Assessing the Diagnostic Values of the Neutrophil-to-Lymphocyte Ratio (NLR) and Systematic Immunoinflammatory Index (SII) as Biomarkers in Predicting COVID-19 Severity: A Multicentre Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Statistical Analysis
2.3. Ethical Considerations
3. Results
3.1. Participant Characteristics
3.2. Patients’ Baseline Investigations
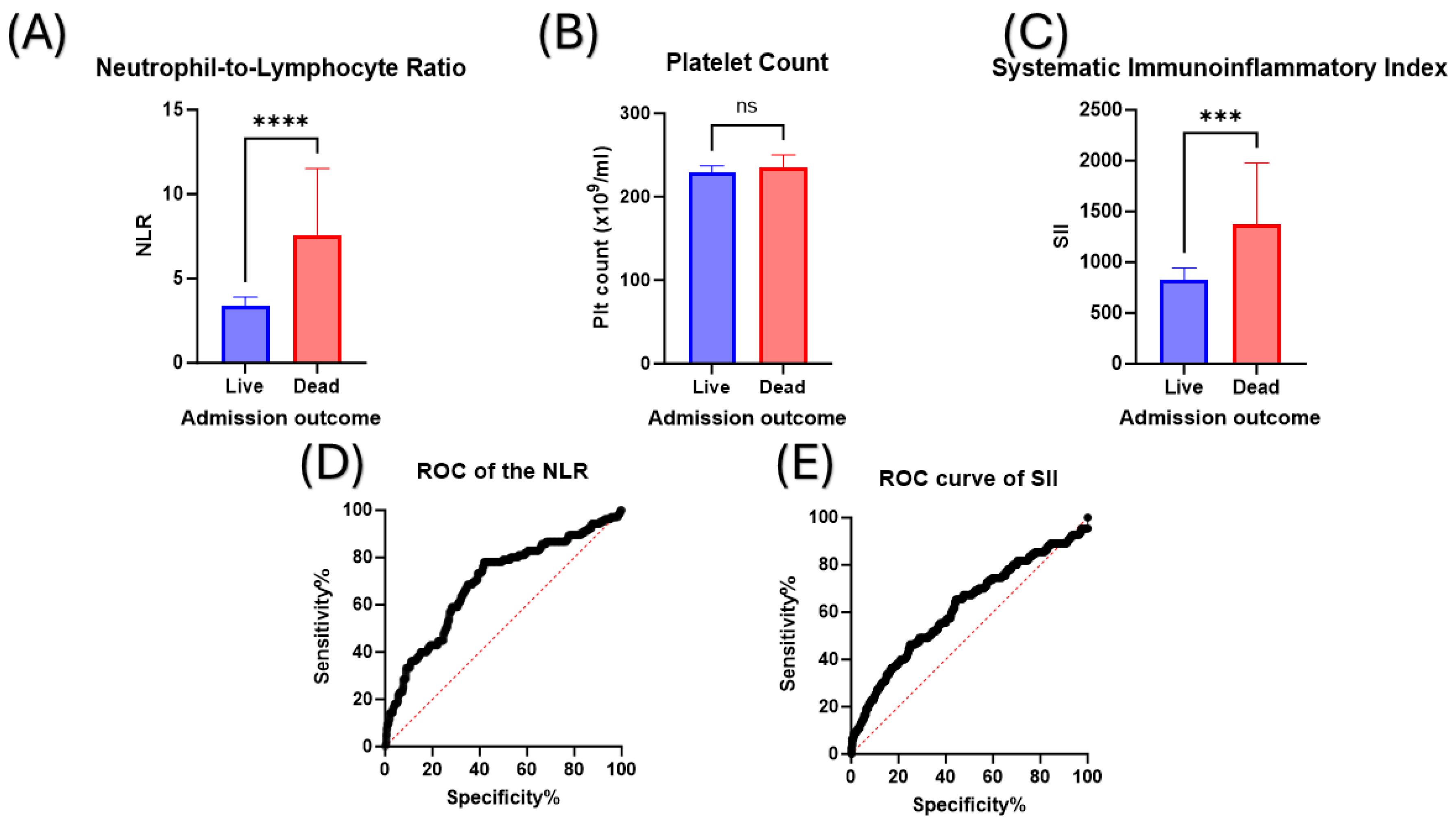
3.3. Non-Survivors Demonstrate a Different Haematological Profile Compared to Survivors
3.4. The Value of Using the Neutrophil-to-Lymphocyte Ratio (NLR) and the Systematic Immunoinflammatory Index (SII) to Discriminate between Survivors and Non-Survivors
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.A. The Progressive Public Measures of Saudi Arabia to Tackle Covid-19 and Limit Its Spread. Int. J. Environ. Res. Public Health 2021, 18, 783. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. Covid-19: WHO declares end of global health emergency. BMJ 2023, 381, 1041. [Google Scholar] [CrossRef] [PubMed]
- Anka, A.U.; Tahir, M.I.; Abubakar, S.D.; Alsabbagh, M.; Zian, Z.; Hamedifar, H.; Sabzevari, A.; Azizi, G. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand. J. Immunol. 2021, 93, e12998. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Karp Leaf, R.S.; Dzik, W.H.; Carlson, J.C.T.; Fogerty, A.E.; Waheed, A.; Goodarzi, K.; Bendapudi, P.K.; Bornikova, L.; Gupta, S.; et al. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020, 136, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Papachristou, S.; Papanas, N. COVID-19 and the kidney: Time to take a closer look. Int. Urol. Nephrol. 2022, 54, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Abo-Haded, H.M.; Alshengeti, A.M.; Alawfi, A.D.; Khoshhal, S.Q.; Al-Harbi, K.M.; Allugmani, M.D.; El-Agamy, D.S. Cytokine Profiling among Children with Multisystem Inflammatory Syndrome versus Simple COVID-19 Infection: A Study from Northwest Saudi Arabia. Biology 2022, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Koffman, J.; Gross, J.; Etkind, S.N.; Selman, L.E. Clinical uncertainty and Covid-19: Embrace the questions and find solutions. Palliat. Med. 2020, 34, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.A.; Allam, A.A.; Sayed, A.I.; Alraey, M.A.; Joseph, M.V. The use of neutrophil-to-lymphocyte ratio (NLR) as a marker for COVID-19 infection in Saudi Arabia. Saudi Med. J. 2021, 42, 370–376. [Google Scholar] [CrossRef]
- Sayed, A.A.; Nozha, O.M. Al Developing a COVID-19 Mortality Prediction (CoMPred) Indicator for ICU Diabetic Patients Treated with Tocilizumab in Saudi Arabia: A Proof-of-Concept Study. Biomedicines 2023, 11, 2649. [Google Scholar] [CrossRef]
- Ministry of Health. Saudi MoH Protocol for Patients Suspected of/Confirmed with COVID-19. Available online: https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/MOH-therapeutic-protocol-for-COVID-19.pdf (accessed on 5 August 2022).
- Armstrong, R.A.; Kane, A.D.; Kursumovic, E.; Oglesby, F.C.; Cook, T.M. Mortality in patients admitted to intensive care with COVID-19: An updated systematic review and meta-analysis of observational studies. Anaesthesia 2021, 76, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Weinberger, B.; Grubeck-Loebenstein, B. The aging of the immune system. Transpl. Int. 2009, 22, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef] [PubMed]
- Alhoufie, S.T.; Mumena, W.A.; Alsharif, N.; Makhdoom, H.M.; Almutawif, Y.A.; Alfarouk, K.O.; Alharbi, M.Z.; Aljabri, K.; Aljifri, A. Epidemiological Characteristics and Outcomes Predictors for Intensive Care Unit COVID-19 Patients in Al-Madinah, Saudi Arabia. Retrospective Cohort Study. Infect. Drug Resist. 2023, 16, 5573–5586. [Google Scholar] [CrossRef] [PubMed]
- Mobarki, A.A.; Dobie, G.; Saboor, M.; Madkhali, A.M.; Akhter, M.S.; Hakamy, A.; Humran, A.; Hamali, Y.; Jackson, D.E.; Hamali, H.A. MPR and NLR as Prognostic Markers in ICU-Admitted Patients with COVID-19 in Jazan, Saudi Arabia. Infect. Drug Resist. 2021, 14, 4859–4864. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-Y.; Wang, R.-R.; Zhang, D.-W.; Chen, S.-H.; Tan, Y.-Y.; Zhang, W.-T.; Han, M.-F.; Fei, G.-H. Differential Characteristics of Patients for Hospitalized Severe COVID-19 Infected by the Omicron Variants and Wild Type of SARS-CoV-2 in China. J. Inflamm. Res. 2023, 16, 3063–3078. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, J.; Yang, L.; Hu, J.; Yao, Y. Value of the Neutrophil-Lymphocyte Ratio in Predicting COVID-19 Severity: A Meta-analysis. Dis. Markers 2021, 2021, 2571912. [Google Scholar] [CrossRef] [PubMed]
- Dymicka-Piekarska, V.; Dorf, J.; Milewska, A.; Łukaszyk, M.; Kosidło, J.W.; Kamińska, J.; Wolszczak-Biedrzycka, B.; Naumnik, W. Neutrophil/Lymphocyte Ratio (NLR) and Lymphocyte/Monocyte Ratio (LMR)—Risk of Death Inflammatory Biomarkers in Patients with COVID-19. J. Inflamm. Res. 2023, 16, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Regolo, M.; Vaccaro, M.; Sorce, A.; Stancanelli, B.; Colaci, M.; Natoli, G.; Russo, M.; Alessandria, I.; Motta, M.; Santangelo, N.; et al. Neutrophil-to-Lymphocyte Ratio (NLR) Is a Promising Predictor of Mortality and Admission to Intensive Care Unit of COVID-19 Patients. J. Clin. Med. 2022, 11, 2235. [Google Scholar] [CrossRef]
- Regolo, M.; Sorce, A.; Vaccaro, M.; Colaci, M.; Stancanelli, B.; Natoli, G.; Motta, M.; Isaia, I.; Castelletti, F.; Giangreco, F.; et al. Assessing Humoral Immuno-Inflammatory Pathways Associated with Respiratory Failure in COVID-19 Patients. J. Clin. Med. 2023, 12, 4057. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Sayed, A.A. The Cost-Effectiveness of Requesting a Complete Blood Count (CBC) in the Management of COVID-19 in Saudi Arabia. Healthcare 2022, 10, 1780. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Italiano, J.E.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, R.; Singh, J.; Jain, K.; Paul, D. Thrombocytopenia in COVID-19: Focused Summary of Current Understanding of Mechanisms and Clinical Implications. J. Pediatr. Hematol. Oncol. 2021, 43, 243–248. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, Q.; Xu, J. Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 2020, 99, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.; Baos, S.; Cappel-Porter, H.; Carson-Stevens, A.; Clout, M.; Culliford, L.; Emmett, S.R.; Garstang, J.; Gbadamoshi, L.; Hallis, B.; et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): A multicentre, randomised, controlled, phase 4 trial. Lancet 2021, 398, 2277–2287. [Google Scholar] [CrossRef]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A.R.; Asghar, M.S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun. Inflamm. Dis. 2023, 11, e807. [Google Scholar] [CrossRef] [PubMed]
- Elrashdy, F.; Tambuwala, M.M.; Hassan, S.S.; Adadi, P.; Seyran, M.; Abd El-Aziz, T.M.; Rezaei, N.; Lal, A.; Aljabali, A.A.A.; Kandimalla, R.; et al. Autoimmunity roots of the thrombotic events after COVID-19 vaccination. Autoimmun. Rev. 2021, 20, 102941. [Google Scholar] [CrossRef]
- Cines, D.B.; Greinacher, A. Vaccine-induced immune thrombotic thrombocytopenia. Blood 2023, 141, 1659–1665. [Google Scholar] [CrossRef]
- Fois, A.G.; Paliogiannis, P.; Scano, V.; Cau, S.; Babudieri, S.; Perra, R.; Ruzzittu, G.; Zinellu, E.; Pirina, P.; Carru, C.; et al. The Systemic Inflammation Index on Admission Predicts In-Hospital Mortality in COVID-19 Patients. Molecules 2020, 25, 5725. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, W.; Ye, B.; Chen, C.; Huang, R.; Wu, F.; Wei, Q.; Zhang, W.; Hu, J. Changes of hematological and immunological parameters in COVID-19 patients. Int. J. Hematol. 2020, 112, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, H.; Mohammadshahi, J.; Javaheri, N.; Fouladi, N.; Mirzazadeh, Y.; Aslani, M.R. Role of leukocytes and systemic inflammation indexes (NLR, PLR, MLP, dNLR, NLPR, AISI, SIR-I, and SII) on admission predicts in-hospital mortality in non-elderly and elderly COVID-19 patients. Front. Med. 2022, 9, 916453. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Pérez, I.A.; Buendía-Roldán, I.; Pérez-Rubio, G.; Chávez-Galán, L.; Hernández-Zenteno, R.d.J.; Aguilar-Duran, H.; Fricke-Galindo, I.; Zaragoza-García, O.; Falfán-Valencia, R.; Guzmán-Guzmán, I.P. Outcome predictors in COVID-19: An analysis of emergent systemic inflammation indices in Mexican population. Front. Med. 2022, 9, 1000147. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, A.; Taşkın, Ö.; Demir, U.; Soylu, V.G. Predictive Role of Biomarkers in COVID-19 Mortality. Cureus 2023, 15, e34173. [Google Scholar] [CrossRef] [PubMed]
- Cakirca, G.; Cakirca, T.D.; Bindal, A.; Olcen, M. Inflammation-based Indices Predicting Mortality in COVID-19. J. Coll. Physicians Surg. Pak. 2023, 33, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Citu, C.; Gorun, F.; Motoc, A.; Sas, I.; Gorun, O.M.; Burlea, B.; Tuta-Sas, I.; Tomescu, L.; Neamtu, R.; Malita, D.; et al. The Predictive Role of NLR, d-NLR, MLR, and SIRI in COVID-19 Mortality. Diagnostics 2022, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Kaushik, A.; Kujawska, M.; Batiha, G.E.-S. Hemolytic anemia in COVID-19. Ann. Hematol. 2022, 101, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ismail, L.; Taha, M.J.J.; Abuawwad, M.T.; Al-Bustanji, Y.; Al-Shami, K.; Nashwan, A.; Yassin, M. COVID-19 and Anemia: What Do We Know So Far? Hemoglobin 2023, 47, 122–129. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Borrelli de Andreis, F.; Aronico, N.; Lenti, M.V.; Barteselli, C.; Merli, S.; Pellegrino, I.; Coppola, L.; Cremonte, E.M.; Croce, G.; et al. Anemia in patients with Covid-19: Pathogenesis and clinical significance. Clin. Exp. Med. 2021, 21, 239–246. [Google Scholar] [CrossRef]
- Oh, S.M.; Skendelas, J.P.; Macdonald, E.; Bergamini, M.; Goel, S.; Choi, J.; Segal, K.R.; Vivek, K.; Nair, S.; Leff, J. On-admission anemia predicts mortality in COVID-19 patients: A single center, retrospective cohort study. Am. J. Emerg. Med. 2021, 48, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Setia, M. Methodology series module 3: Cross-sectional studies. Indian J. Dermatol. 2016, 61, 261. [Google Scholar] [CrossRef] [PubMed]
- Wolszczak-Biedrzycka, B.; Dorf, J.; Wojewódzka-Żelezniakowicz, M.; Żendzian-Piotrowska, M.; Dymicka-Piekarska, V.; Matowicka-Karna, J.; Maciejczyk, M. Unveiling COVID-19 Secrets: Harnessing Cytokines as Powerful Biomarkers for Diagnosis and Predicting Severity. J. Inflamm. Res. 2023, 16, 6055–6070. [Google Scholar] [CrossRef] [PubMed]
- Wolszczak-Biedrzycka, B.; Dorf, J.; Milewska, A.; Łukaszyk, M.; Naumnik, W.; Kosidło, J.W.; Dymicka-Piekarska, V. The Diagnostic Value of Inflammatory Markers (CRP, IL6, CRP/IL6, CRP/L, LCR) for Assessing the Severity of COVID-19 Symptoms Based on the MEWS and Predicting the Risk of Mortality. J. Inflamm. Res. 2023, 16, 2173–2188. [Google Scholar] [CrossRef]
| Characteristic (Unit) | Values |
|---|---|
| Age (years) | 41 (27–57) * |
| Gender | Male: 426 |
| Female: 429 | |
| Nationality | Saudi: 550 |
| Non-Saudi: 305 | |
| Admission outcome | Alive: 742 |
| Deceased: 113 |
| Investigation (Unit) | Patients’ Readings | Reference Levels |
|---|---|---|
| RBC count (×106/mL) | 4.74 (4.35–5.13) | Male: 4.0–5.9 |
| Female: 3.8–5.2 | ||
| Haemoglobin (g/dL) | 13.40 (11.99–14.60) | Male: 13.8–17.2 |
| Female: 12.1–15.1 | ||
| Haematocrit (%) | 40.70 (37.13–44.10) | Male: 40–54 |
| Female: 36–48 | ||
| MCV (fl) | 86.25 (81.53–89.80) | 80–100 |
| MCH (pg) | 28.60 (26.80–30) | 27–31 |
| Platelet count (×106/mL) | 229.5 (182–290.5) | 150–450 |
| WBC (×103/mL) | 6.24 (4.57–9.29) | 4–11 |
| Neutrophil count (×103/mL) | 4.28 (2.71–7.49) | 2.5–7 |
| Lymphocyte count (×103/mL) | 1.07 (0.69–1.59) | 1–4.8 |
| Monocyte Count (×103/mL) | 0.33 (0.23–0.48) | 0.2–0.8 |
| Eosinophil count (×103/mL) | 0.04 (0.01–0.08) | 0.03–0.35 |
| Characteristics/Investigation (Unit) | Survivors (n = 742) | Non-Survivors (n = 113) | p-Value |
|---|---|---|---|
| Age (years) | 38 (27–52) | 62 (49–75.50) | <0.0001 |
| Gender | Male: 361 | Male: 65 | 0.08 ^ |
| Female: 381 | Female: 48 | ||
| RBC count (×106/mL) | 4.76 (4.37–5.14) | 4.60 (4.09–5.08) | 0.09 |
| Haemoglobin (g/dL) | 13.50 (12.20–14.70) | 12.88 (11.60–14.35) | 0.007 |
| Haematocrit (%) | 41 (37.30–44.20) | 39.70 (35.70–43.30) | 0.046 |
| MCV (fl) | 86.20 (81.70–89.80) | 86.70 (80.90–89.90) | 0.73 |
| MCH (pg) | 28.70 (26.90–30) | 28.10 (26.40–29.90) | 0.23 |
| Platelet count (×106/mL) | 228.5 (185–294) | 235 (160.9–285) | 0.46 |
| WBC count (×103/mL) | 5.96 (4.45–8.56) | 8.33 (5.78–11.60) | <0.0001 |
| Neutrophil count (×103/mL) | 3.93 (2.57–6.63) | 6.16 (4.16–9.86) | <0.0001 |
| Lymphocyte count (×103/mL) | 1.13 (0.74–1.66) | 0.76 (0.53–1.25) | <0.0001 |
| Monocyte Count (×103/mL) | 0.33 (0.23–0.48) | 0.33 (0.20–0.53) | 0.32 |
| Eosinophil count (×103/mL) | 0.04 (0.01–0.08) | 0.02 (0.00–0.06) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed, A.A. Assessing the Diagnostic Values of the Neutrophil-to-Lymphocyte Ratio (NLR) and Systematic Immunoinflammatory Index (SII) as Biomarkers in Predicting COVID-19 Severity: A Multicentre Comparative Study. Medicina 2024, 60, 602. https://doi.org/10.3390/medicina60040602
Sayed AA. Assessing the Diagnostic Values of the Neutrophil-to-Lymphocyte Ratio (NLR) and Systematic Immunoinflammatory Index (SII) as Biomarkers in Predicting COVID-19 Severity: A Multicentre Comparative Study. Medicina. 2024; 60(4):602. https://doi.org/10.3390/medicina60040602
Chicago/Turabian StyleSayed, Anwar A. 2024. "Assessing the Diagnostic Values of the Neutrophil-to-Lymphocyte Ratio (NLR) and Systematic Immunoinflammatory Index (SII) as Biomarkers in Predicting COVID-19 Severity: A Multicentre Comparative Study" Medicina 60, no. 4: 602. https://doi.org/10.3390/medicina60040602
APA StyleSayed, A. A. (2024). Assessing the Diagnostic Values of the Neutrophil-to-Lymphocyte Ratio (NLR) and Systematic Immunoinflammatory Index (SII) as Biomarkers in Predicting COVID-19 Severity: A Multicentre Comparative Study. Medicina, 60(4), 602. https://doi.org/10.3390/medicina60040602







