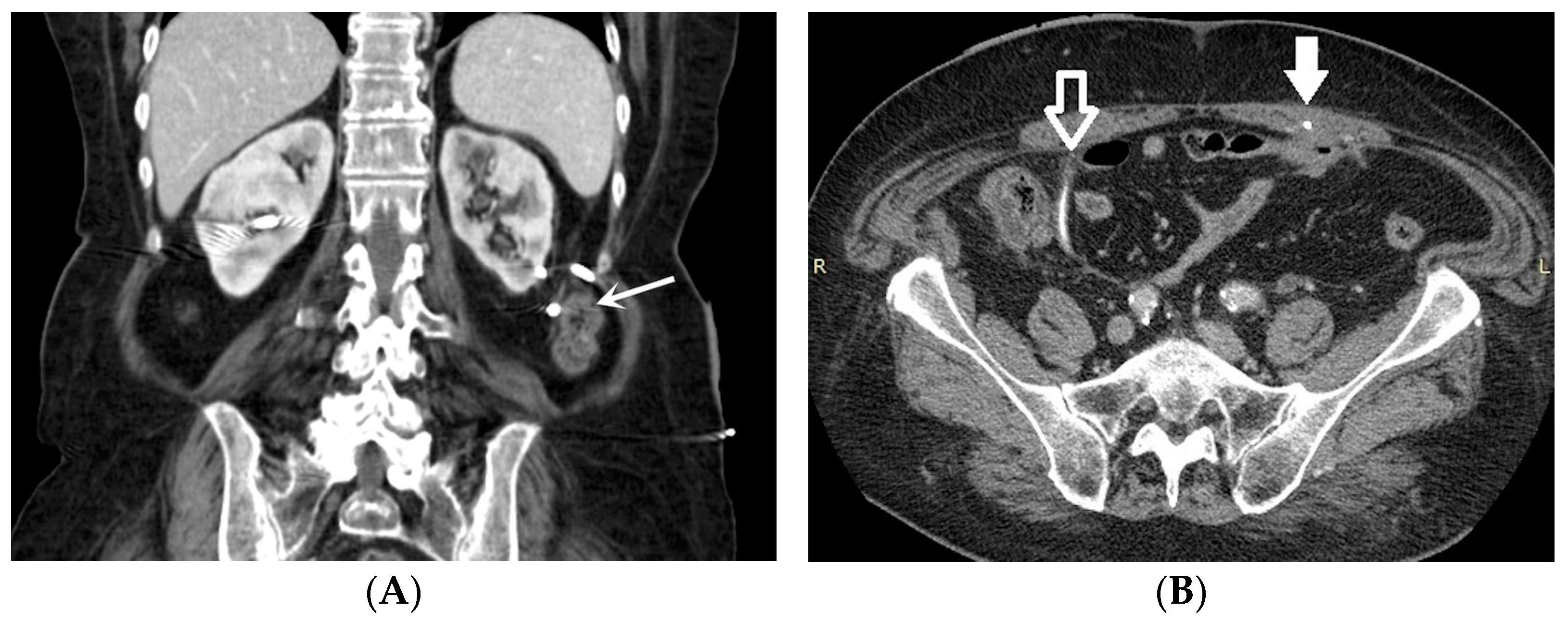
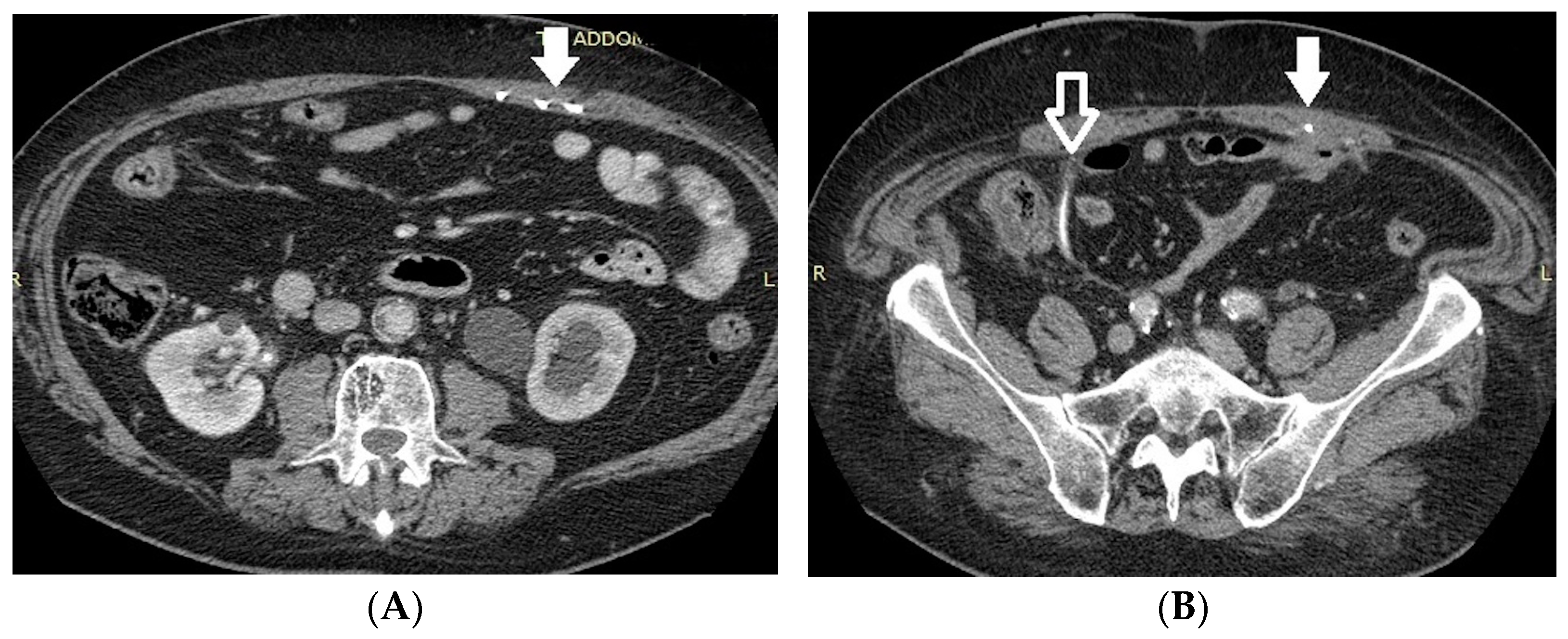
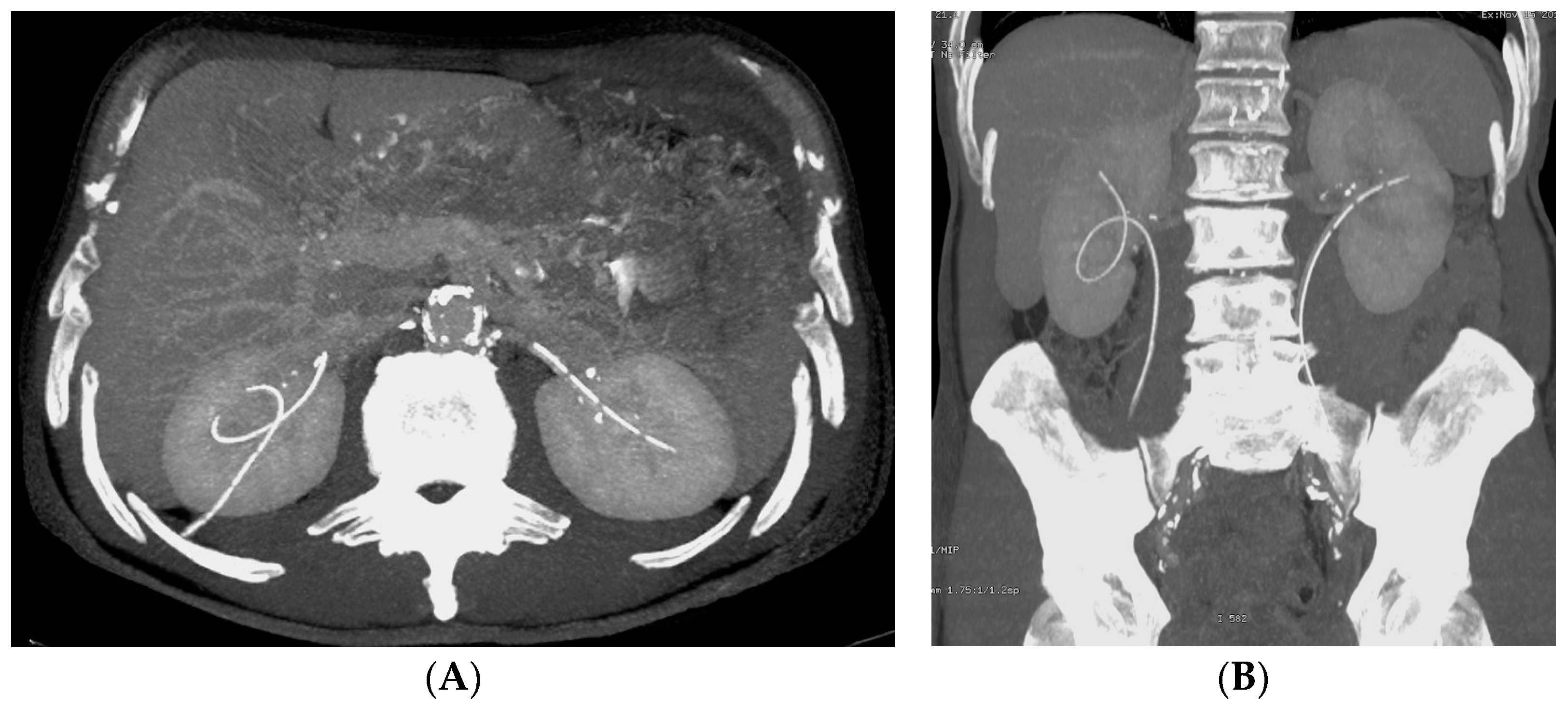
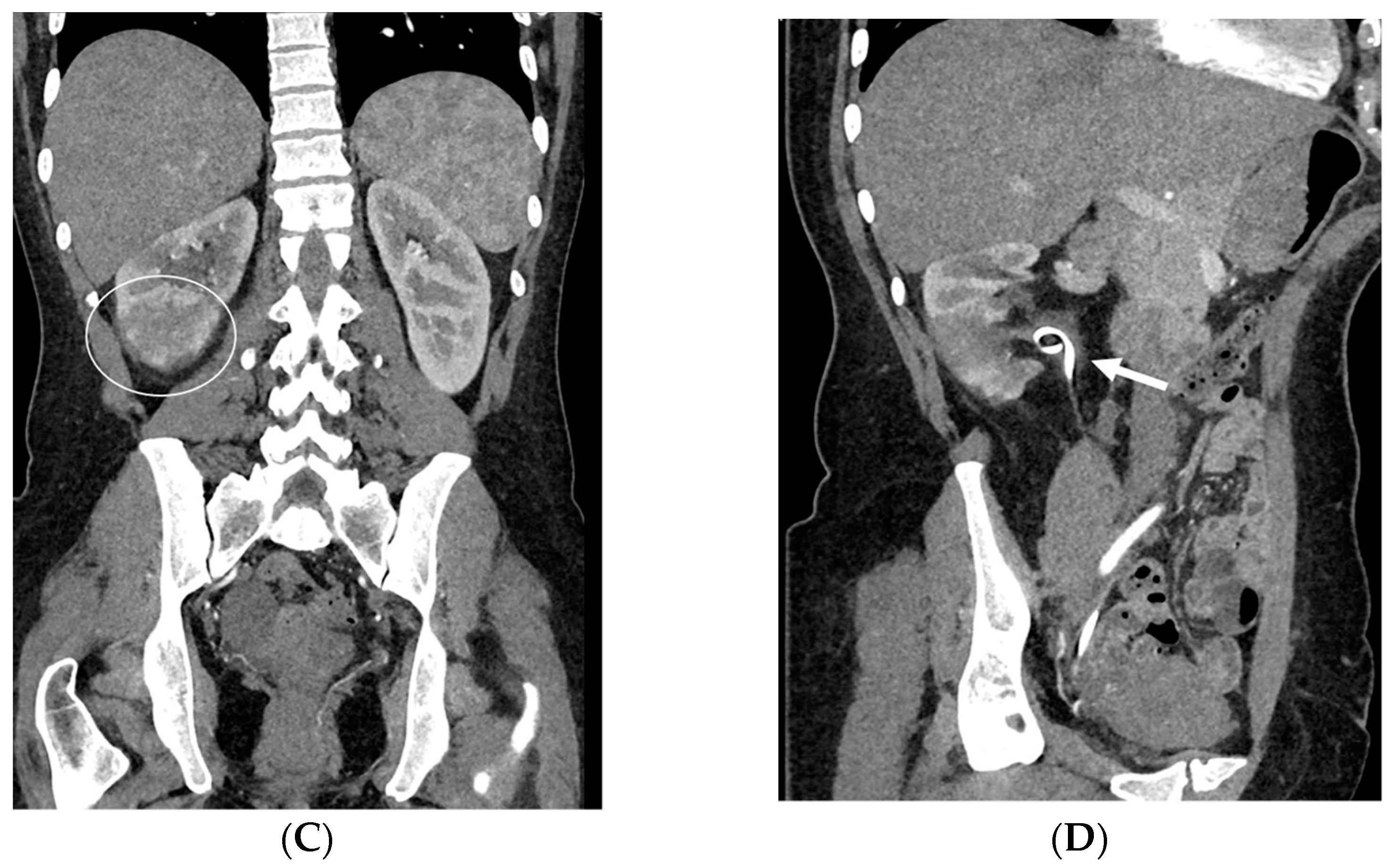
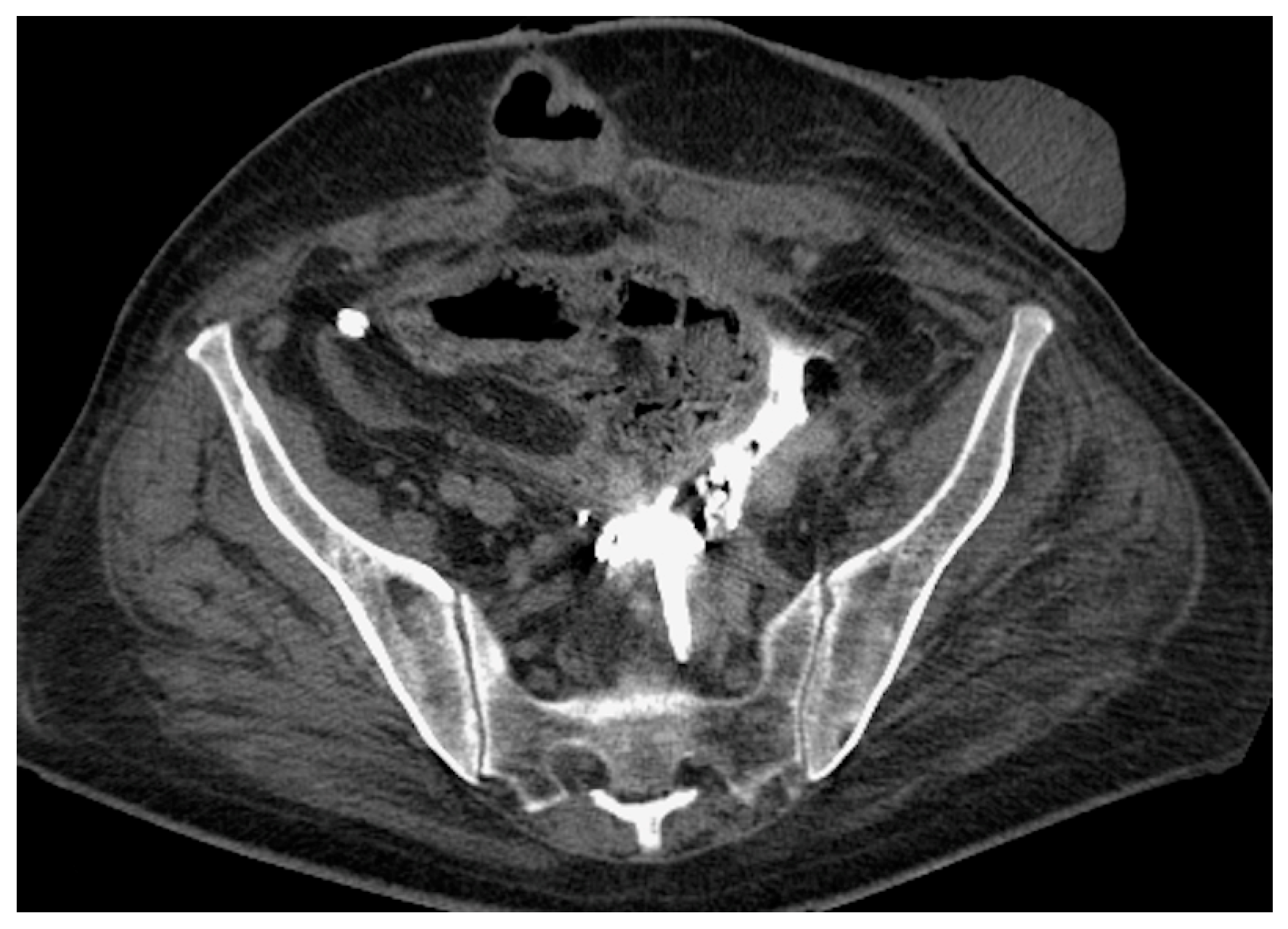
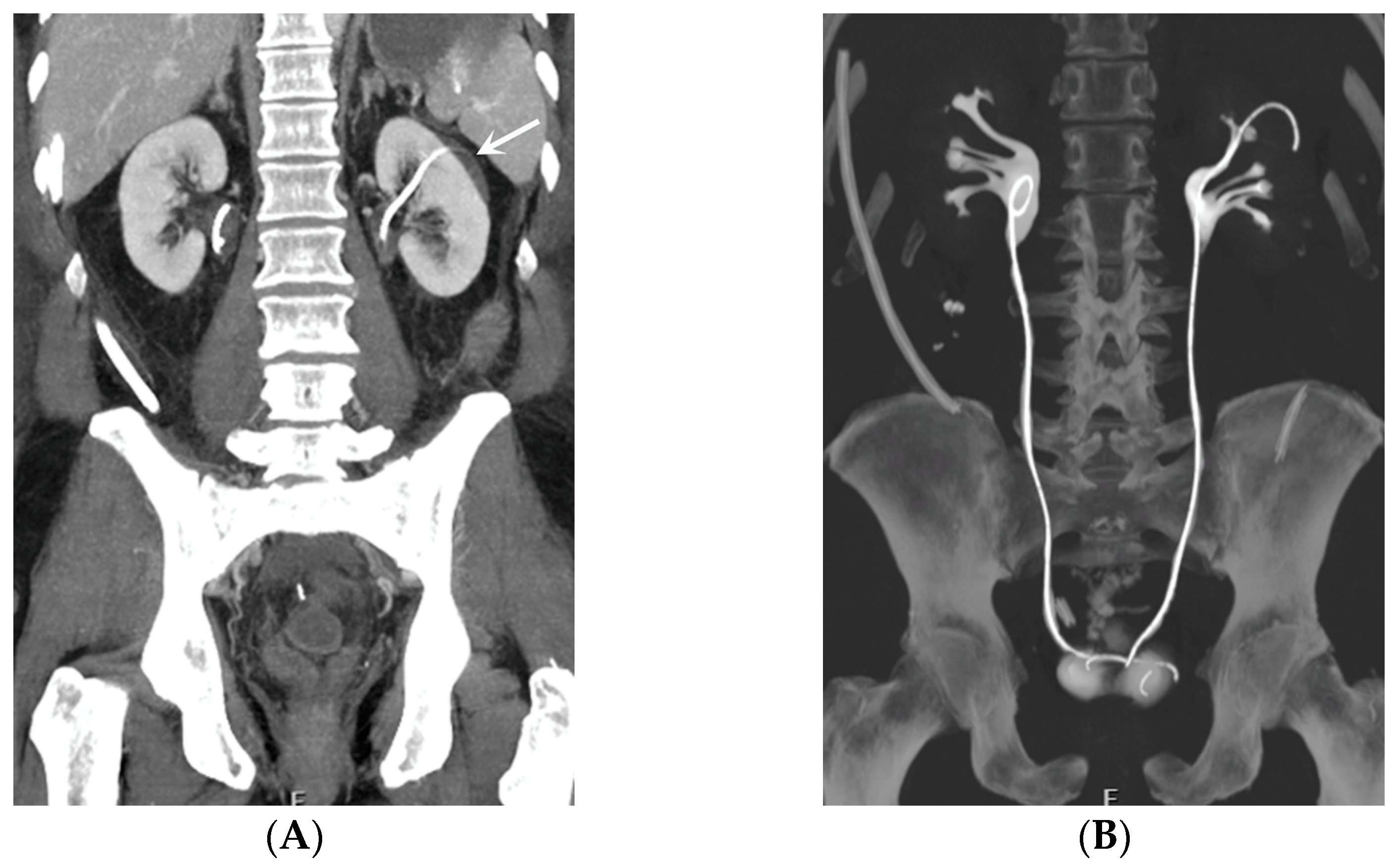
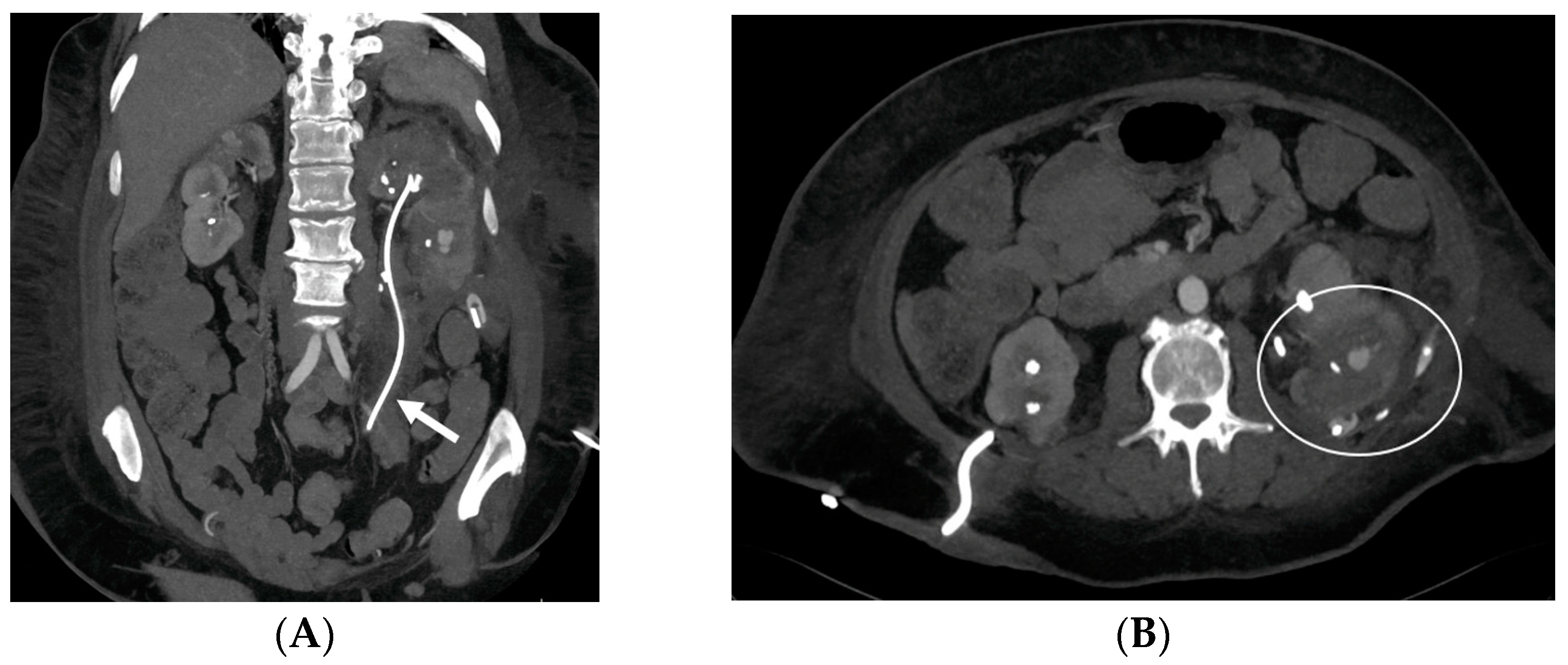
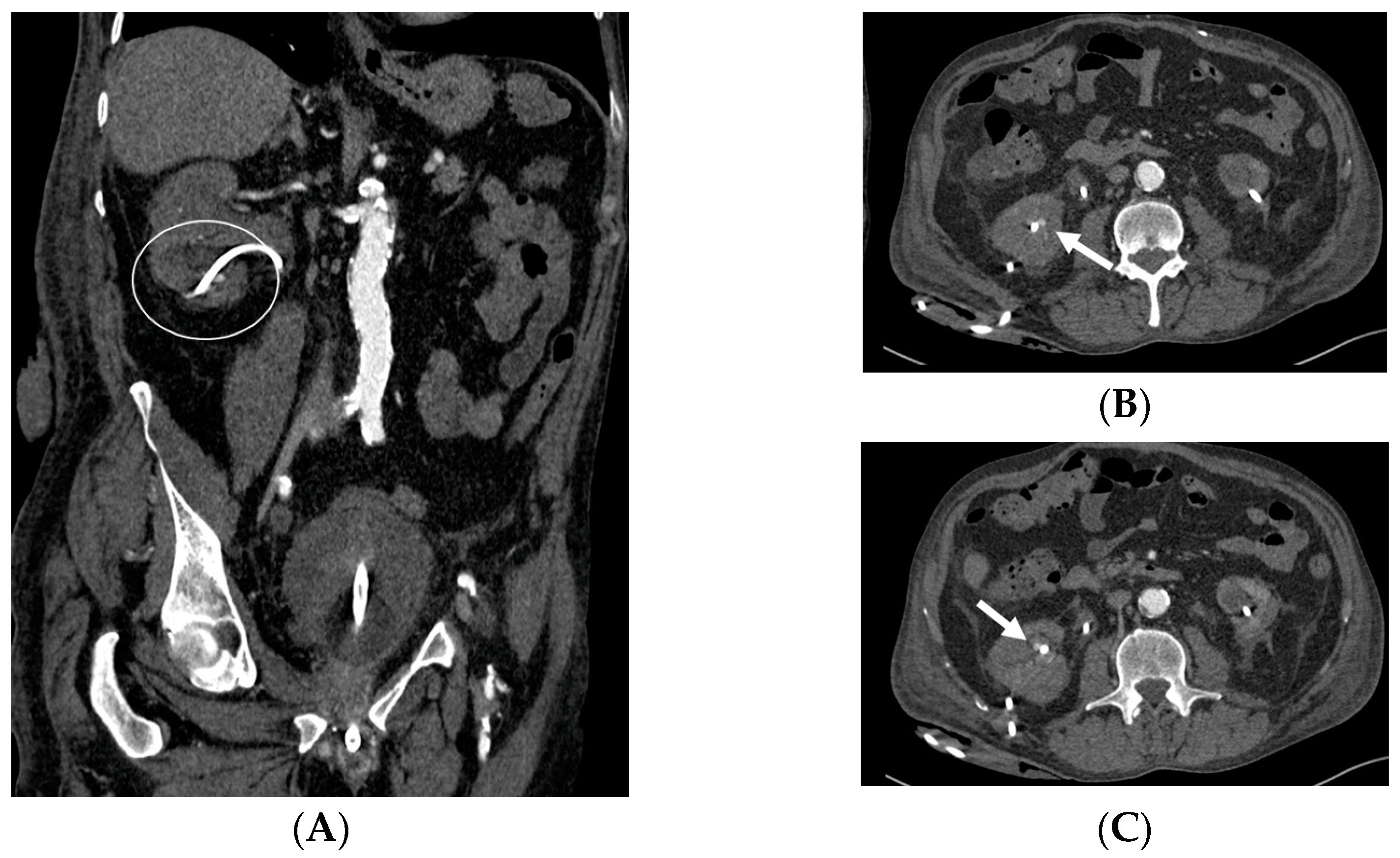
- Malposition: The malposition of a stent is defined as an incorrect position relative to initial placement, while displacement presents a subsequent occurrence in a device that was previously located in the correct position. A stent improperly positioned might assume a sub-pyelic position when the proximal end fails to reach the renal pelvis and a supravesical position when the distal end is detected within the ureter. The origins of this complication predominantly stem from the placement technique, whether it be endoscopy- or fluoroscopy-guided insertion. This underscores the need to verify the accurate positioning of the stent post-placement. Ensuring an adequate length is essential to reduce the occurrence of this complication (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6) [21,22].
- Obstruction of the stent lumen: It is a potential occurrence after insertion into the urinary tract. Luminal blockage may arise from hematuria related to the technique or from elevated urine viscosity and debris associated with insertion in an infected system. Clinical manifestations of flank pain can indicate the potential for a stent to be obstructed, but they can also be attributed to a stent that is functioning properly. The evaluation of renal function through blood studies may not necessarily reveal an acute obstruction, particularly if the obstruction is unilateral. Indeed, it is widely recognized that, in patients with a long-standing upper tract obstruction, the placement of a stent may not completely restore the renal collecting system to a normal appearance. During voiding cystography, it is common for most patent stents to exhibit reflux, and this can serve as an indicator of patency. Color Doppler US has also been utilized to assess stent jets [2,3].
- Encrustation: Encrustations may develop in the case of long-standing catheters, when the need for obstruction relief is necessary for a long time or when the stent has just been “forgotten”, as for patients lost to follow-up. Encrustation most frequently develops in the endovesical part of a double-J catheter. It is important to identify the presence and extent of the encrustation in order to plan treatment strategies [23,24].
- Stent fracture: Due to the “hostile” environment of human urine, a stent fracture can occur. Over time, polyethylene was eliminated as a material because stents made of it turned brittle and fractured in relatively short indwelling times. Stent fractures have also been documented with newer materials. Interestingly, fenestration sites are where most fractures occur. Encrustation probably contributes to stent fragmentation, and the prevalence of both complications is directly proportional to the residence period [2,25,26].
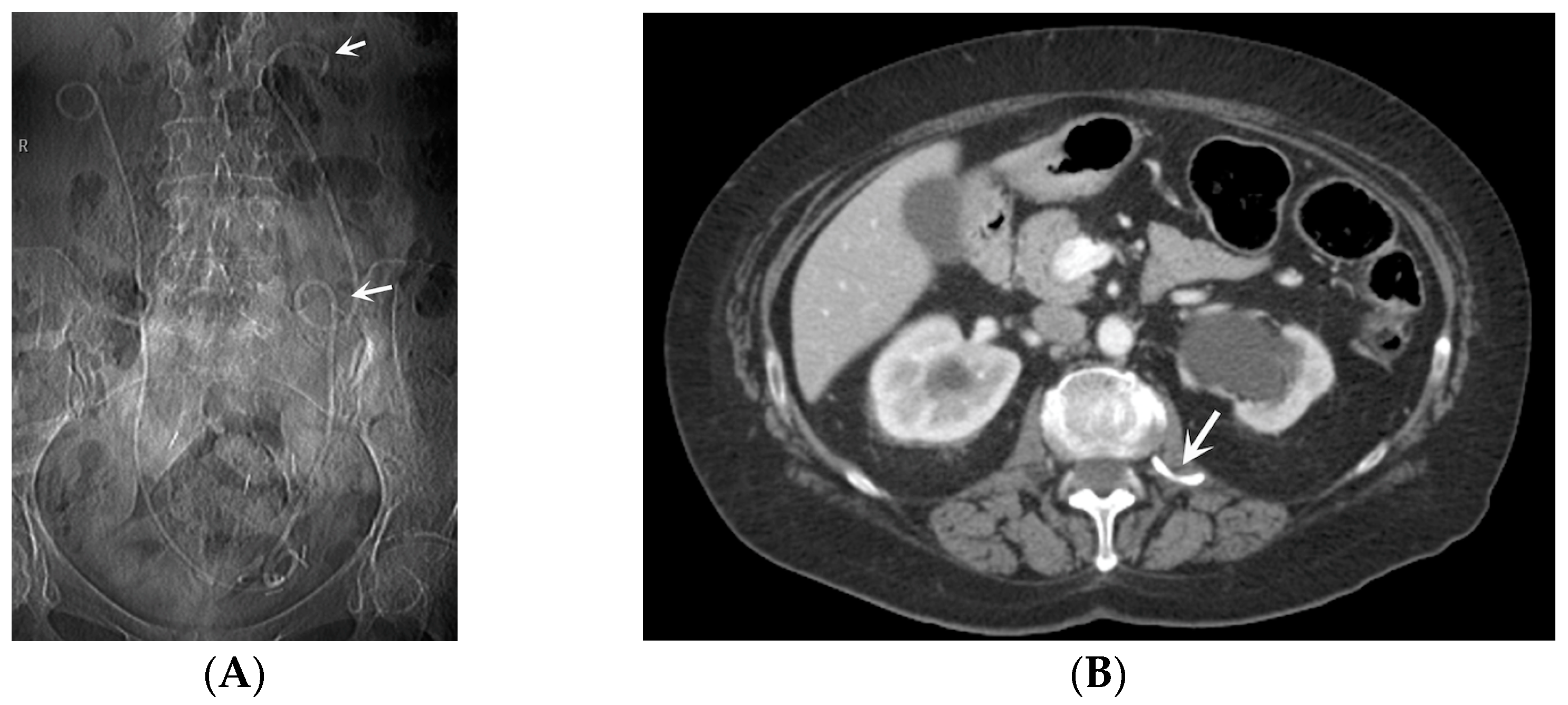
- Stent Knotting: It is a rare complication. Most of these knots involve the proximal end of the stent near the coil, but every portion can be affected [27,28]. Previous reports have attributed knot formation to the excessive length of the stent, stent shape (double-J or multicoil), and flexibility or anatomical abnormalities, such as cystocele and ileal conduits. An abdominal X-ray and, especially, a CT scan are more sensitive than other imaging modalities in identifying a ruptured or knotted stent as well as its migration (Figure 7) [29,30].
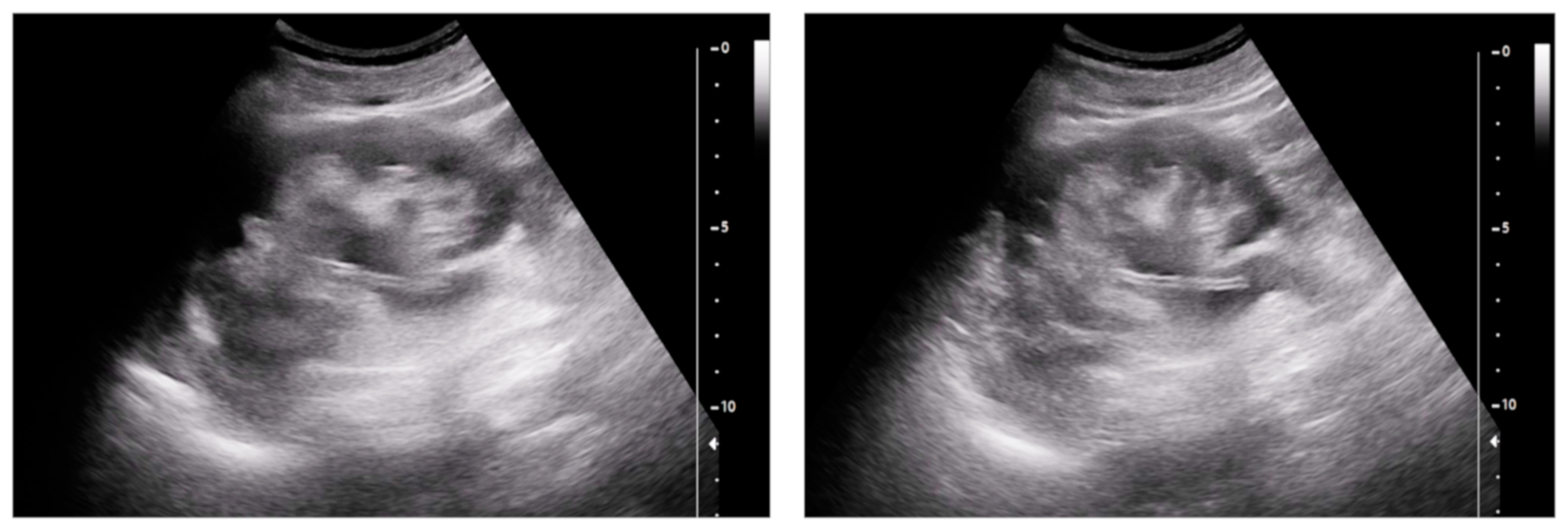
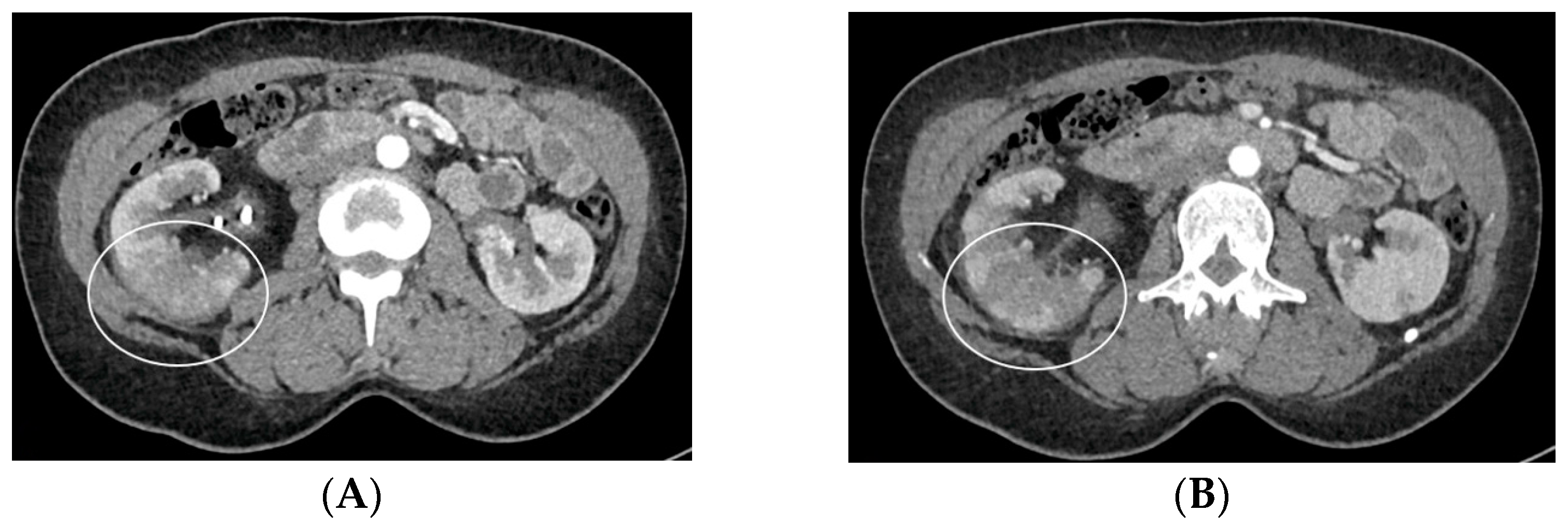
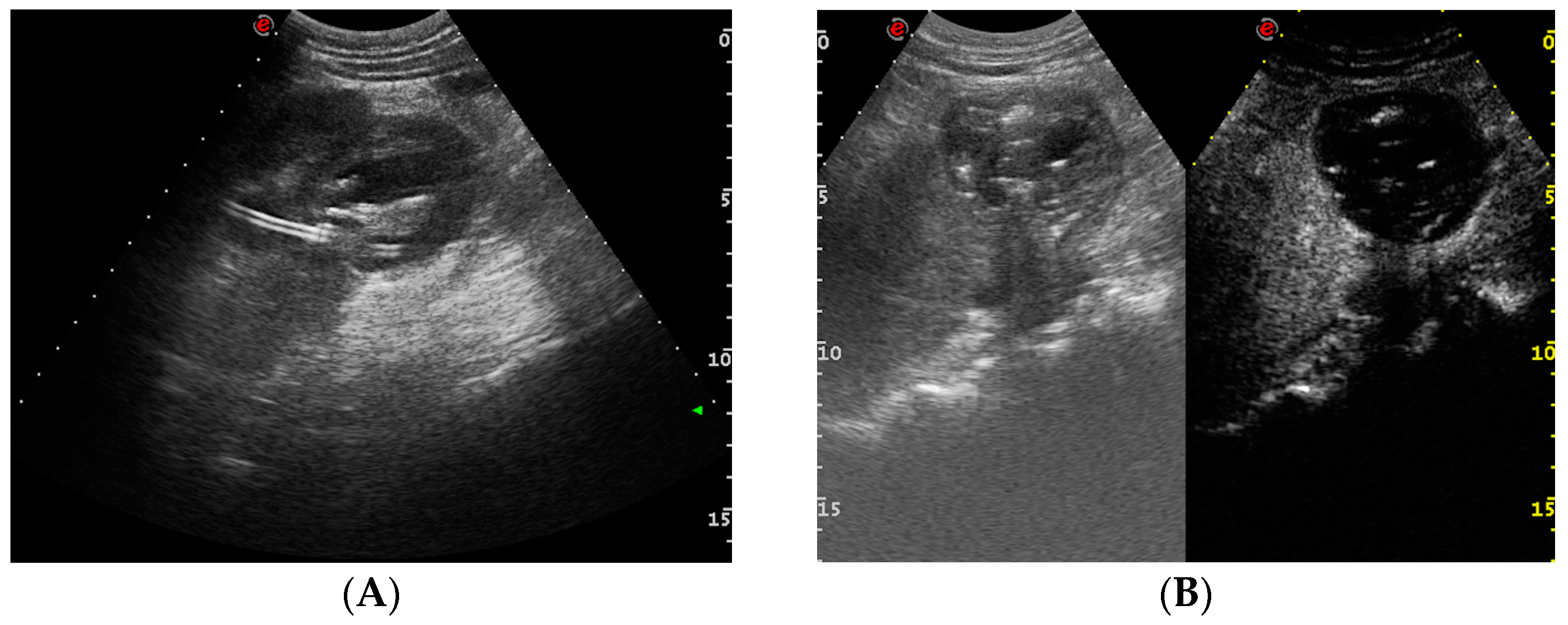
- Urinary tract infection: Stent colonization by bacteria, with an overall incidence ranging from 42% to 90%, is a significant clinical challenge that can lead to a urinary tract infection. In some instances, this infection can result in complications, such as acute pyelonephritis (Figure 8) and renal failure [31,32]. For most patients experiencing a ureteral obstruction, stent placement is carried out with antibiotic prophylaxis, typically administered as a single dose concurrent with the procedure. In cases where a urinary tract infection is already known, the insertion of the stent should be delayed whenever possible until the appropriate treatment with culture-specific antibiotics allows for urine sterilization [32]. US serves as the first-line diagnostic tool to assess the urinary tract in patients presenting with the symptoms of pyelonephritis. Unfortunately, pyelonephritis lacks clear gray-scale findings useful during characterization [33]. Consequently, most patients with clinically suspected pyelonephritis have negative results from US. In cases where imaging is deemed necessary, CT emerges as the preferred modality, providing comprehensive anatomical and physiological information and accurately delineating both intra- and extra-renal pathological conditions. The presence of urinary tract gas, calculi, hemorrhage, renal enlargement, inflammatory masses, and obstruction can be easily detected by CT. Specifically, the affected regions may show a lower attenuation due to edema with pockets of higher attenuation representing the foci of hemorrhage. However, these findings are frequently absent, and unenhanced CT images may appear normal. It is only after the administration of contrast material that the diagnostic features of pyelonephritis become evident. In advanced stages, sepsis is a potential complication, occasionally presenting a critical issue in a debilitated cancer patient. The close monitoring of the patients after the procedure is imperative, with a heightened awareness of the potential for sepsis. The incidence of sepsis following catheter insertion varies between 1.5% and 7%, particularly in patients with pyelonephritis [1,2].
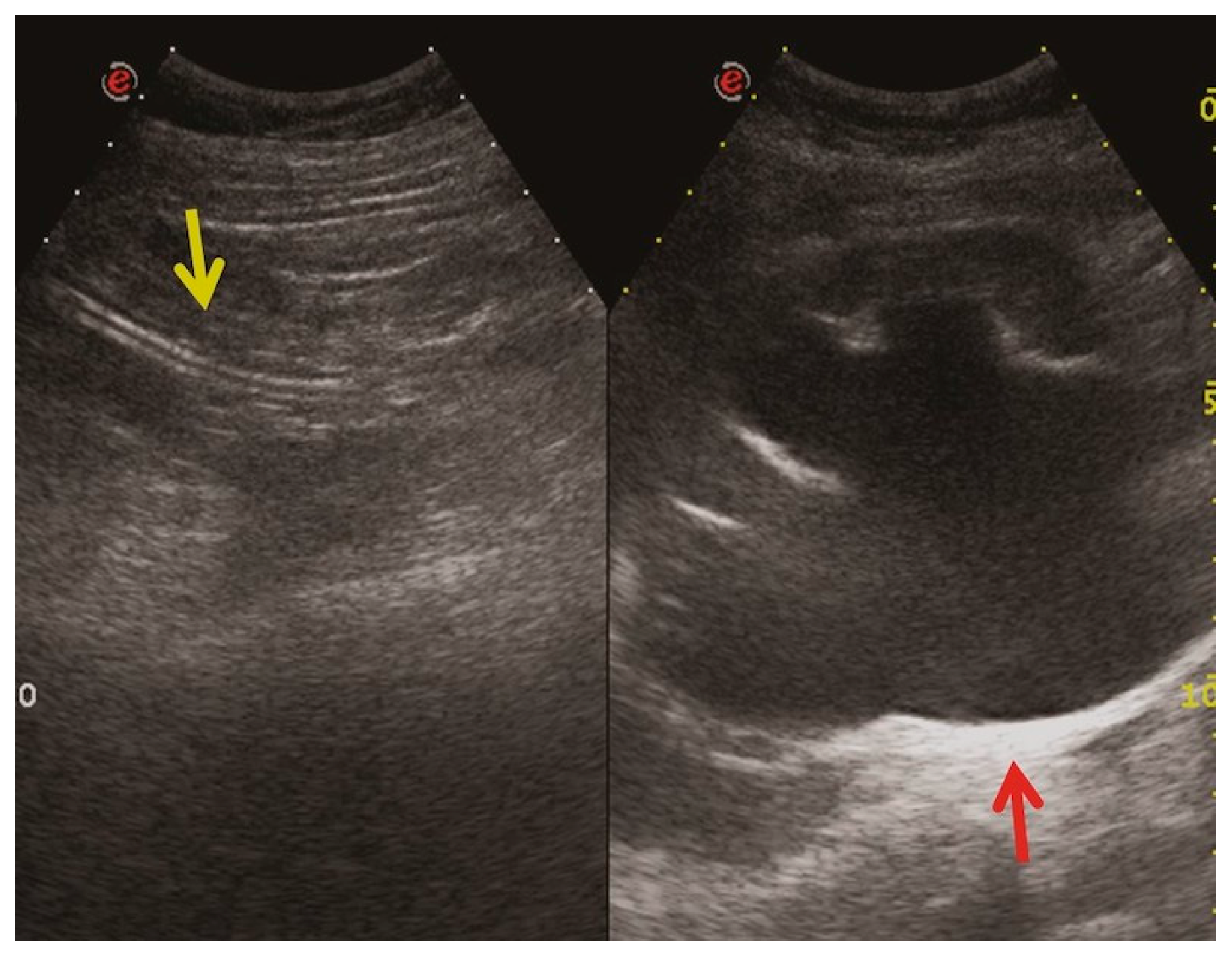
- Urine leakage: Stents crafted from more rigid materials have the potential to perforate the ureter, collecting system, and kidney parenchyma during placement, leading to the formation of fluid collections or urine leakage, often resulting in a urinoma [35] (Figure 9). A urinoma is a collection of extravasated urine outside of the urinary tract. The optimal diagnostic imaging studies for this condition include contrast material-enhanced CT with delayed imaging (10–20 min), CT–cystography, and a retrograde urethrography [36,37]. In a CT scan, a urinoma may manifest as a restricted or unrestrained collection within the intra- or retro-peritoneal compartment, with the latter occurrence being more prevalent. Its attenuation values can vary from 0 to 20 HUs before the administration of intravenous contrast, subsequently intensifying up to 200 HUs after contrast administration (Figure 9). The irritation from the urine may lead to the formation of a fibrous capsule surrounding the urinoma, which can occasionally become calcified. Not infrequently, a dystrophic calcification of the urinoma may occur, which results from an inflammatory and fibrotic reaction to the extravasated urine [38]. Urinomas, typically small initially, are often resolved on their own without intervention. However, in cases of significant injury or a larger urinoma failing to reabsorb, urological or interventional radiological procedures may be necessary. Neglecting intervention can lead to complications, such as abscess, electrolyte imbalance, hydronephrosis, and urosepsis [37,38].
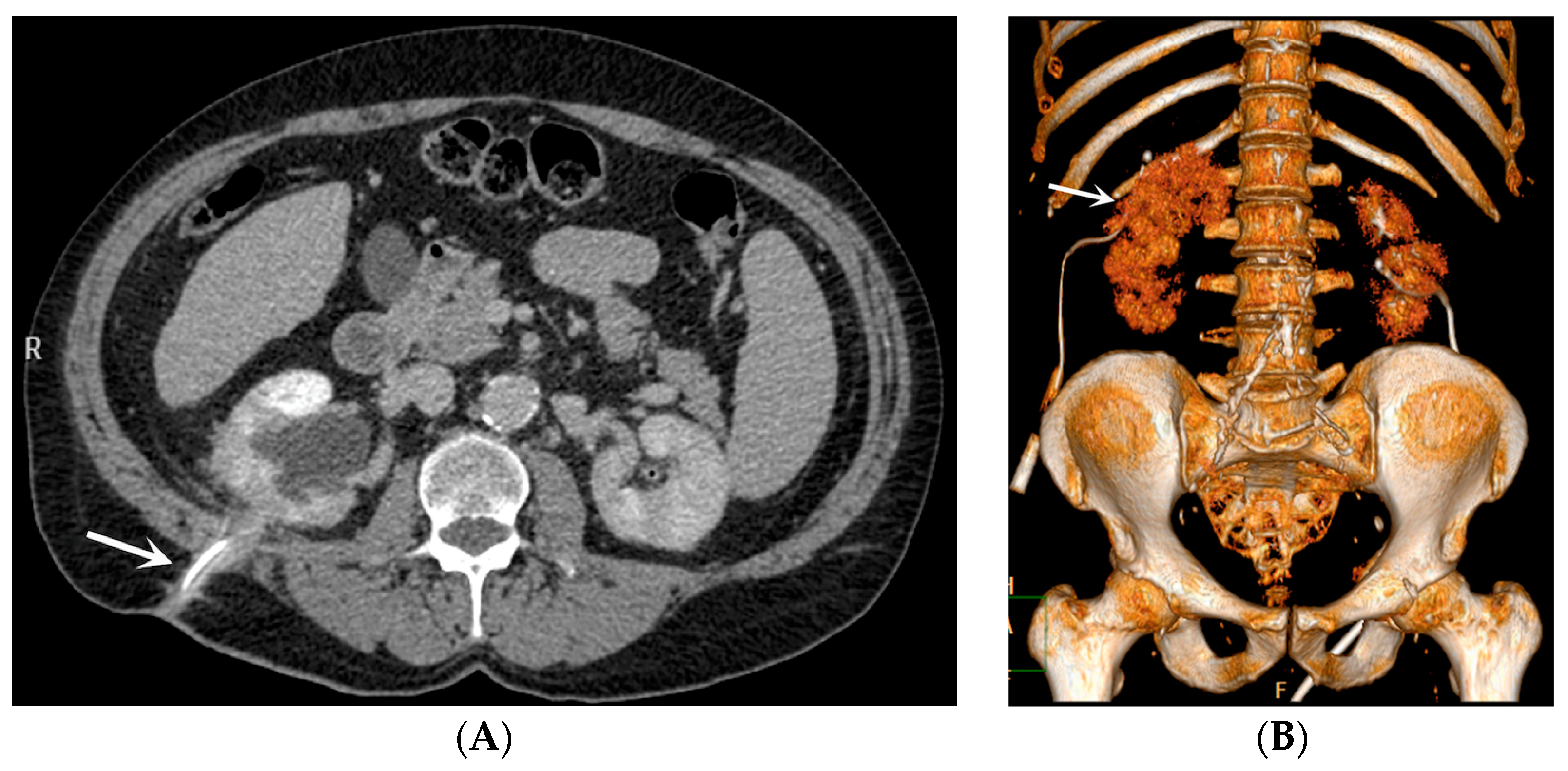
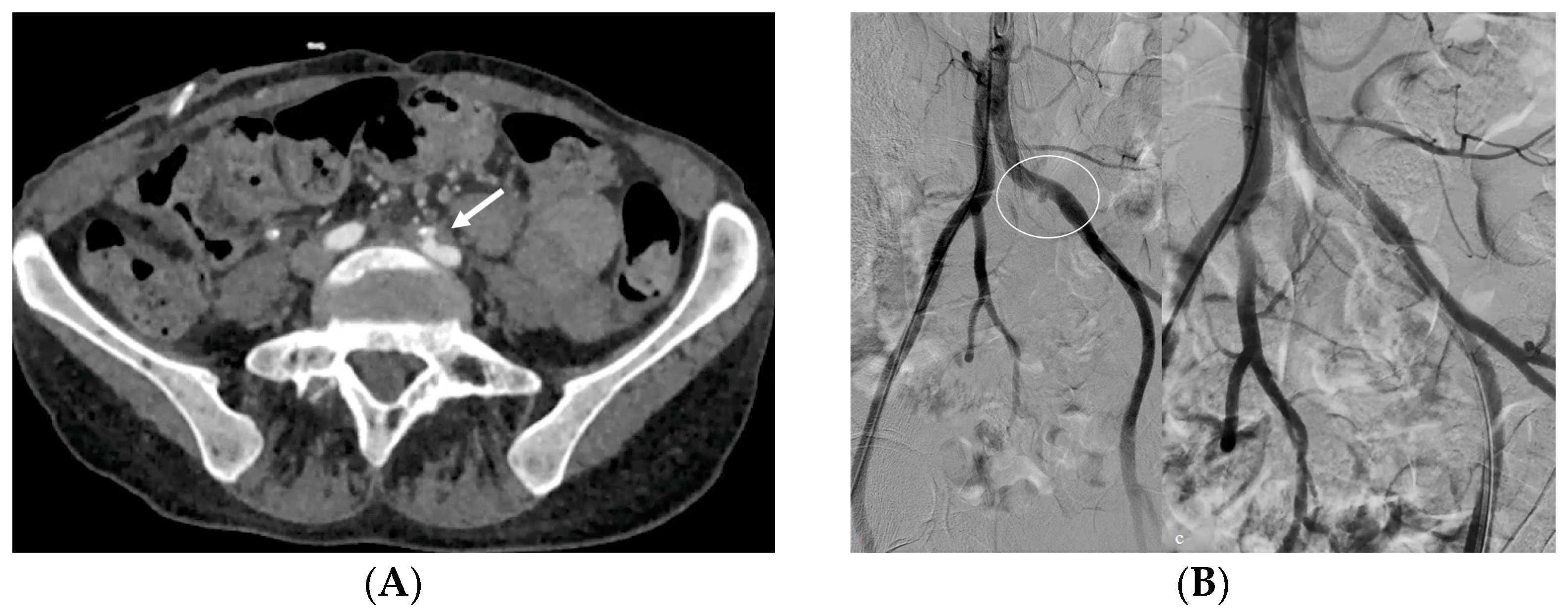
- Bleeding: The erosion of the stent into the arterial system is a rare and feared complication of ureteral stent placement, which can cause hematomas (Figure 10 and Figure 11), active bleeding, or pseudoaneurysm (Figure 12, Figure 13 and Figure 14). To avoid mortality from these complications, a high level of clinical suspicion is essential. Intermittent hematuria in a patient with a stent is typically the usual clinical scenario. However, massive hematuria and circulatory collapse can occur due to the manipulation of the ureteral stent [2,39].
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adamo, R.; Saad, W.E.; Brown, D.B. Management of nephrostomy drains and ureteral stents. Tech. Vasc. Interv. Radiol. 2009, 12, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Dyer, R.B.; Chen, M.Y.; Zagoria, R.J.; Regan, J.D.; Hood, C.G.; Kavanagh, P.V. Complications of ureteral stent placement. Radiographics 2002, 22, 1005–1022. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.H.; Lee, S.W.; Jeon, H.G.; Kim, H.H.; Oh, S.J. Percutaneous nephrostomy versus indwelling ureteral stents in the management of extrin-sic ureteral obstruction in advanced malignancies: Are there differences? Urology 2004, 64, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Saad, W.E.; Moorthy, M.; Ginat, D. Percutaneous nephrostomy: Native and transplanted kidneys. Tech. Vasc. Interv. Radiol. 2009, 12, 172–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yoon, C.J.; Lee, S.; Lee, J.H.; Choi, W.S.; Lee, C.H. Comparison between antegrade versus retrograde ureteral stent placement for malignant ureteral obstruction. J. Vasc. Interv. Radiol. 2022, 33, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Turgut, B.; Bayraktar, A.M.; Bakdık, S.; Hamarat, M.B.; Öncü, F.; Gönen, M.; Tolu, I. Placement of double-J stent in patients with malignant ureteral obstruction: Antegrade or retrograde approach? Clin. Radiol. 2019, 74, 976.e11–976.e17. [Google Scholar] [CrossRef]
- Dyer, R.B.; Regan, J.D.; Kavanagh, P.V.; Khatod, E.G.; Chen, M.Y.; Zagoria, R.J. Percutaneous nephrostomy with extensions of the technique: Step by step. Radiographics 2002, 22, 503–525. [Google Scholar] [CrossRef]
- Hunter, T.B.; Taljanovic, M.S. Medical devices of the abdomen and pelvis. Radiographics 2005, 25, 503–523. [Google Scholar] [CrossRef]
- Borin, J.F.; Melamud, O.; Clayman, R.V. Initial experience with full-length metal stent to relieve malignant ureteral obstruction. J. Endourol. 2006, 20, 300–304. [Google Scholar] [CrossRef]
- Monga, M. The dwell time of indwelling ureteral stents-the clock is ticking but when should we set the alarm? J. Urol. 2011, 185, 387. [Google Scholar] [CrossRef]
- Goldsmith, Z.G.; Wang, A.J.; Bañez, L.L.; Lipkin, M.E.; Ferrandino, M.N.; Preminger, G.M.; Inman, B.A. Outcomes of metallic stents for malignant ureteral obstruction. J. Urol. 2012, 188, 851–855. [Google Scholar] [CrossRef]
- Kunkel, G.; Patel, H.; Kaldany, A.; Allu, S.; Elsamra, S.; Cancian, M. Pelvic radiation-induced urinary strictures: Etiology and management of a challenging disease. World J. Urol. 2023, 41, 1459–1468. [Google Scholar] [CrossRef]
- Fabiano de Oliveira Leite, T.; Vatanabe Pazinato, L.; Mauricio da Motta Leal Filho, J. Percutaneous insertion of bilateral double J in pelvic cancer patients: Indications, complications, technique of antegrade ureteral stenting. Gynecol. Oncol. Rep. 2021, 38, 100864. [Google Scholar] [CrossRef]
- Lau, M.W.; Temperley, D.E.; Mehta, S.; Johnson, R.J.; Barnard, R.J.; Clarke, N.W. Urinary tract obstruction and nephrostomy drainage in pelvic malignant disease. Br. J. Urol. 1995, 76, 565–569. [Google Scholar] [CrossRef]
- Tlili, G.; Ammar, H.; Dziri, S.; Ben Ahmed, K.; Farhat, W.; Arem, S.; Acacha, E.; Gupta, R.; Rguez, A.; Jaidane, M. Antegrade double-J stent placement for the treatment of malignant obstructive uropathy: A retrospective cohort study. Ann. Med. Surg. 2021, 69, 102726. [Google Scholar] [CrossRef]
- Jepperson, M.A.; Thiel, D.D.; Cernigliaro, J.G.; Broderick, G.A.; Parker, A.S.; Haley, W.E. Determination of ureter stent appearance on dual-energy computed tomography scan. Urology 2012, 80, 986–989. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heinitz, S.; Müller, J.; Jenderka, K.V.; Schlögl, H.; Stumvoll, M.; Blüher, M.; Blank, V.; Karlas, T. The application of high-performance ultrasound probes increases anatomic depiction in obese patients. Sci. Rep. 2023, 13, 16297. [Google Scholar] [CrossRef] [PubMed]
- Corvino, A.; Catalano, O.; Faggian, G.; Delli Pizzi, A.; Tafuri, D.; Corvino, F.; Borzelli, A.; Picchi, S.G.; Lassandro, G.; Boccatonda, A.; et al. Multiparametric Ultrasound Diagnostic Approach to Malignancy-Mimicking Adenomatoid Tumors of the Scrotum: Is Strain Elastography Enough? Medicina 2023, 59, 1261. [Google Scholar] [CrossRef] [PubMed]
- Pollard, S.G.; Macfarlane, R. Symptoms arising from Double-J ureteral stents. J. Urol. 1988, 139, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.B.; Stainthorpe, A.; Keeley, F.X., Jr.; MacDonagh, R.; Timoney, A.G. Indwelling ureteral stents: Evaluation of quality of life to aid outcome analysis. J. Endourol. 2001, 15, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.B.; Newns, N.; Stainthorpe, A.; MacDonagh, R.P.; Keeley, F.X., Jr.; Timoney, A.G. Ureteral stent symptom questionnaire: Development and validation of a multidimensional quality of life measure. J. Urol. 2003, 169, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fentes, D.; Aranda-Pérez, J.; de la Cruz, J.E.; Soria, F. Indications, Complications and Side Effects of Ureteral Stents. In Urinary Stents; Soria, F., Rako, D., de Graaf, P., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Tomer, N.; Garden, E.; Small, A.; Palese, M. Ureteral stent encrustation: Epidemiology, pathophysiology, management and current technology. J. Urol. 2021, 205, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Lange, D.; Bidnur, S.; Hoag, N.; Chew, B.H. Ureteral stent-associated complications—Where we are and where we are going. Nat. Rev. Urol. 2015, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zisman, A.; Siegel, Y.I.; Siegmann, A.; Lindner, A. Spontaneous ureteral stent fragmentation. J. Urol. 1995, 153 Pt 1, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A. Breakage of a silicone double pigtail ureteral stent as a long-term complication. J. Urol. 1993, 150, 1898–1899. [Google Scholar] [CrossRef] [PubMed]
- Jendouzi, O.; Lamghari, A.; Jamali, M.; Harchaoui, A.; Alami, M.; Ameur, A. Knotted double j ureteral stent: A case report and literature review. Pan Afr. Med. J. 2022, 43, 5. [Google Scholar] [CrossRef] [PubMed]
- Corvino, A.; Catalano, F.; Cipolletta Campanile, A.; Cocco, G.; Delli Pizzi, A.; Corvino, F.; Varelli, C.; Catalano, O. Interventional Ultrasound in Dermatology: A Pictorial Overview Focusing on Cutaneous Melanoma Patients. J. Ultrasound Med. 2022, 41, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Bhirud, P.; Giridhar, V.; Hegde, P. Midureteric knotted stent removed by percutaneous access! Urol. Ann. 2012, 4, 106–107. [Google Scholar] [CrossRef]
- Jones, C.D.; McGahan, J.P. Computed tomographic evaluation and guided correction of malpositioned nephrostomy catheters. Abdom. Imaging 1999, 24, 422–425. [Google Scholar] [CrossRef]
- Kehinde, E.O.; Rotimi, V.O.; Al-Hunayan, A.; Abdul-Halim, H.; Boland, F.; Al-Awadi, K.A. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J. Endourol. 2004, 18, 891–896. [Google Scholar] [CrossRef]
- Cormio, L.; Vuopio-Varkila, J.; Siitonen, A.; Talja, M.; Ruutu, M. Bacterial adhesion and biofilm formation on various double-J stents in vivo and in vitro. Scand. J. Urol. Nephrol. 1996, 30, 19–24. [Google Scholar] [CrossRef]
- Vourganti, S.; Agarwal, P.K.; Bodner, D.R.; Dogra, V.S. Ultrasonographic evaluation of renal infections. Radiol. Clin. North. Am. 2006, 44, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.D.; Wagner, B.J.; Travis, M.D. Pyelonephritis: Radiologic-pathologic review. Radiographics 2008, 28, 255–277; quiz 327–328. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.; Buckley, O.; Torreggiani, W.C. Sonographic appearance of metallic ureteric stents. J. Ultrasound Med. 2009, 28, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Tonolini, M.; Campari, A.; Bianco, R. Common and unusual diseases involving the iliopsoas muscle compartment: Spectrum of cross-sectional imaging findings. Abdom. Imaging 2012, 37, 118–139. [Google Scholar] [CrossRef]
- Titton, R.L.; Gervais, D.A.; Hahn, P.F.; Harisinghani, M.G.; Arellano, R.S.; Mueller, P.R. Urine leaks and urinomas: Diagnosis and imaging-guided intervention. Radiographics 2003, 23, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Gayer, G.; Zissin, R.; Apter, S.; Garniek, A.; Ramon, J.; Kots, E.; Hertz, M. Urinomas caused by ureteral injuries: CT appearance. Abdom. Imaging 2002, 27, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.D.; Kumaravel, M.; Censullo, M.L.; Cohen, A.M.; Kievlan, D.S.; West, O.C. Multidetector CT evaluation of active extravasation in blunt abdominal and pelvic trauma patients. Radiographics 2008, 28, 1603–1616. [Google Scholar] [CrossRef]
- Corvino, A.; Catalano, O.; de Magistris, G.; Corvino, F.; Giurazza, F.; Raffaella, N.; Vallone, G. Usefulness of doppler techniques in the diagnosis of peripheral iatrogenic pseudoaneurysms secondary to minimally invasive interventional and surgical procedures: Imaging findings and diagnostic performance study. J. Ultrasound 2020, 23, 563–573. [Google Scholar] [CrossRef]
- Corvino, A.; Granata, V.; Tafuri, D.; Cocco, G.; Catalano, O. Incidental Focal Spleen Lesions: Integrated Imaging and Pattern Recognition Approach to the Differential Diagnosis. Diagnostics 2023, 13, 2536. [Google Scholar] [CrossRef]
- Goldwasser, J.; Wahdat, R.; Espinosa, J.; Lucerna, A. Urinoma: Prompt Diagnosis and Treatment Can Prevent Abscess Formation, Hydronephrosis, and a Progressive Loss of Renal Function. Case Rep. Emerg. Med. 2018, 2018, 5456738. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.R.; Li, G.; Hyams, E.S.; Shah, O. Delayed Decompression of Obstructing Stones with Urinary Tract Infection is Associated with Increased Odds of Death. J. Urol. 2020, 204, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Geavlete, P.; Georgescu, D.; Mulțescu, R.; Stanescu, F.; Cozma, C.; Geavlete, B. Ureteral stent complications—Experience on 50,000 procedures. J. Med. Life. 2021, 14, 769–775. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corvino, A.; Basile, L.; Cocco, G.; Delli Pizzi, A.; Tafuri, D.; Corvino, F.; Catalano, O. Complications Subsequent to Urinary Tract Stent Placement: An Overview Focusing on the Imaging of Cancer Patients. Medicina 2024, 60, 338. https://doi.org/10.3390/medicina60020338
Corvino A, Basile L, Cocco G, Delli Pizzi A, Tafuri D, Corvino F, Catalano O. Complications Subsequent to Urinary Tract Stent Placement: An Overview Focusing on the Imaging of Cancer Patients. Medicina. 2024; 60(2):338. https://doi.org/10.3390/medicina60020338
Chicago/Turabian StyleCorvino, Antonio, Luigi Basile, Giulio Cocco, Andrea Delli Pizzi, Domenico Tafuri, Fabio Corvino, and Orlando Catalano. 2024. "Complications Subsequent to Urinary Tract Stent Placement: An Overview Focusing on the Imaging of Cancer Patients" Medicina 60, no. 2: 338. https://doi.org/10.3390/medicina60020338
APA StyleCorvino, A., Basile, L., Cocco, G., Delli Pizzi, A., Tafuri, D., Corvino, F., & Catalano, O. (2024). Complications Subsequent to Urinary Tract Stent Placement: An Overview Focusing on the Imaging of Cancer Patients. Medicina, 60(2), 338. https://doi.org/10.3390/medicina60020338







