Overview of Cochrane Systematic Reviews for Rehabilitation Interventions in Individuals with Upper Limb Fractures: A Mapping Synthesis
Abstract
1. Introduction
2. Materials and Methods
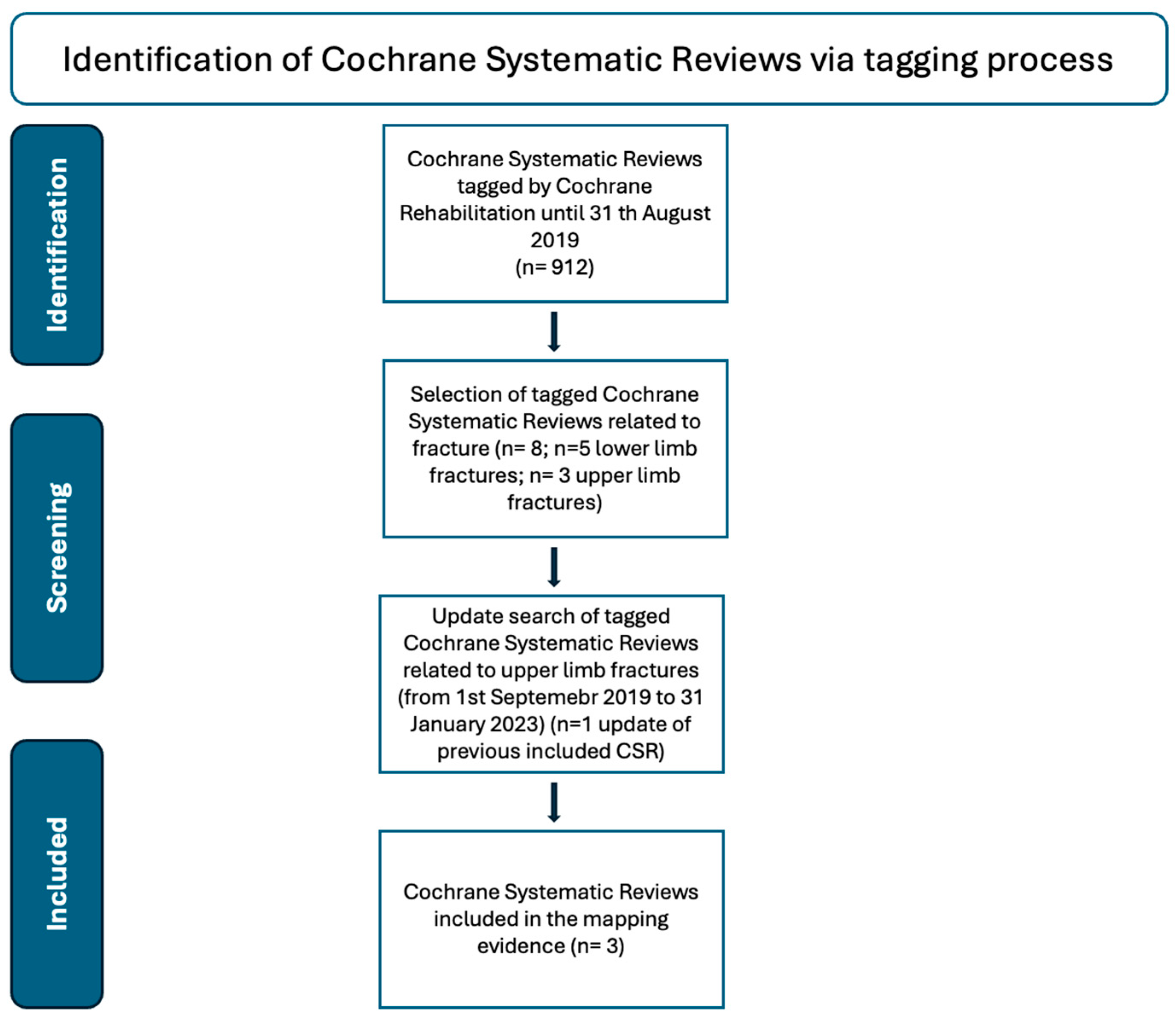
2.1. Search Strategy
2.2. Assessment of Methodological Quality of Included Reviews
2.3. Data Extraction and Quality of Evidence Appraisal
2.4. Summarizing Evidence within a Map
3. Results
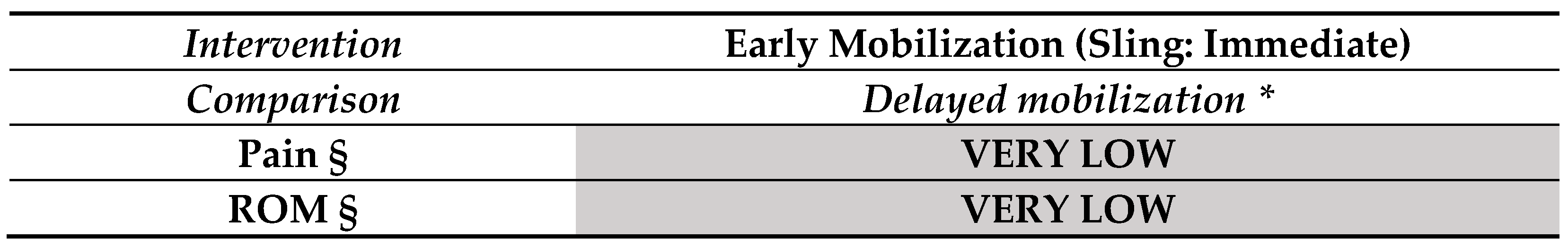
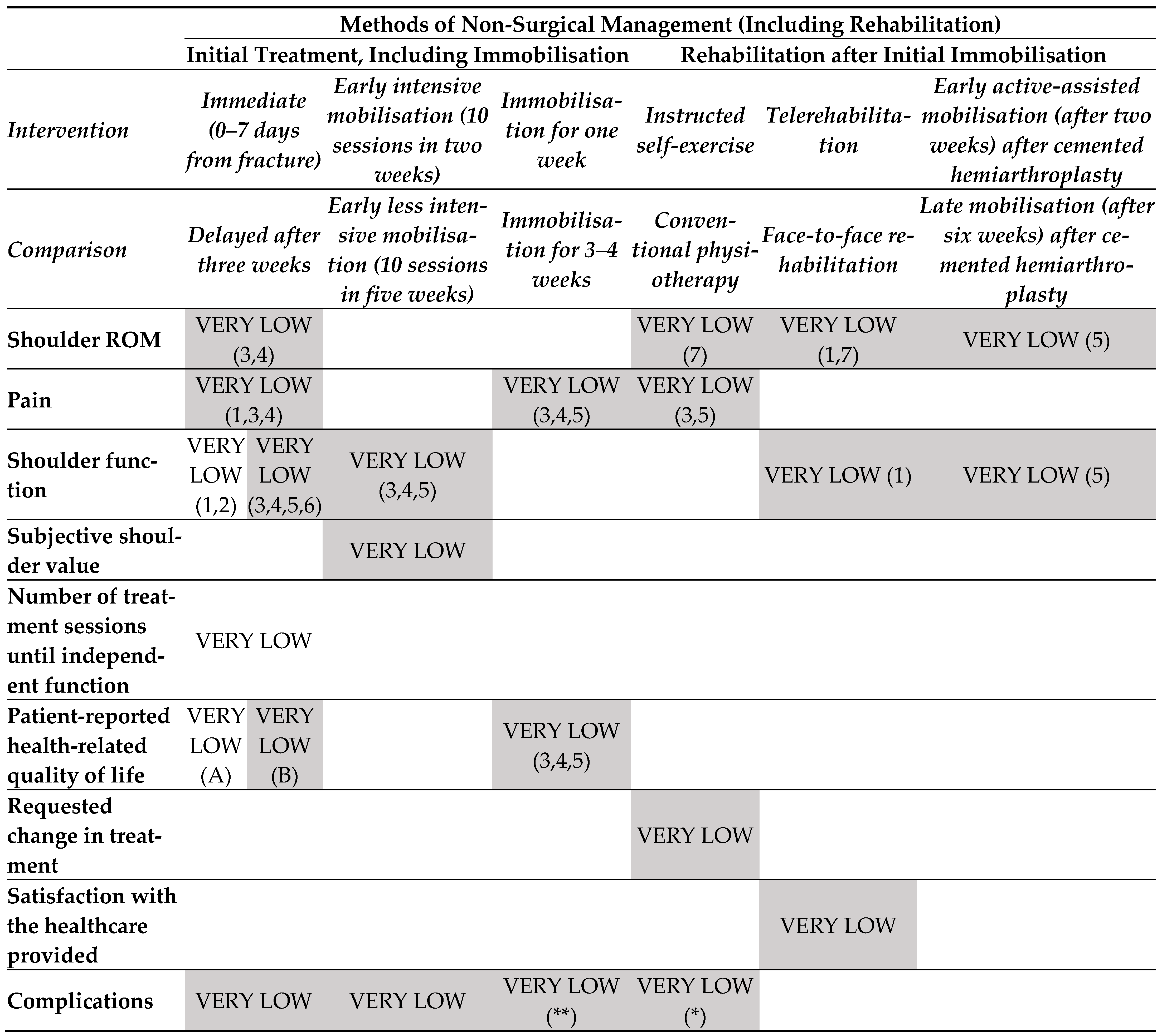
3.1. Findings in Distal Radial Fractures
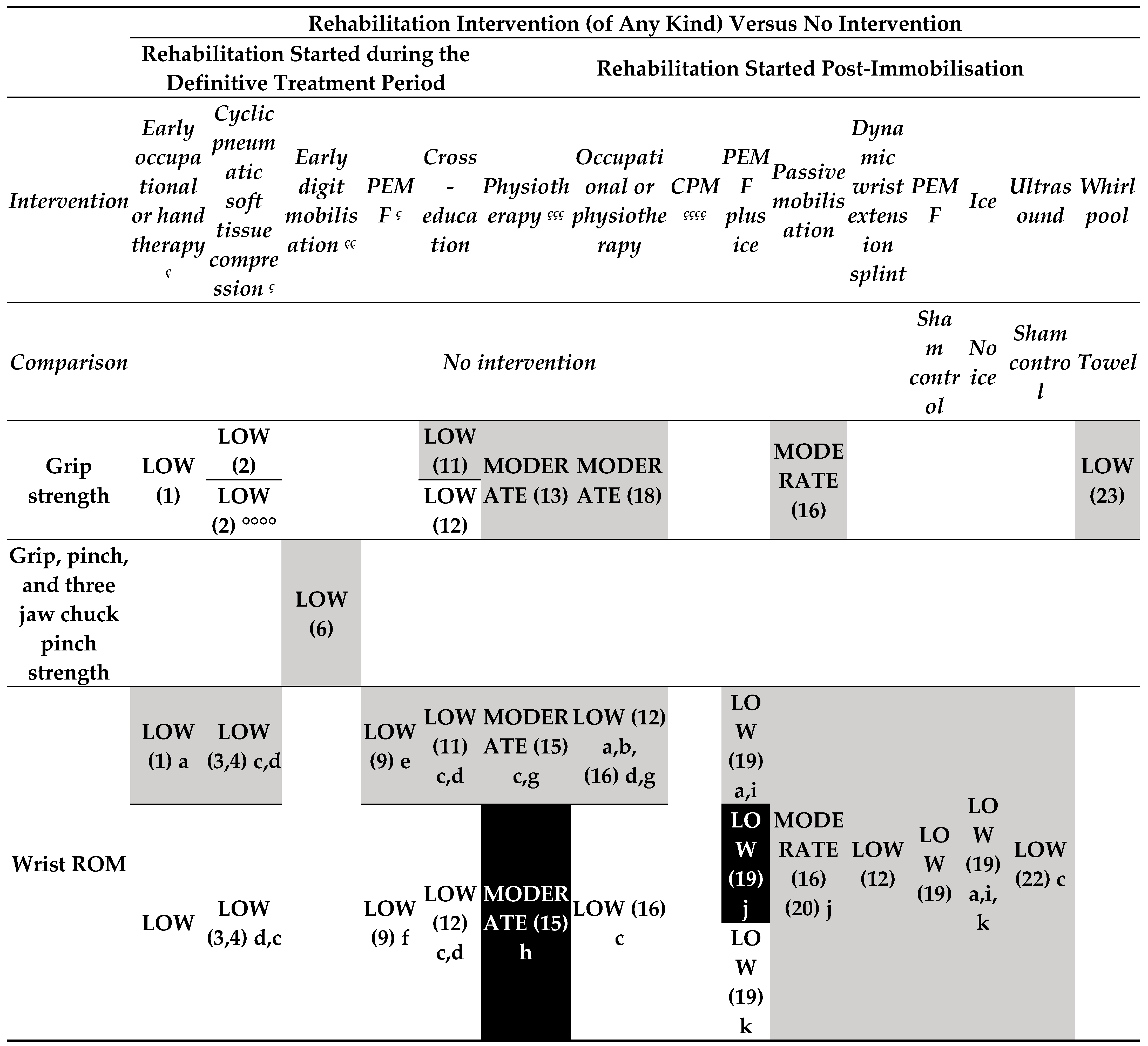
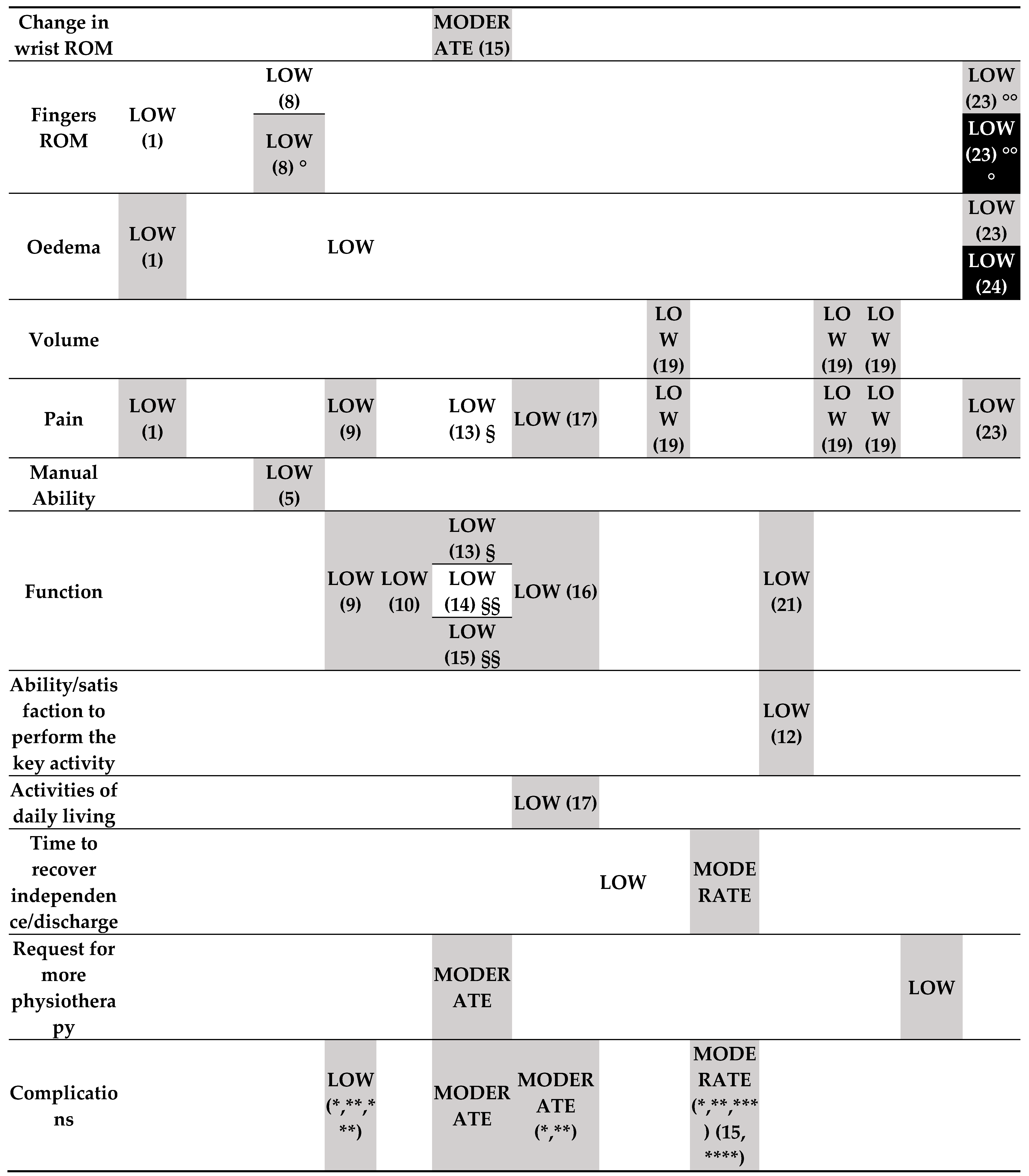
- Rehabilitation intervention versus no intervention (started during the treatment period) (Figure 3.1).
- o
- Low-certainty evidence
- Rehabilitation intervention versus no intervention (started post-immobilisation) (Figure 3.1).
- One rehabilitation intervention (post-immobilization) versus another rehabilitation intervention (Figure 3.2).
- o
- Low-certainty evidence
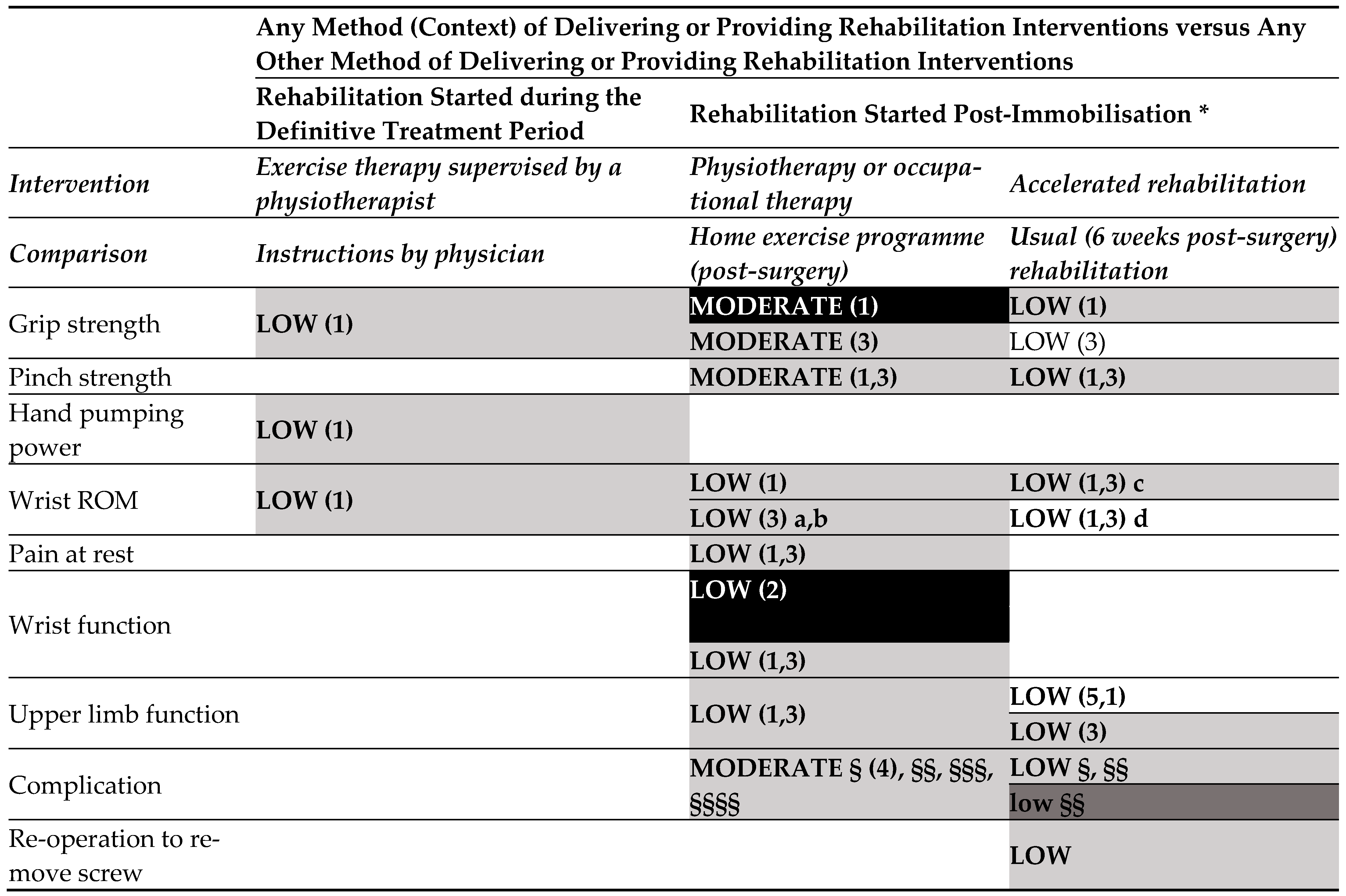
- Any method of delivering or providing rehabilitation interventions versus any other method of delivering or providing rehabilitation interventions (started during the definitive treatment period) (Figure 3.3).
- o
- Low-certainty evidence
- Any method of delivering or providing rehabilitation interventions versus any other method of delivering or providing rehabilitation interventions (started post-immobilisation).
3.2. Findings in Elbow Fractures (Figure 4)
3.3. Findings in Proximal Humeral Fractures (Figure 5)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gimigliano, F.; Negrini, S. The World Health Organization “Rehabilitation 2030: A call for action”. Eur. J. Phys. Rehabil. Med. 2017, 53, 155–168. [Google Scholar] [CrossRef]
- Rauch, A.; Negrini, S.; Cieza, A. Toward Strengthening Rehabilitation in Health Systems: Methods Used to Develop a WHO Package of Rehabilitation Interventions. Arch. Phys. Med. Rehabil. 2019, 100, 2205–2211. [Google Scholar] [CrossRef]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef]
- GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Bergh, C.; Wennergren, D.; Möller, M.; Brisby, H. Fracture incidence in adults in relation to age and gender: A study of 27,169 fractures in the Swedish Fracture Register in a well-defined catchment area. PLoS ONE 2020, 15, e0244291. [Google Scholar] [CrossRef]
- Dielwart, C.; Harmer, L.; Thompson, J.; Seymour, R.B.; Karunakar, M.A. Management of Closed Diaphyseal Humerus Fractures in Patients with Injury Severity Score ≥17. J. Orthop. Trauma 2017, 31, 220–224. [Google Scholar] [CrossRef]
- Lauder, A.; Richard, M.J. Management of distal humerus fractures. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 745–762. [Google Scholar] [CrossRef] [PubMed]
- Schierenbeck, M.; Grözinger, M.; Reichardt, B.; Jansen, O.; Kauczor, H.U.; Campbell, G.M.; Sedaghat, S. Detecting Bone Marrow Edema of the Extremities on Spectral Computed Tomography Using a Three-Material Decomposition. Diagnostics 2023, 13, 2745. [Google Scholar] [CrossRef] [PubMed]
- Mellstrand Navarro, C.; Brolund, A.; Ekholm, C.; Heintz, E.; Hoxha Ekström, E.; Josefsson, P.O.; Leander, L.; Nordström, P.; Zidén, L.; Stenström, K. Treatment of radius or ulna fractures in the elderly: A systematic review covering effectiveness, safety, economic aspects and current practice. PLoS ONE 2019, 14, e0214362. [Google Scholar] [CrossRef] [PubMed]
- Mills, L.; Tsang, J.; Hopper, G. The multifactorial aetiology of fracture nonunion and the importance of searching for latent infection. Bone Jt. Res. 2016, 5, 512–519. [Google Scholar] [CrossRef]
- Gimigliano, F.; Liguori, S.; Moretti, A.; Toro, G.; Rauch, A.; Negrini, S.; other members of the Technical Working Group; Iolascon, G. Systematic review of clinical practice guidelines for adults with fractures: Identification of best evidence for rehabilitation to develop the WHO’s Package of Interventions for Rehabilitation. J. Orthop. Traumatol. 2020, 21, 20, Erratum in J. Orthop. Traumatol. 2021, 22, 7. [Google Scholar] [CrossRef]
- Gimigliano, F.; Liguori, S.; Moretti, A.; Toro, G.; Rauch, A.; Negrini, S.; Iolascon, G.; Technical Working Group. A systematic review of Clinical Practice Guidelines for the management of fractures in the pediatric population: Identification of best evidence for rehabilitation to develop the WHO’s Package of Interventions for Rehabilitation. Eur. J. Phys. Rehabil. Med. 2022, 58, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Hetrick, S.E.; Parker, A.G.; Callahan, P.; Purcell, R. Evidence mapping: Illustrating an emerging methodology to improve evidence-based practice in youth mental health. J. Eval. Clin. Pract. 2010, 16, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Liguori, S.; Young, V.M.; Arienti, C.; Pollini, E.; Patrini, M.; Gimigliano, F.; Negrini, S.; Kiekens, C. Overview of Cochrane systematic reviews for rehabilitation interventions in individuals with cerebral palsy: A mapping synthesis. Dev. Med. Child Neurol. 2023, 65, 1280–1291. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Levack, W.M.M.; Rathore, F.A.; Pollet, J.; Negrini, S. One in 11 Cochrane Reviews Are on Rehabilitation Interventions, According to Pragmatic Inclusion Criteria Developed by Cochrane Rehabilitation. Arch. Phys. Med. Rehabil. 2019, 100, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Handoll, H.H.; Elliott, J.; Thillemann, T.M.; Aluko, P.; Brorson, S. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst. Rev. 2022, 6, CD000434. [Google Scholar] [CrossRef]
- Harding, P.; Rasekaba, T.; Smirneos, L.; Holland, A.E. Early mobilisation for elbow fractures in adults. Cochrane Database Syst. Rev. 2011, 2011, CD008130. [Google Scholar] [CrossRef] [PubMed]
- Handoll, H.H.; Elliott, J. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst. Rev. 2015, 2015, CD003324. [Google Scholar] [CrossRef]
- Ikpeze, T.C.; Smith, H.C.; Lee, D.J.; Elfar, J.C. Distal Radius Fracture Outcomes and Rehabilitation. Geriatr. Orthop. Surg. Rehabil. 2016, 7, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Gashaw, M.; Aragaw, F.M.; Zemed, A.; Endalew, M.; Tsega, N.T.; Asratie, M.H.; Belay, D.G. Distal and/or Proximal Joint Stiffness Among Post-Fracture Patients Treated in University of Gondar Comprehensive Specialized Hospital, Gondar, Ethiopia. Orthop. Res. Rev. 2022, 14, 157–167. [Google Scholar] [CrossRef]
- Wilcke, M.K.; Abbaszadegan, H.; Adolphson, P.Y. Patient-perceived outcome after displaced distal radius fractures. A comparison between radiological parameters, objective physical variables, and the DASH score. J. Hand Ther. 2007, 20, 290–298. [Google Scholar] [CrossRef]
- American Academy of Orthopaedic Surgeons. The Treatment of Distal Radius Fractures. 2009. Available online: https://www.aaos.org/globalassets/quality-and-practice-resources/distal-radius/distal-radius-fractures-clinical-practice-guideline.pdf (accessed on 28 February 2023).
- Danish Health Authority. National Clinical Guideline on the Treatment of Distal Radial Fractures. 2016. Available online: https://www.sst.dk/da/udgivelser/2014/~/media/22E568AA633C49A9A0A128D5FDC4D8B7.ashx (accessed on 28 February 2023).
- Meijer, H.A.W.; Graafland, M.; Obdeijn, M.C.; van Dieren, S.; Goslings, J.C.; Schijven, M.P.; ReValidate! Collaborative Study Group. Serious game versus standard care for rehabilitation after distal radius fractures: A protocol for a multicentre randomised controlled trial. BMJ Open 2021, 11, e042629. [Google Scholar] [CrossRef]
- Hackl, M.; Leschinger, T.; Uschok, S.; Mueller, L.P.; Wegmann, K. Rehabilitation of elbow fractures and dislocations. Obere Extrem. 2017, 12, 201–207. [Google Scholar] [CrossRef]
- Unsworth-White, J.; Koka, R.; Churchill, M.; D’Arcy, J.C.; James, S.E. The non-operative management of radial head fractures: A randomized trial of three treatments. Injury 1994, 25, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Macdermid, J.C.; Vincent, J.I.; Kieffer, L.; Kieffer, A.; Demaiter, J.; Macintosh, S. A survey of practice patterns for rehabilitation post elbow fracture. Open Orthop. J. 2012, 6, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Rodríguez, S.; Domínguez-Prado, D.M.; García-Reza, A.; Fernández-Fernández, D.; Pérez-Alfonso, E.; García-Piñeiro, J.; Castro-Menéndez, M. Epidemiology of proximal humerus fractures. J. Orthop. Surg. Res. 2021, 16, 402. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Razzano, C.; Albino, P.; Mezzoprete, R. Immediate intensive mobilization compared with immediate conventional mobilization for the impacted osteoporotic conservatively treated proximal humeral fracture: A randomized controlled trial. Musculoskelet. Surg. 2017, 101 (Suppl. S2), 137–143. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.P.; Gutbrod, J.; Cahill, M.; Shi, L. Optimal Treatment of Proximal Humeral Fractures in the Elderly: Risks and Management Challenges. Orthop. Res. Rev. 2023, 15, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.P.; Gutbrod, J.; Strelzow, J.A.; Maassen, N.H.; Shi, L. Management of Proximal Humerus Fractures in Adults—A Scoping Review. J. Clin. Med. 2022, 11, 6140. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liguori, S.; Moretti, A.; Toro, G.; Arienti, C.; Patrini, M.; Kiekens, C.; Negrini, S.; Iolascon, G.; Gimigliano, F. Overview of Cochrane Systematic Reviews for Rehabilitation Interventions in Individuals with Upper Limb Fractures: A Mapping Synthesis. Medicina 2024, 60, 469. https://doi.org/10.3390/medicina60030469
Liguori S, Moretti A, Toro G, Arienti C, Patrini M, Kiekens C, Negrini S, Iolascon G, Gimigliano F. Overview of Cochrane Systematic Reviews for Rehabilitation Interventions in Individuals with Upper Limb Fractures: A Mapping Synthesis. Medicina. 2024; 60(3):469. https://doi.org/10.3390/medicina60030469
Chicago/Turabian StyleLiguori, Sara, Antimo Moretti, Giuseppe Toro, Chiara Arienti, Michele Patrini, Carlotte Kiekens, Stefano Negrini, Giovanni Iolascon, and Francesca Gimigliano. 2024. "Overview of Cochrane Systematic Reviews for Rehabilitation Interventions in Individuals with Upper Limb Fractures: A Mapping Synthesis" Medicina 60, no. 3: 469. https://doi.org/10.3390/medicina60030469
APA StyleLiguori, S., Moretti, A., Toro, G., Arienti, C., Patrini, M., Kiekens, C., Negrini, S., Iolascon, G., & Gimigliano, F. (2024). Overview of Cochrane Systematic Reviews for Rehabilitation Interventions in Individuals with Upper Limb Fractures: A Mapping Synthesis. Medicina, 60(3), 469. https://doi.org/10.3390/medicina60030469







