Alcoholic Liver Disease/Nonalcoholic Fatty Liver Disease Index at Diagnosis Is Associated with All-Cause Mortality during Follow-Up in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis
Abstract
1. Introduction
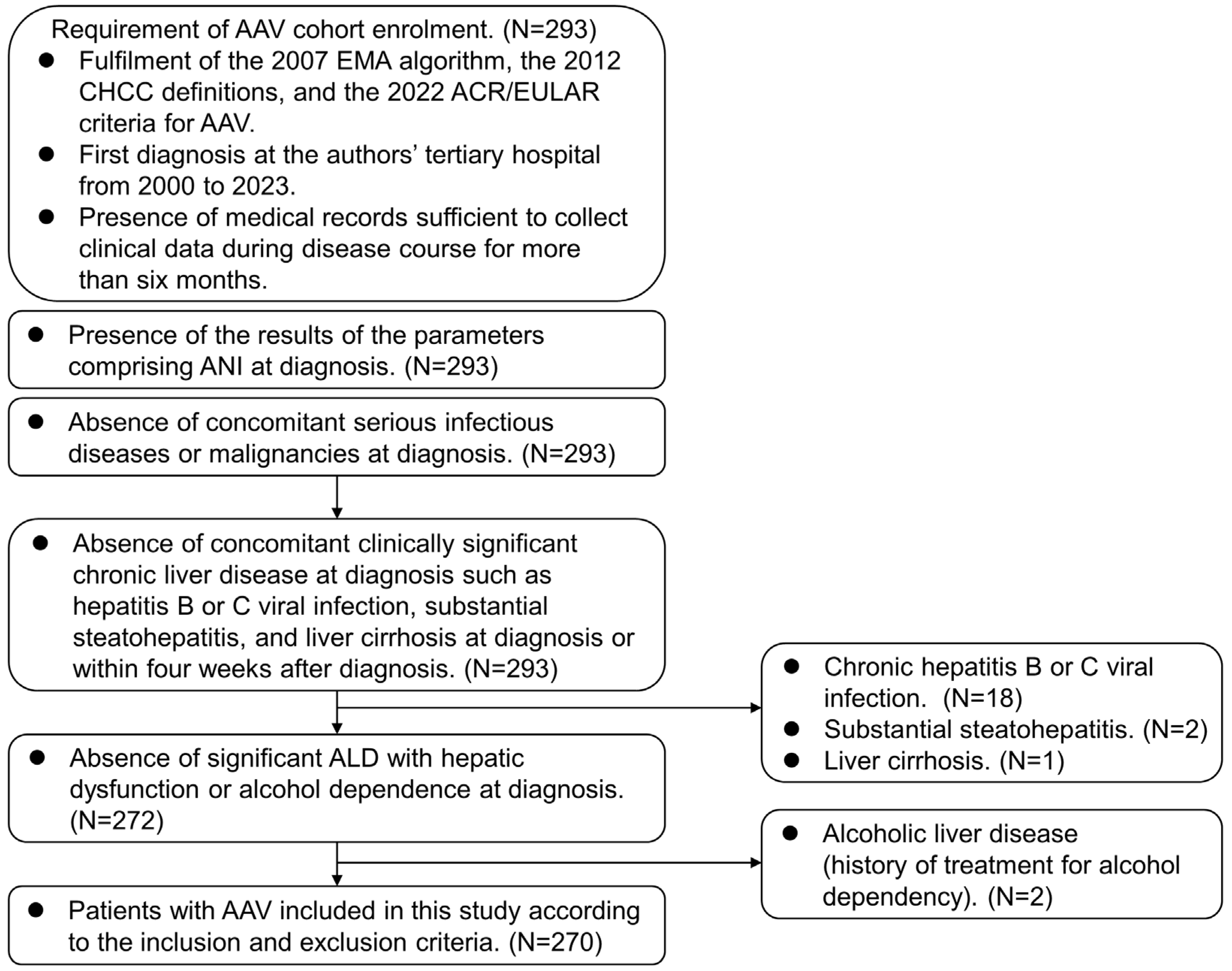
2. Materials and Methods
2.1. Patients
2.2. Ethical Approval
2.3. Equation for ANI
2.4. Data at AAV Diagnosis
2.5. Data during Follow-Up
2.6. Statistical Analyses
3. Results
3.1. Characteristics
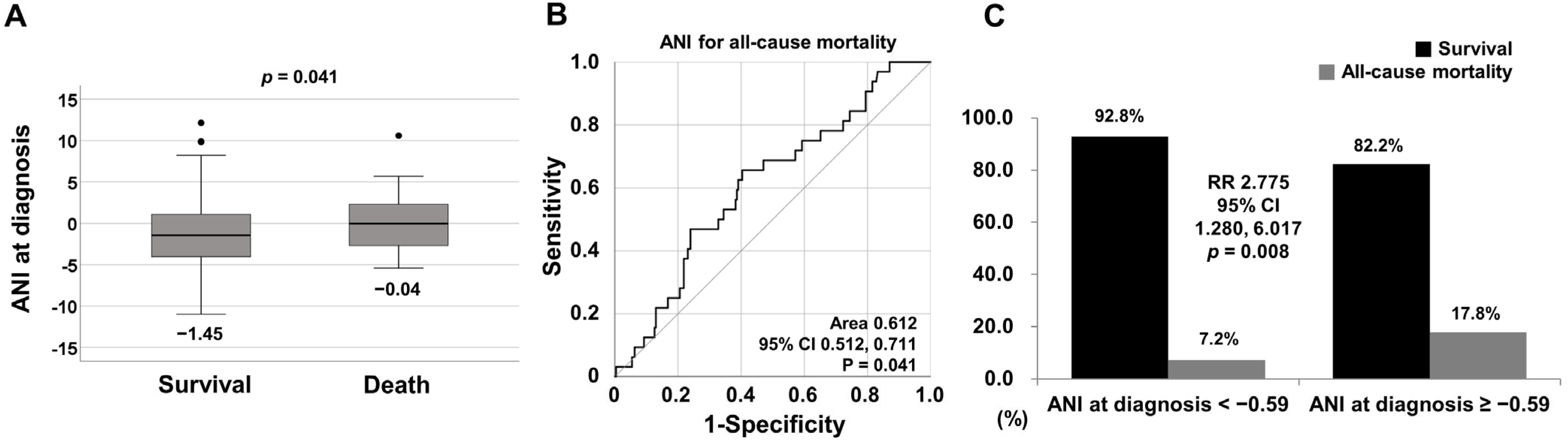
3.2. Comparison of ANI at Diagnosis between Surviving and Deceased Patients
3.3. Optimal Cut-off of ANI for All-Cause Mortality
3.4. Relative Risk of ANI at Diagnosis ≥ −0.59 for All-Cause Mortality
3.5. Comparison of Cumulative Patient Survival Rates
3.6. Cox Hazards Model Analysis for All-Cause Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef]
- Li, B.; Zhang, C.; Zhan, Y.T. Nonalcoholic Fatty Liver Disease Cirrhosis: A Review of Its Epidemiology, Risk Factors, Clinical Presentation, Diagnosis, Management, and Prognosis. Can. J. Gastroenterol. Hepatol. 2018, 2018, 2784537. [Google Scholar] [CrossRef]
- Idalsoaga, F.; Kulkarni, A.V.; Mousa, O.Y.; Arrese, M.; Arab, J.P. Non-alcoholic Fatty Liver Disease and Alcohol-Related Liver Disease: Two Intertwined Entities. Front. Med. 2020, 7, 448. [Google Scholar] [CrossRef]
- Dunn, W.; Angulo, P.; Sanderson, S.; Jamil, L.H.; Stadheim, L.; Rosen, C.; Malinchoc, M.; Kamath, P.S.; Shah, V.H. Utility of a new model to diagnose an alcohol basis for steatohepatitis. Gastroenterology 2006, 131, 1057–1063. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Watts, R.; Lane, S.; Hanslik, T.; Hauser, T.; Hellmich, B.; Koldingsnes, W.; Mahr, A.; Segelmark, M.; Cohen-Tervaert, J.W.; Scott, D. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 2007, 66, 222–227. [Google Scholar] [CrossRef]
- Suppiah, R.; Robson, J.C.; Grayson, P.C.; Ponte, C.; Craven, A.; Khalid, S.; Judge, A.; Hutchings, A.; Merkel, P.; Luqmani, R.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann. Rheum. Dis. 2022, 81, 321–326. [Google Scholar] [CrossRef]
- Robson, J.C.; Grayson, P.C.; Ponte, C.; Suppiah, R.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Watts, R.A.; Merkel, P.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann. Rheum. Dis. 2022, 81, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Grayson, P.C.; Ponte, C.; Suppiah, R.; Robson, J.C.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Luqmani, R.A.; Watts, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis. Ann. Rheum. Dis. 2022, 81, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Yazici, H.; Tascilar, K.; Yazici, Y. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria sets for three types of antineutrophilic cytoplasmic antibody-associated vasculitis. Curr. Opin. Rheumatol. 2023, 35, 1–5. [Google Scholar] [CrossRef]
- Mukhtyar, C.; Lee, R.; Brown, D.; Carruthers, D.; Dasgupta, B.; Dubey, S.; Flossmann, O.; Hall, C.; Hollywood, J.; Jayne, D.; et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann. Rheum. Dis. 2009, 68, 1827–1832. [Google Scholar] [CrossRef]
- Guillevin, L.; Pagnoux, C.; Seror, R.; Mahr, A.; Mouthon, L.; Toumelin, P.L.; French Vasculitis Study Group. The Five-Factor Score revisited: Assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine 2011, 90, 19–27. [Google Scholar] [CrossRef]
- Park, P.G.; Pyo, J.Y.; Ahn, S.S.; Choi, H.J.; Song, J.J.; Park, Y.B.; Huh, J.H.; Lee, S.-W. Fatty Liver Index Independently Predicts All-Cause Mortality in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis but No Substantial Liver Disease. Front. Cardiovasc. Med. 2022, 9, 848121. [Google Scholar] [CrossRef] [PubMed]
- Park, P.G.; Pyo, J.Y.; Ahn, S.S.; Song, J.J.; Park, Y.B.; Huh, J.H.; Lee, S.-W. New index using triglyceride glucose-body mass index for predicting mortality in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Front. Med. 2023, 10, 1168016. [Google Scholar] [CrossRef] [PubMed]
- Whang, J.Y.; Park, P.G.; Park, Y.B.; Huh, J.H.; Lee, S.W. Non-alcoholic fatty liver disease fibrosis score is a useful index for predicting all-cause mortality in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Front. Med. 2023, 10, 1217937. [Google Scholar] [CrossRef] [PubMed]
- Willeke, P.; Schlüter, B.; Limani, A.; Becker, H.; Schotte, H. Liver involvement in ANCA-associated vasculitis. Clin. Rheumatol. 2016, 35, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, F.; Xie, X.; Chen, B.; Yu, Q.; Wei, Y.; Ge, Y. Relationship Between Gender and 1-Year Mortality in ANCA-Associated Vasculitis Patients: A Single-Center Retrospective Analysis and Meta-Analysis. Front. Med. 2022, 9, 945011. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Atkinson, C.; Bhalla, K.; Birbeck, G.; Burstein, R.; Chou, D.; Chugh, S.S.; Cohen, A.; Colson, K.E.; Cooper, L.T.; et al. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef]
- Kim, N.H.; Lee, J.; Kim, T.J.; Kim, N.H.; Choi, K.M.; Baik, S.H.; Choi, D.S.; Pop-Busui, R.; Park, Y.; Kim, S.G. Body Mass Index and Mortality in the General Population and in Subjects with Chronic Disease in Korea: A Nationwide Cohort Study (2002–2010). PLoS ONE 2015, 10, e0139924. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; Prasad, M.; Norat, T.; Janszky, I.; Tonstad, S.; Romundstad, P.; Vatten, L.J. BMI and all cause mortality: Systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016, 353, i2156. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, P. Elevated AST/ALT ratio is associated with all-cause mortality in patients with stable coronary artery disease: A secondary analysis based on a retrospective cohort study. Sci. Rep. 2022, 12, 9231. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, C.; Hu, L.; Li, M.; Zhou, W.; Wang, T.; Zhu, L.; Bao, H.; Cheng, X. The association between AST/ALT ratio and all-cause and cardiovascular mortality in patients with hypertension. Medicine 2021, 100, e26693. [Google Scholar] [CrossRef]
- Choi, C.B.; Park, Y.B.; Lee, S.W. Antineutrophil Cytoplasmic Antibody-Associated Vasculitis in Korea: A Narrative Review. Yonsei Med. J. 2019, 60, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.P.; Chang, C.C.; Kor, C.T.; Yang, Y.; Wen, Y.K.; Chiu, P.F. Mean Corpuscular Volume and Mortality in Patients with CKD. Clin. J. Am. Soc. Nephrol. 2017, 12, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Kimachi, M.; Kurita, N.; Joki, N.; Nangaku, M. Low rather than high mean corpuscular volume is associated with mortality in Japanese patients under hemodialysis. Sci. Rep. 2020, 10, 15663. [Google Scholar] [CrossRef] [PubMed]
- Ganne-Carrié, N.; Nahon, P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J. Hepatol. 2019, 70, 284–293. [Google Scholar] [CrossRef]
- Testino, G.; Leone, S.; Borro, P. Alcohol and hepatocellular carcinoma: A review and a point of view. World J. Gastroenterol. 2014, 20, 15943–15954. [Google Scholar] [CrossRef]
- Mazzotta, A.D.; Pascale, A.; Cano, L.; Rosmorduc, O.; Allard, M.; Cunha, A.S.; Adam, R.; Cherqui, D.; Vibert, E.; Golse, N. Number of hepatocellular carcinoma nodules in patients listed for liver transplantation within alpha-fetoprotein score: A new prognostic risk factor. Transpl. Int. 2021, 34, 954–963. [Google Scholar] [CrossRef]

| Variables | Values |
|---|---|
| At diagnosis | |
| Demographic data | |
| Age (years) | 61.0 (50.0–69.3) |
| Male sex (N, (%)) | 93 (34.4) |
| Female sex (N, (%)) | 177 (66.6) |
| BMI (kg/m2) | 22.4 (20.2–24.5) |
| Smoking history (N, (%)) | 9 (3.3) |
| AAV Subtypes (N, (%)) | |
| MPA | 152 (56.3) |
| GPA | 66 (24.4) |
| EGPA | 52 (19.3) |
| ANCA positivity (N, (%)) | |
| MPO-ANCA (or P-ANCA) positivity | 191 (70.7) |
| PR3-ANCA (or C-ANCA) positivity | 41 (15.2) |
| AAV-specific indices | |
| BVAS | 12.0 (7.0–18.0) |
| FFS | 1.0 (0–2.0) |
| Acute-phase proteins | |
| ESR (mm/h) | 60.5 (22.0–96.0) |
| CRP (mg/L) | 13.8 (1.6–68.9) |
| Comorbidities at diagnosis (N, (%)) | |
| Type 2 diabetes mellitus | 72 (26.7) |
| Arterial hypertension | 110 (40.7) |
| Hyperlipidaemia | 55 (20.4) |
| ANI-related variables | |
| MCV (fL) | 90.4 (87.5–93.9) |
| Adjusted MCV * (fL) | 92.0 (92.0–93.9) |
| AST (IU/L) | 18.0 (15.0–24.0) |
| ALT (IU/L) | 16.0 (11.0–25.0) |
| AST/ALT | 1.2 (0.9–1.6) |
| Adjusted AST/ALT ** | 1.2 (0.9–1.6) |
| BMI (kg/m2) | 22.4 (20.2–24.5) |
| Male sex | 93 (34.4) |
| ANI | −1.15 (−3.89–1.55) |
| During follow-up | |
| All-cause mortality | |
| All-cause mortality (N, (%)) | 32 (11.9) |
| Follow-up duration based on all-cause mortality (months) | 47.8 (15.7–82.1) |
| Medications administered during follow-up (N, (%)) | |
| Glucocorticoid | 254 (94.1) |
| Cyclophosphamide | 153 (56.7) |
| Rituximab | 45 (16.7) |
| Azathioprine | 146 (54.1) |
| Mycophenolate mofetil | 51 (18.9) |
| Tacrolimus | 23 (8.5) |
| Methotrexate | 23 (8.5) |
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1.089 | 1.048, 1.133 | <0.001 | 1.061 | 1.017, 1.107 | 0.006 |
| Male sex * | 2.913 | 1.441, 5.891 | 0.003 | |||
| BMI * | 1.114 | 1.015, 1.223 | 0.024 | |||
| Smoking history | 1.902 | 0.453, 7.980 | 0.380 | |||
| MPO-ANCA (or P-ANCA) positivity | 2.0117 | 0.827, 4.918 | 0.123 | |||
| PR3-ANCA (or C-ANCA) positivity | 0.170 | 0.023, 1.248 | 0.082 | |||
| BVAS | 1.086 | 1.037, 1.138 | <0.001 | 1.051 | 0.993, 1.112 | 0.088 |
| FFS | 2.019 | 1.444, 2.823 | <0.001 | 1.286 | 0.844, 1.959 | 0.242 |
| ESR | 1.012 | 1.003, 1.022 | 0.010 | 1.000 | 0.988, 1.012 | 0.962 |
| CRP | 1.009 | 1.004, 1.014 | <0.001 | 1.004 | 0.998, 1.011 | 0.214 |
| Type 2 diabetes mellitus | 1.090 | 0.514, 2.311 | 0.822 | |||
| Arterial hypertension | 1.374 | 0.686, 2.752 | 0.370 | |||
| Hyperlipidaemia | 1.761 | 0.833, 3.723 | 0.139 | |||
| ANI ≥ −0.59 | 2.550 | 1.228, 5.296 | 0.012 | 2.479 | 1.149, 5.349 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, M.; Rah, W.; Song, J.J.; Park, Y.-B.; Lee, S.-W. Alcoholic Liver Disease/Nonalcoholic Fatty Liver Disease Index at Diagnosis Is Associated with All-Cause Mortality during Follow-Up in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Medicina 2024, 60, 381. https://doi.org/10.3390/medicina60030381
Cho M, Rah W, Song JJ, Park Y-B, Lee S-W. Alcoholic Liver Disease/Nonalcoholic Fatty Liver Disease Index at Diagnosis Is Associated with All-Cause Mortality during Follow-Up in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Medicina. 2024; 60(3):381. https://doi.org/10.3390/medicina60030381
Chicago/Turabian StyleCho, Minsuk, Woongchan Rah, Jason Jungsik Song, Yong-Beom Park, and Sang-Won Lee. 2024. "Alcoholic Liver Disease/Nonalcoholic Fatty Liver Disease Index at Diagnosis Is Associated with All-Cause Mortality during Follow-Up in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis" Medicina 60, no. 3: 381. https://doi.org/10.3390/medicina60030381
APA StyleCho, M., Rah, W., Song, J. J., Park, Y.-B., & Lee, S.-W. (2024). Alcoholic Liver Disease/Nonalcoholic Fatty Liver Disease Index at Diagnosis Is Associated with All-Cause Mortality during Follow-Up in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Medicina, 60(3), 381. https://doi.org/10.3390/medicina60030381






