Disparities and Outcomes in the First and Second Year of the Pandemic on Events of Acute Myocardial Infarction in Coronavirus Disease 2019 Patients
Abstract
1. Introduction
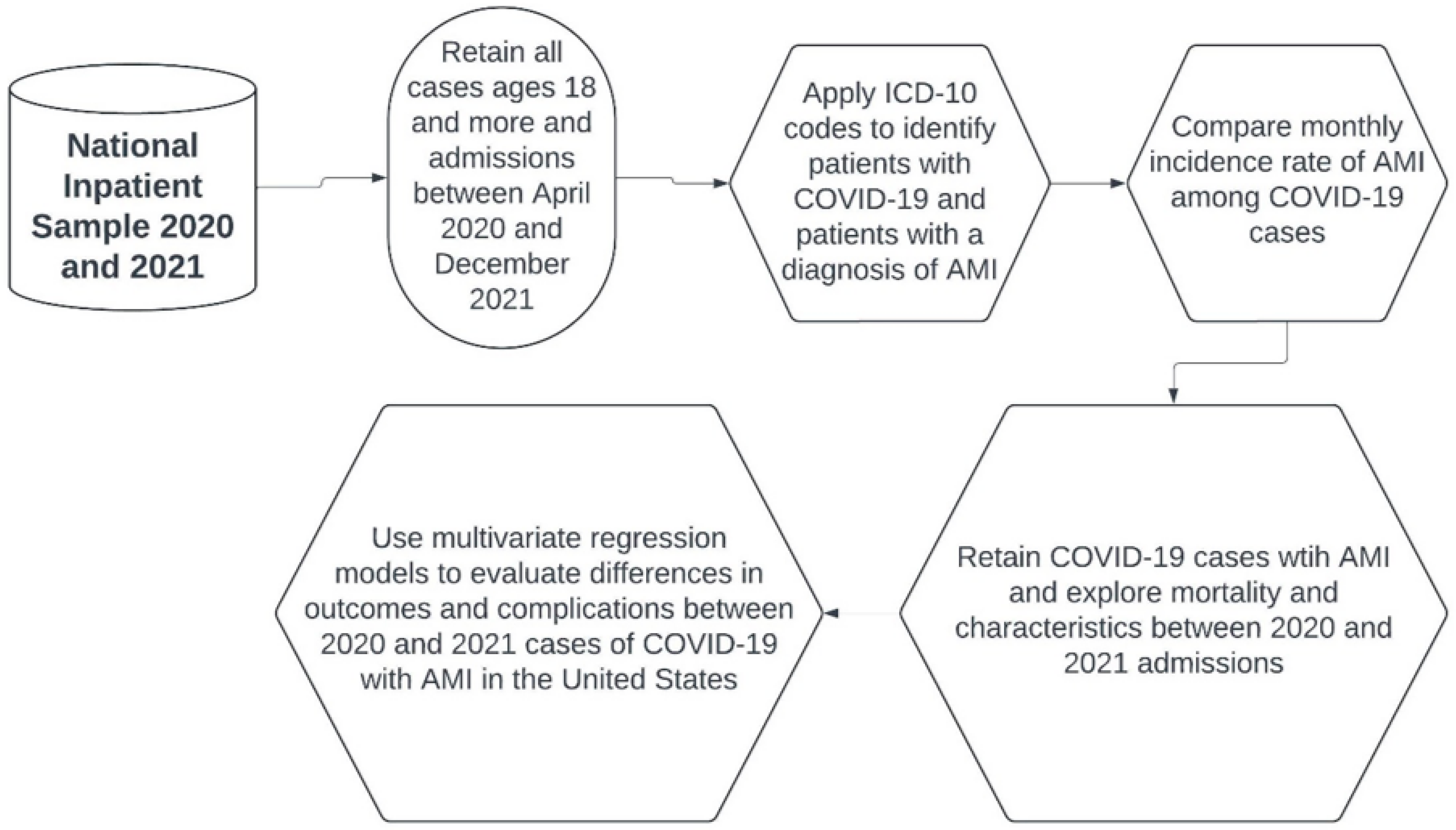
2. Materials and Methods
2.1. Data Source
2.2. Sample and Statistical Analysis
3. Results
3.1. AMI Trend among COVID-19 Cases
3.2. Comparing AMI Cases in COVID-19 Patients in 2020 vs. 2021
3.3. Baseline Characteristics
3.4. Trends in Mortality
3.5. Events of Cardiac Arrhythmias
3.6. Complications and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 1 February 2024).
- Mohamed-Ahmed, O.; Aboutaleb, H.; Latif, S.; Watson, H.L.; Handley, R.; Humphreys, E.; Gedik, F.G.; De Sa, J.; Zhang, Y.; Bhatia, T.; et al. Reviewing essential public health functions in the Eastern Mediterranean Region post COVID-19 pandemic: A foundation for system resilience. BMJ Glob. Health 2024, 9, e013782. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Senguttuvan, N.B.; Raymond, G.; Sankar, S.; Mukherjee, A.G.; Kunale, M.; Kodiveri Muthukaliannan, G.; Baxi, S.; Mani, R.R.; Rajagopal, M.; et al. COVID-19 Outcomes in Patients Hospitalised with Acute Myocardial Infarction (AMI): A Protocol for Systematic Review and Meta-Analysis. COVID 2022, 2, 138–147. [Google Scholar] [CrossRef]
- Toscano, O.; Cosentino, N.; Campodonico, J.; Bartorelli, A.L.; Marenzi, G. Acute Myocardial Infarction During the COVID-19 Pandemic: An Update on Clinical Characteristics and Outcomes. Front. Cardiovasc. Med. 2021, 8, 648290. [Google Scholar] [CrossRef] [PubMed]
- Rus, M.; Ardelean, A.I.; Andronie-Cioara, F.L.; Filimon, G.C. Acute Myocardial Infarction during the COVID-19 Pandemic: Long-Term Outcomes and Prognosis-A Systematic Review. Life 2024, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Lindmark, K.; Fors Connolly, A.M. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: A self-controlled case series and matched cohort study. Lancet 2021, 398, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Katsoularis, I.; Jerndal, H.; Kalucza, S.; Lindmark, K.; Fonseca-Rodríguez, O.; Connolly, A.F. Risk of arrhythmias following COVID-19: Nationwide self-controlled case series and matched cohort study. Eur. Heart J. Open 2023, 3, oead120. [Google Scholar] [CrossRef]
- Adeghate, E.A.; Eid, N.; Singh, J. Mechanisms of COVID-19-induced heart failure: A short review. Heart Fail. Rev. 2021, 26, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Beetler, D.J.; Di Florio, D.N.; Musigk, N.; Heidecker, B.; Cooper, L.T., Jr. COVID-19, Myocarditis and Pericarditis. Circ. Res. 2023, 132, 1302–1319. [Google Scholar] [CrossRef]
- Warren-Gash, C.; Davidson, J.A.; Strongman, H.; Herrett, E.; Smeeth, L.; Breuer, J.; Banerjee, A. Severe COVID-19 outcomes by cardiovascular risk profile in England in 2020: A population-based cohort study. Lancet Reg. Health. Eur. 2023, 27, 100604. [Google Scholar] [CrossRef]
- Fedorowski, A.; Fanciulli, A.; Raj, S.R.; Sheldon, R.; Shibao, C.A.; Sutton, R. Cardiovascular autonomic dysfunction in post-COVID-19 syndrome: A major health-care burden. Nat. Rev. Cardiol. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Shrestha, A.B.; Mehta, A.; Pokharel, P.; Mishra, A.; Adhikari, L.; Shrestha, S.; Yadav, R.S.; Khanal, S.; Sah, R.; Nowrouzi-Kia, B.; et al. Long COVID Syndrome and Cardiovascular Manifestations: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Onyeaka, H.; Anumudu, C.K.; Al-Sharify, Z.T.; Egele-Godswill, E.; Mbaegbu, P. COVID-19 pandemic: A review of the global lockdown and its far-reaching effects. Sci. Prog. 2021, 104, 368504211019854. [Google Scholar] [CrossRef] [PubMed]
- Soraci, L.; Lattanzio, F.; Soraci, G.; Gambuzza, M.E.; Pulvirenti, C.; Cozza, A.; Corsonello, A.; Luciani, F.; Rezza, G. COVID-19 Vaccines: Current and Future Perspectives. Vaccines 2022, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Moras, E.; Gandhi, K.D.; Bertasi, R.; Bertasi, T.; Dominguez, A.C. A-32|The Effect of COVID-19 on Outcomes of Non-ST Elevation Myocardial Infarction (NSTEMI): Results from the National Inpatient Sample Database. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100696. [Google Scholar] [CrossRef]
- Majeed, H.; Gangu, K.; Sagheer, S.; Garg, I.; Khan, U.; Shuja, H.; Bobba, A.; Chourasia, P.; Shekhar, R.; Avula, S.R.; et al. COVID-19 and NSTEMI Outcomes among Hospitalized Patients in the United States and Racial Disparities in Mortality: Insight from National Inpatient Sample Database. Vaccines 2022, 10, 2024. [Google Scholar] [CrossRef] [PubMed]
- Nanavaty, D.; Sinha, R.; Kaul, D.; Sanghvi, A.; Kumar, V.; Vachhani, B.; Singh, S.; Devarakonda, P.; Reddy, S.; Verghese, D. Impact of COVID-19 on Acute Myocardial Infarction: A National Inpatient Sample Analysis. Curr Probl Cardiol 2024, 49, 102030. [Google Scholar] [CrossRef] [PubMed]
- Shaka, H.; Ilelaboye, A.I.; DeAngelo, S.; Gwira-Tamattey, E.; Vardar, U. Increased national mortality in acute myocardial infarction hospitalization during the COVID-19 pandemic. Proc. (Bayl. University. Med. Cent.) 2023, 36, 298–303. [Google Scholar] [CrossRef] [PubMed]
- HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP); Agency for Healthcare Research and Quality: Rockville, MD, USA, 2020–2021. Available online: www.hcup-us.ahrq.gov/nisoverview.jsp (accessed on 1 February 2024).
- Trend Weights for Hcup Nis Data. Available online: https://hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp (accessed on 1 February 2024).
- Insam, C.; Paccaud, F.; Marques-Vidal, P. Trends in hospital discharges, management and in-hospital mortality from acute myocardial infarction in Switzerland between 1998 and 2008. BMC Public Health 2013, 13, 270. [Google Scholar] [CrossRef]
- Ahmed, R.; Shahbaz, H.; Ramphul, K.; Mactaggart, S.; Dulay, M.S.; Okafor, J.; Azzu, A.; Khattar, R.; Wells, A.U.; Wechalekar, K.; et al. Racial disparities among patients with cardiac sarcoidosis and arrhythmias in the United States: A propensity matched-analysis from the national inpatient sample database 2016–2020. Curr. Probl. Cardiol. 2024, 49, 102450. [Google Scholar] [CrossRef]
- Sawatari, H.; Chahal, A.A.; Ahmed, R.; Collinss, G.B.; Deshpande, S.; Khanji, M.Y.; Provedenciae, R.; Khan, H.; Wafa, S.E.I.; Salloum, M.N.; et al. Impact of Cardiac Implantable Electronic Devices on Cost and Length of Stay in Patients With Surgical Aortic Valve Replacement and Transcutaneous Aortic Valve Implantation. Am. J. Cardiol. 2023, 192, 69–78. [Google Scholar] [CrossRef]
- Data Organizations Participating in HCUP. Available online: https://www.hcup-us.ahrq.gov/partners.jsp (accessed on 1 February 2024).
- Li, P.; Lu, X.; Teng, C.; Hadley, M.; Cai, P.; Dai, Q.; Wang, B. The Association Between Hyperlipidemia and In-Hospital Outcomes in Takotsubo Cardiomyopathy. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fan, Y.; Zhao, W.; Li, B.; Pan, N.; Lou, Z.; Zhang, M. In-Hospital Outcomes of Acute Myocardial Infarction With Essential Thrombocythemia and Polycythemia Vera: Insights From the National Inpatient Sample. J. Am. Heart Assoc. 2022, 11, e027352. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Li, S.; Dong, Y.; Paliwal, N.; Wang, Y. Epidemiology and the Impact of Acute Kidney Injury on Outcomes in Patients with Rhabdomyolysis. J. Clin. Med. 2021, 10, 1950. [Google Scholar] [CrossRef]
- Taneja, V.; Stein, D.J.; Feuerstein, J.D. Impact of Cirrhosis on Outcomes in Inflammatory Bowel Disease Hospitalizations. J. Clin. Gastroenterol. 2022, 56, 718–723. [Google Scholar] [CrossRef]
- Elixhauser Comorbidity Software Refined for ICD-10-CM. Available online: https://hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp (accessed on 1 February 2024).
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits 2019, 12, 188–197. [Google Scholar]
- COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 1 February 2024).
- World/Countries/United States. Available online: https://www.worldometers.info/coronavirus/country/us/ (accessed on 1 February 2024).
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- COVID-19 Restrictions. Available online: https://eu.usatoday.com/storytelling/coronavirus-reopening-america-map/ (accessed on 1 February 2024).
- Yao, H.; Wang, J.; Liu, W. Lockdown Policies, Economic Support, and Mental Health: Evidence From the COVID-19 Pandemic in United States. Front. Public Health 2022, 10, 857444. [Google Scholar] [CrossRef]
- Li, Y.; Undurraga, E.A.; Zubizarreta, J.R. Effectiveness of Localized Lockdowns in the COVID-19 Pandemic. Am. J. Epidemiol. 2022, 191, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.H. European and US lockdowns and second waves during the COVID-19 pandemic. Math. Biosci. 2020, 330, 108472. [Google Scholar] [CrossRef]
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef]
- Choi, J.Y.; Smith, D.M. SARS-CoV-2 Variants of Concern. Yonsei Med. J. 2021, 62, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Twohig, K.A.; Harris, R.J.; Seaman, S.R.; Flannagan, J.; Allen, H.; Charlett, A.; De Angelis, D.; Dabrera, G.; Presanis, A.M. Risk of hospital admission for patients with SARS-CoV-2 variant B.1.1.7: Cohort analysis. BMJ (Clin. Res. Ed.) 2021, 373, n1412. [Google Scholar] [CrossRef]
- Gbolahan, O.; Bonatsos, V.; Mukherjee, S.; Raza, A. Are patients’ fears of catching COVID-19 during an emergency hospital admission with an acute urological problem justified?—A UK epicentre experience. J. Public Health 2023, 45, 488–490. [Google Scholar] [CrossRef]
- Einav, S.; Tankel, J. The unseen pandemic: Treatment delays and loss to follow-up due to fear of COVID. J. Anesth. Analg. Crit. Care 2022, 2, 5. [Google Scholar] [CrossRef]
- Kontos, M.C.; Rennyson, S.L.; Chen, A.Y.; Alexander, K.P.; Peterson, E.D.; Roe, M.T. The association of myocardial infarction process of care measures and in-hospital mortality: A report from the NCDR®. Am. Heart J. 2014, 168, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Conroy, A.; Khosla, A.; Rubens, M.; Saxena, A.; Ramamoorthy, V.; Roy, M.; Appunni, S.; Doke, M.; Ahmed, M.A.; et al. Prevalence and effects of acute myocardial infarction on hospital outcomes among COVID-19 patients. Coron. Artery Dis. 2024, 35, 38–43. [Google Scholar] [CrossRef]
- Tertulien, T.; Broughton, S.T.; Swabe, G.; Essien, U.R.; Magnani, J.W. Association of Race and Ethnicity on the Management of Acute Non-ST-Segment Elevation Myocardial Infarction. J. Am. Heart Assoc. 2022, 11, e025758. [Google Scholar] [CrossRef]
- Gomez, L.E.; Bernet, P. Diversity improves performance and outcomes. J. Natl. Med. Assoc. 2019, 111, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Verma, R.; Lohana, P.; Lohana, A.; Ramphul, K. Acute myocardial infarction in COVID-19 patients. A review of cases in the literature. Arch. Med. Sci. Atheroscler. Dis. 2021, 6, e169–e175. [Google Scholar] [CrossRef] [PubMed]
- Bosomworth, J.; Khan, Z. Analysis of Gender-Based Inequality in Cardiovascular Health: An Umbrella Review. Cureus 2023, 15, e43482. [Google Scholar] [CrossRef]
- Burgess, S.N. Understudied, Under-Recognized, Underdiagnosed, and Undertreated: Sex-Based Disparities in Cardiovascular Medicine. Circulation. Cardiovasc. Interv. 2022, 15, e011714. [Google Scholar] [CrossRef] [PubMed]
- Schulte, K.J.; Mayrovitz, H.N. Myocardial Infarction Signs and Symptoms: Females vs. Males. Cureus 2023, 15, e37522. [Google Scholar] [CrossRef] [PubMed]
- Senney, G.T.; Steckel, R.H. Developmental Origins of Cardiovascular Disease: Understanding High Mortality Rates in the American South. Int. J. Environ. Res. Public Health 2021, 18, 13192. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, M.J.; Hsia, R.Y.; Shen, Y.C. Not All Insurance Is Equal: Differential Treatment and Health Outcomes by Insurance Coverage Among Nonelderly Adult Patients with Heart Attack. J. Am. Heart Assoc. 2018, 7, e008152. [Google Scholar] [CrossRef] [PubMed]
- Provisional Mortality Data—United States. 2021. Available online: https://www.cdc.gov/mmwr/volumes/71/wr/mm7117e1.htm (accessed on 1 February 2024).
- Duong, B.V.; Larpruenrudee, P.; Fang, T.; Hossain, S.I.; Saha, S.C.; Gu, Y.; Islam, M.S. Is the SARS-CoV-2 Omicron Variant Deadlier and More Transmissible Than Delta Variant? Int. J. Environ. Res. Public Health 2022, 19, 4586. [Google Scholar] [CrossRef] [PubMed]
- Reece, S.; CarlLee, S.; Scott, A.J.; Willis, D.E.; Rowland, B.; Larsen, K.; Holman-Allgood, I.; McElfish, P.A. Hesitant adopters: COVID-19 vaccine hesitancy among diverse vaccinated adults in the United States. Infect. Med. 2023, 2, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ramphul, K.; Lohana, P.; Verma, R.; Sombans, S. The impact of COVID-19 on cardiology departments. Arch. Med. Sci. Atheroscler. Dis. 2021, 6, e1–e2. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; Mamas, M.A. Cardiovascular Health Care Implications of the COVID-19 pandemic. Heart Fail. Clin. 2023, 19, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.F.; Strobel, R.J.; Young, A.M.; Wisniewski, A.M.; Ahmad, R.M.; Mehaffey, J.H.; Hawkins, R.B.; Yarboro, L.T.; Quader, M.; Teman, N.R. Cardiac Surgery Outcomes During the COVID-19 Pandemic Worsened Across All Socioeconomic Statuses. Ann. Thorac. Surg. 2023, 115, 1511–1518. [Google Scholar] [CrossRef]
- Yang, X.; Shi, F.; Zhang, J.; Gao, H.; Chen, S.; Olatosi, B.; Weissman, S.; Li, X. Disease severity of COVID-19 in different phases of the pandemic: Do healthcare workers have better outcomes? Vaccine X 2023, 15, 100377. [Google Scholar] [CrossRef]
- Bian, L.; Gao, Q.; Gao, F.; Wang, Q.; He, Q.; Wu, X.; Mao, Q.; Xu, M.; Liang, Z. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev. Vaccines 2021, 20, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Polubriaginof, F.C.G.; Ryan, P.; Salmasian, H.; Shapiro, A.W.; Perotte, A.; Safford, M.M.; Hripcsak, G.; Smith, S.; Tatonetti, N.P.; Vawdrey, D.K. Challenges with quality of race and ethnicity data in observational databases. J. Am. Med. Inform. Assoc. JAMIA 2019, 26, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.; Panagopoulos, C.; van der Linden, S. Political polarization on COVID-19 pandemic response in the United States. Personal. Individ. Differ. 2021, 179, 110892. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.T. The political economy of early COVID-19 interventions in U.S. states: Comment. J. Econ. Dyn. Control 2022, 140, 104304. [Google Scholar] [CrossRef]
- Rahman, M.M.; Thill, J.C. Associations between COVID-19 Pandemic, Lockdown Measures and Human Mobility: Longitudinal Evidence from 86 Countries. Int. J. Environ. Res. Public Health 2022, 19, 7317. [Google Scholar] [CrossRef]

| Time | Total Number of AMI Cases | Total Number of COVID-19 Cases | AMI Events among COVID-19 Cases |
|---|---|---|---|
| Apr-20 | 4525 | 123,925 | 3.65% |
| May-20 | 2145 | 60,865 | 3.52% |
| Jun-20 | 1775 | 59,035 | 3.01% |
| Jul-20 | 3545 | 118,725 | 2.99% |
| Aug-20 | 2515 | 75,970 | 3.31% |
| Sep-20 | 1785 | 55,635 | 3.21% |
| Oct-20 | 3130 | 98,525 | 3.18% |
| Nov-20 | 7265 | 207,120 | 3.51% |
| Dec-20 | 8050 | 220,060 | 3.66% |
| Jan-21 | 10,520 | 261,279 | 4.03% |
| Feb-21 | 3790 | 103,270 | 3.67% |
| Mar-21 | 2445 | 75,705 | 3.23% |
| Apr-21 | 2725 | 93,035 | 2.93% |
| May-21 | 1765 | 55,215 | 3.20% |
| Jun-21 | 715 | 26,825 | 2.67% |
| Jul-21 | 2005 | 79,275 | 2.53% |
| Aug-21 | 7055 | 227,059 | 3.11% |
| Sep-21 | 6645 | 181,289 | 3.67% |
| Oct-21 | 4410 | 111,135 | 3.97% |
| Nov-21 | 4865 | 112,515 | 4.32% |
| Dec-21 | 8505 | 195,530 | 4.35% |
| Overall | 90,180 | 2,541,992 | 3.55% |
| Variable | AMI among COVID-19 Patients in 2020 (n = 34,735) (%) | AMI among COVID-19 Patients in 2021 (n = 55,445) (%) | p-Value |
|---|---|---|---|
| Mean age (±SD) | 72.38 (13.31) | 70.31 (13.71) | <0.01 |
| Weekend admission | 26.8 | 26.7 | 0.766 |
| Female | 39.3 | 40.5 | <0.01 |
| Primary payer | <0.01 | ||
| Medicare | 69.9 | 66.1 | |
| Medicaid | 8.7 | 9.4 | |
| Private | 15.5 | 17.8 | |
| Race | <0.01 | ||
| White | 55.6 | 65.0 | |
| Black | 17.8 | 16.1 | |
| Hispanic | 18.0 | 12.4 | |
| Median household income | <0.01 | ||
| 0–25th percentile | 36.8 | 36.8 | |
| 26th to 50th percentile (median) | 29.6 | 27.4 | |
| 51st to 75th percentile | 20.5 | 21.7 | |
| 76th to 100th percentile | 13.1 | 14.1 | |
| Hospital characteristics | |||
| Hospital bed size | <0.01 | ||
| Small | 23.5 | 24.7 | |
| Medium | 29.9 | 30.7 | |
| Large | 46.6 | 44.6 | |
| Location/Teaching status | <0.01 | ||
| Rural | 10.9 | 13.1 | |
| Urban non-teaching | 18.0 | 18.5 | |
| Urban teaching | 71.2 | 68.3 | |
| Region of hospital | <0.01 | ||
| Northeast | 18.7 | 16.6 | |
| Midwest | 2.1 | 22.9 | |
| South | 38.7 | 42.8 | |
| West | 16.5 | 17.7 | |
| Comorbidities | |||
| Sarcoidosis | 0.3 | 0.3 | 0.791 |
| SLE | 0.6 | 0.5 | 0.495 |
| Rheumatoid arthritis | 2.1 | 2.2 | 0.290 |
| Hyperthyroidism | 0.6 | 0.6 | 0.615 |
| Hypothyroidism | 14.1 | 13.2 | <0.01 |
| Hypertension | 27.9 | 28.1 | 0.543 |
| Dyslipidemia | 53.2 | 49.4 | <0.01 |
| Smoking | 28.1 | 30.1 | <0.01 |
| Diabetes | 49.0 | 45.9 | <0.01 |
| CKD | 40.5 | 39.2 | <0.01 |
| Prior CABG | 8.4 | 7.6 | <0.01 |
| Prior PCI | 10.7 | 9.2 | <0.01 |
| Family history of CAD | 4.5 | 4.0 | <0.01 |
| Peripheral vascular disease | 4.4 | 4.3 | 0.604 |
| Prior stroke | 8.3 | 7.6 | <0.01 |
| Cirrhosis | 5.2 | 5.8 | <0.01 |
| Alcohol abuse | 1.8 | 2.4 | <0.01 |
| Prior MI | 9.4 | 8.8 | <0.01 |
| Obesity | 20.4 | 25.5 | <0.01 |
| Drug abuse | 2.0 | 2.8 | <0.01 |
| COPD | 20.7 | 21.9 | <0.01 |
| Mean CCI Score | 5.04 (3.35) | 4.91 (3.36) | <0.01 |
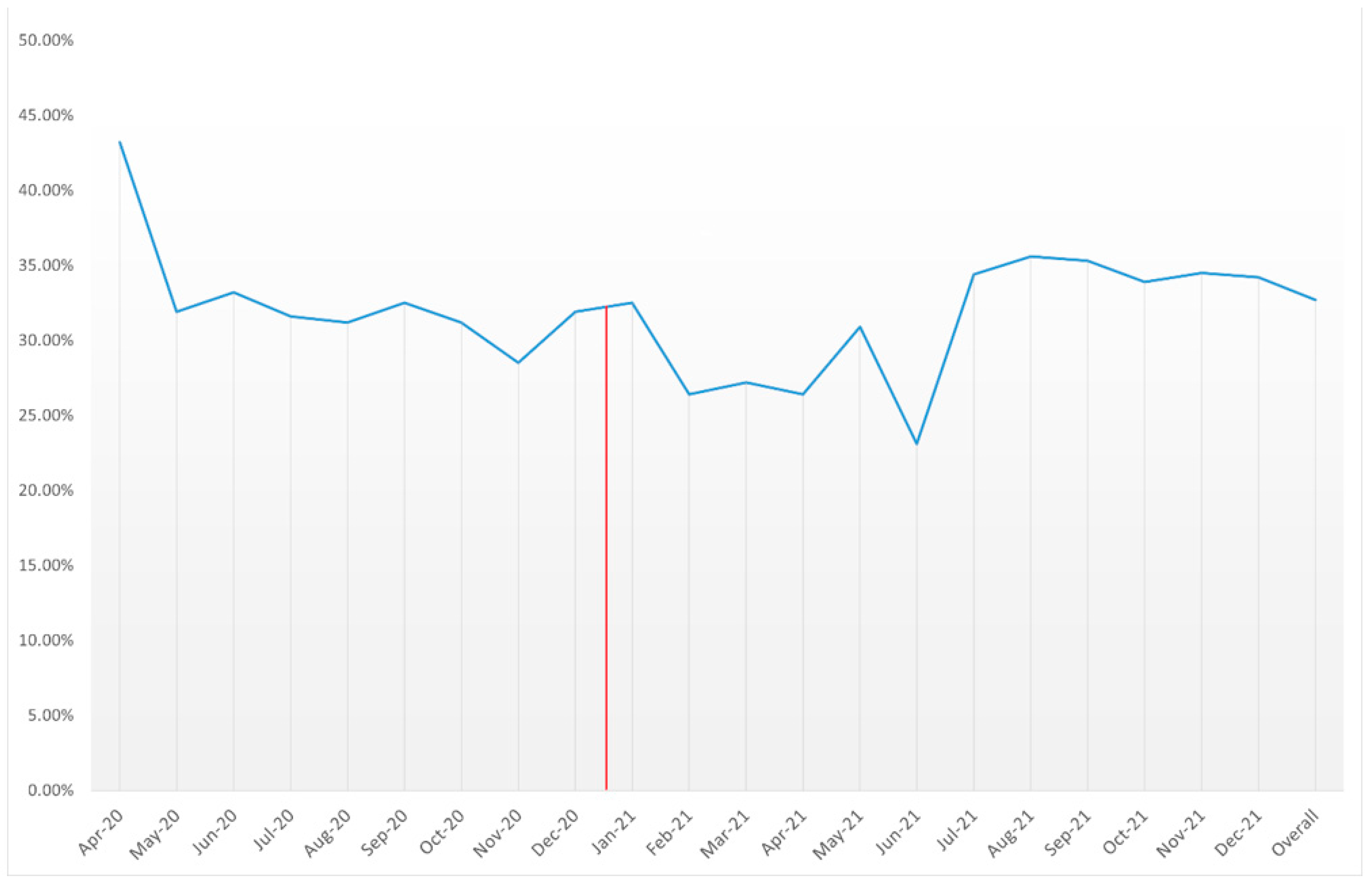
| Time | Mortality of AMI Patients with COVID-19 |
|---|---|
| Apr-20 | 43.20% |
| May-20 | 31.90% |
| Jun-20 | 33.20% |
| Jul-20 | 31.60% |
| Aug-20 | 31.20% |
| Sep-20 | 32.50% |
| Oct-20 | 31.20% |
| Nov-20 | 28.50% |
| Dec-20 | 31.90% |
| Jan-21 | 32.50% |
| Feb-21 | 26.40% |
| Mar-21 | 27.20% |
| Apr-21 | 26.40% |
| May-21 | 30.90% |
| Jun-21 | 23.10% |
| Jul-21 | 34.40% |
| Aug-21 | 35.60% |
| Sep-21 | 35.30% |
| Oct-21 | 33.90% |
| Nov-21 | 34.50% |
| Dec-21 | 34.20% |
| Overall | 32.70% |
| Cardiac Arrhythmias | ||||
|---|---|---|---|---|
| Variable | p-Value | aOR | Lower 95% CI | Upper 95% CI |
| Atrial fibrillation | 0.424 | 1.013 | 0.982 | 1.045 |
| Supraventricular tachycardia | 0.527 | 1.023 | 0.954 | 1.096 |
| Ventricular tachycardia | <0.001 | 1.126 | 1.062 | 1.194 |
| Complications | ||||
| PCI | <0.001 | 1.627 | 1.454 | 1.822 |
| Cardiogenic shock | 0.841 | 1.008 | 0.931 | 1.092 |
| IABP | 0.565 | 0.902 | 0.636 | 1.281 |
| AKI | <0.001 | 1.078 | 1.047 | 1.110 |
| AIS | <0.001 | 1.215 | 1.113 | 1.328 |
| Cardiac arrest | <0.001 | 1.106 | 1.050 | 1.166 |
| Invasive mechanical ventilation | <0.001 | 1.133 | 1.096 | 1.172 |
| Died | 0.043 | 1.032 | 1.001 | 1.064 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhaliwal, J.S.; Sekhon, M.S.; Rajotia, A.; Dang, A.K.; Singh, P.P.; Bilal, M.; Sakthivel, H.; Ahmed, R.; Verma, R.; Ramphul, K.; et al. Disparities and Outcomes in the First and Second Year of the Pandemic on Events of Acute Myocardial Infarction in Coronavirus Disease 2019 Patients. Medicina 2024, 60, 597. https://doi.org/10.3390/medicina60040597
Dhaliwal JS, Sekhon MS, Rajotia A, Dang AK, Singh PP, Bilal M, Sakthivel H, Ahmed R, Verma R, Ramphul K, et al. Disparities and Outcomes in the First and Second Year of the Pandemic on Events of Acute Myocardial Infarction in Coronavirus Disease 2019 Patients. Medicina. 2024; 60(4):597. https://doi.org/10.3390/medicina60040597
Chicago/Turabian StyleDhaliwal, Jasninder Singh, Manraj S. Sekhon, Arush Rajotia, Ashujot K. Dang, Prabh Partap Singh, Maham Bilal, Hemamalini Sakthivel, Raheel Ahmed, Renuka Verma, Kamleshun Ramphul, and et al. 2024. "Disparities and Outcomes in the First and Second Year of the Pandemic on Events of Acute Myocardial Infarction in Coronavirus Disease 2019 Patients" Medicina 60, no. 4: 597. https://doi.org/10.3390/medicina60040597
APA StyleDhaliwal, J. S., Sekhon, M. S., Rajotia, A., Dang, A. K., Singh, P. P., Bilal, M., Sakthivel, H., Ahmed, R., Verma, R., Ramphul, K., & Sethi, P. S. (2024). Disparities and Outcomes in the First and Second Year of the Pandemic on Events of Acute Myocardial Infarction in Coronavirus Disease 2019 Patients. Medicina, 60(4), 597. https://doi.org/10.3390/medicina60040597






