A Comprehensive Review: Robot-Assisted Treatments for Gait Rehabilitation in Stroke Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sourse and Search Strategy
2.2. Selection Criteria
2.2.1. Study Types
2.2.2. Participant Types
2.2.3. Intervention and Control Types
2.2.4. Types of Outcome Measurements
2.3. Quality Assessment
3. Results
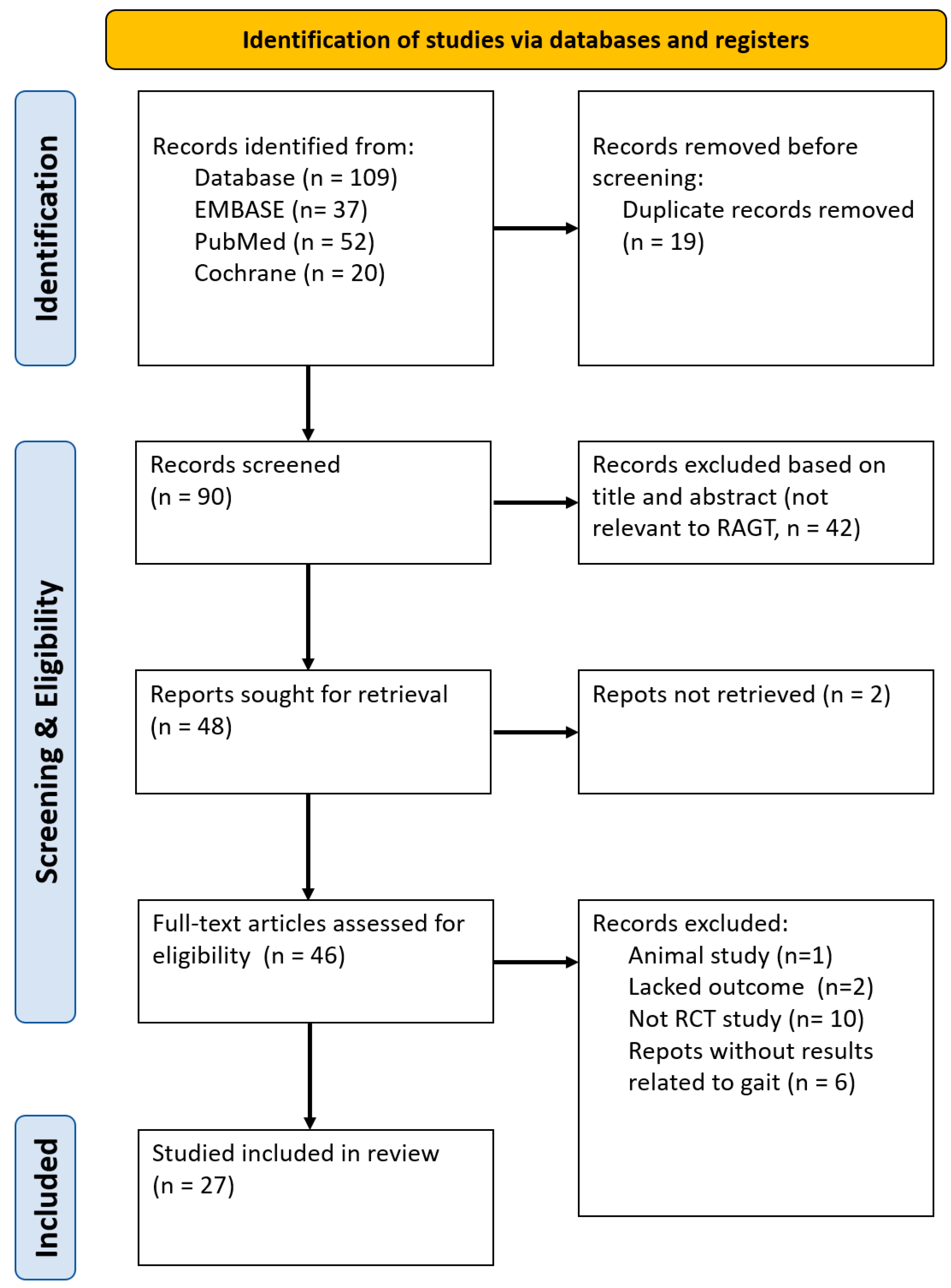
3.1. Literature Search
3.2. Study Characteristics
| Study | Study Design | Sample Size (E/C) | Intervention | Assessment | |
|---|---|---|---|---|---|
| Experimental Group | Control Group | ||||
| Kim (2022) [22] | RCT | 20/20 | Morning walk-assisted gait training (biometric data control group) | Morning walk-assisted gait training (therapist control group) | FAC, 10MWT, TUG, BBS |
| Aprile (2022) [32] | RCT | 19/17 | G-EO system evolution (end-effector system gait/trunk group) | G-EO system evolution (end-effector system gait group) | FAC, 10MWT, 6MWT, TUG, BBS |
| Kim (2020) [16] | RCT | 14/14 | G-EO system evolution | 30% body weight support and a speed of 0.8 km/h | FMA, 10MWT, TUG |
| Kim (2019) [25] | RCT | 10/9 | Lokomat (RAGT + CPT 4 weeks -> CPT 4 weeks) | Lokomat (CPT 4 weeks -> RAGT + CPT 4 weeks) | FAC, 10MWT, FMA-LE |
| Belas (2018) [26] | RCT | 7/8 | Lokomat + CPT | TAGT + CPT | BBS, TUG |
| Tamburella (2019) [27] | RCT | 6/6 | Lokomat + EMGB | Lokomat + Rb | FAC, BBS |
| Alingh (2021) [39] | RCT | 17/15 | AANmDOF Robotic (LOPESII) | CPT | 10MWT, 6MWT, TUG, FMA-LE |
| Yu (2021) [40] | RCT | 27/27 | A3(NX) Gait Training and Evaluation system | Gait training | TUG, FMA |
| Zhang (2023) [41] | RCT | 20/20 | MANBUZHEKANGFU (GR-A1) | CPT | FAC, 6MWT, FMA-LE, |
| Lee (2022) [24] | RCT | 33/10 | Morning walk-assisted gait training (pelvic off n = 11, pelvic control n = 12, CIMT n = 10) | Treadmill + CPT | 10MWT, TUG, BBS |
| Kang (2021) [42] | RCT | 15/15 | SUBAR | CPT | FAC, 10MWT, TUG, BBS |
| Talaty (2023) [28] | RCT | 15/15 | Lokomat + CPT | TAGT + CPT | FAC, 10MWT |
| Mustafaoglu (2020) [29] | RCT | 34/17 | Lokomat (group 1: RAGT + CPT n = 17, group 2: RAGT n = 17) | CPT | 6MWT, FMA-LE |
| Meng (2022) [43] | RCT | 128/61 | Walkbot robotic (group 1: RAGT n = 62, group 2: RGAT + ELLT n = 66) | CPT | FAC, 6MWT, TUG |
| Miyagawa (2023) [44] | RCT | 17/19 | Curara + OT | CPT + OT | 10MWT, 6MWT, BBS |
| Yokota (2023) [34] | RCT | 12/10 | Hybrid assistive limb + CPT | CPT | FAC |
| Bergqvist (2023) [35] | RCT | 27/14 | Hybrid assistive limb + CPT | CPT | FAC, 6MWT, 10MWT, BBS |
| Yeung (2021) [36] | RCT | 30/17 | Dynamixel MX-106R PAAR (power-assisted ankle robot + CT n = 14, swing-controlled ankle robot + CT n = 16) | CT | 10MWT, BBS |
| Palmcrantz (2021) [45] | RCT | 13/28 | Hybrid Assistive Limb | No specific training intervention | 10MWT, 6MWT, FMA, BBS |
| Chang (2023) [10] | RCT | 75/75 | Angel Legs M20 + Gait training | Gait training | FAC, 10MWT, 6MWT, FMA-LE, BBS |
| Louie (2020) [37] | RCT | 20/20 | EksoGT powered robotic exoskeleton + CPT | CPT | 6MWT, BBS, |
| Wright (2021) [38] | RCT | 16/18 | AlterG Bionic Leg orthosis + CPT | CPT | FAC, 6MWT, TUG, BBS, DGI |
| Lee (2023) [23] | RCT | 26/23 | Morning walk + CPT | CT | 10MWT, FMA-LE, BBS |
| Choi (2022) [30] | RCT | 18/6 | Lokomat PRO + NDT (BWS 30% n = 6, 50% n = 6, 70% n = 6) | Treadmill + NDT | 10MWT, TUG, BBS |
| Seo (2018) [33] | RCT | 6/6 | Walkbot + AAN (unaffected limb)/ FA (affected limb) | Walkbot + FA (unaffected limb)/AAN (affected limb) | FAC, FMA-LE |
| Kayabinar (2021) [20] | RCT | 15/15 | VR + RoboGait (Exoskeleton) | RoboGait (Exoskeleton) | FAC, 10MWT, BBS |
| Pournajaf (2022) [31] | RCT | 30/59 | End-effector (G-EO) + + Overground gait training | Exoskeleton (Lokomat) + Overground gait training | 10MWT, 6MWT, TUG |
3.3. Types of Robots Used in Treatment
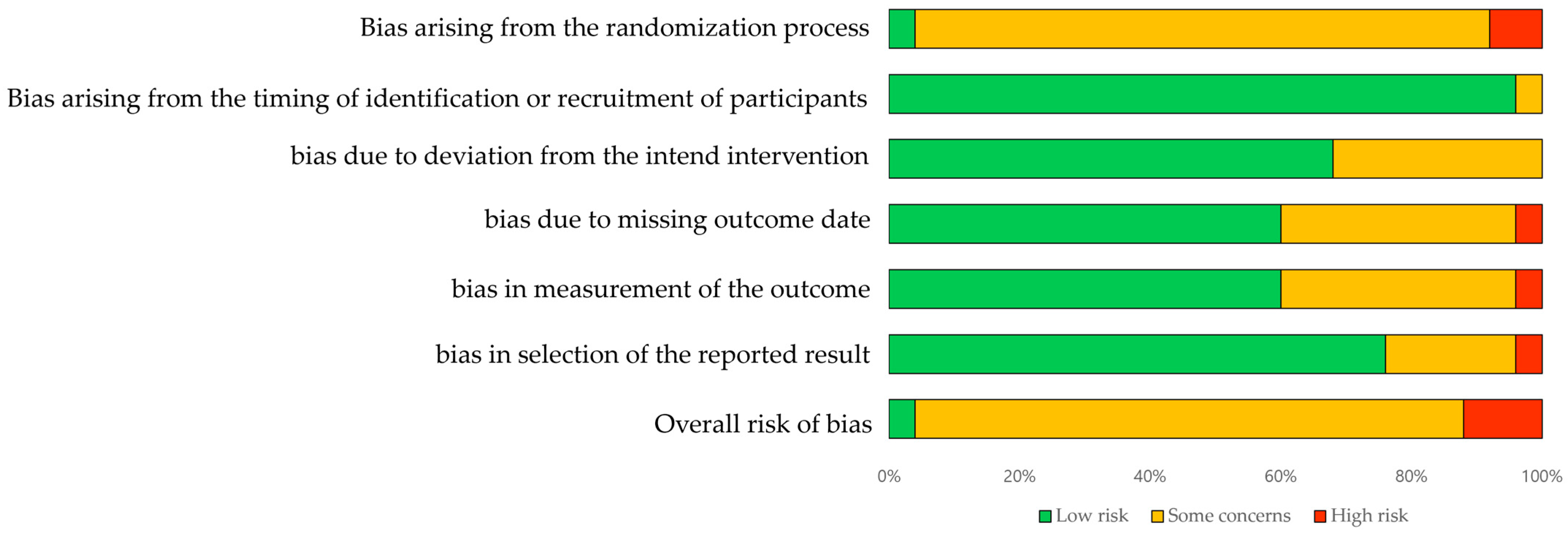
3.4. Quality Assessment
4. Discussion
4.1. Main Findings
4.2. Impact of Lower Extremity Training & Optimal Clinical Application
4.3. Limitations and Suggestions for Further Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Turana, Y.; Tengkawan, J.; Chia, Y.C.; Nathaniel, M.; Wang, J.-G.; Sukonthasarn, A.; Chen, C.-H.; Minh, H.V.; Buranakitjaroen, P.; Shin, J.; et al. Hypertension and Stroke in Asia: A Comprehensive Review from HOPE Asia. J. Clin. Hypertens. 2021, 23, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- Datta Gupta, A.; Visvanathan, R.; Cameron, I.; Koblar, S.A.; Howell, S.; Wilson, D. Efficacy of Botulinum Toxin in Modifying Spasticity to Improve Walking and Quality of Life in Post-Stroke Lower Limb Spasticity—A Randomized Double-Blind Placebo Controlled Study. BMC Neurol. 2019, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.J.; Tang, P.F. Gait Training Strategies to Optimize Walking Ability in People with Stroke: A Synthesis of the Evidence. Expert. Rev. Neurother. 2007, 7, 1417–1436. [Google Scholar] [CrossRef] [PubMed]
- Mikołajewska, E. Bobath and Traditional Approaches in Post-Stroke Gait Rehabilitation in Adults. Biomed. Hum. Kinet. 2017, 9, 27–33. [Google Scholar] [CrossRef]
- Chen, B.-L.; Guo, J.-B.; Liu, M.-S.; Li, X.; Zou, J.; Chen, X.; Zhang, L.-L.; Yue, Y.-S.; Wang, X.-Q. Effect of Traditional Chinese Exercise on Gait and Balance for Stroke: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0135932. [Google Scholar] [CrossRef]
- Chang, W.H.; Kim, Y.-H. Robot-Assisted Therapy in Stroke Rehabilitation. J. Stroke 2013, 15, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Loro, A.; Borg, M.B.; Battaglia, M.; Amico, A.P.; Antenucci, R.; Benanti, P.; Bertoni, M.; Bissolotti, L.; Boldrini, P.; Bonaiuti, D.; et al. Balance Rehabilitation through Robot-Assisted Gait Training in Post-Stroke Patients: A Systematic Review and Meta-Analysis. Brain Sci. 2023, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Nedergård, H.; Arumugam, A.; Sandlund, M.; Bråndal, A.; Häger, C.K. Effect of Robotic-Assisted Gait Training on Objective Biomechanical Measures of Gait in Persons Post-Stroke: A Systematic Review and Meta-Analysis. J. Neuroeng. Rehabil. 2021, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Kim, T.-W.; Kim, H.S.; Hanapiah, F.A.; Kim, D.H.; Kim, D.Y. Exoskeletal Wearable Robot on Ambulatory Function in Patients with Stroke: A Protocol for an International, Multicentre, Randomised Controlled Study. BMJ Open 2023, 13, e065298. [Google Scholar] [CrossRef]
- Moucheboeuf, G.; Griffier, R.; Gasq, D.; Glize, B.; Bouyer, L.; Dehail, P.; Cassoudesalle, H. Effects of Robotic Gait Training after Stroke: A Meta-Analysis. Ann. Phys. Rehabil. Med. 2020, 63, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Heng, H.-M.; Lu, M.-K.; Chou, L.-W.; Meng, N.-H.; Huang, H.-C.; Hamada, M.; Tsai, C.-H.; Chen, J.-C. Changes in Balance, Gait and Electroencephalography Oscillations after Robot-Assisted Gait Training: An Exploratory Study in People with Chronic Stroke. Brain Sci. 2020, 10, 821. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-H.; Lee, S.M.; Ko, M. Comparison of the Effects on Dynamic Balance and Aerobic Capacity between Objective and Subjective Methods of High-Intensity Robot-Assisted Gait Training in Chronic Stroke Patients: A Randomized Controlled Trial. Top. Stroke Rehabil. 2017, 24, 309–313. [Google Scholar] [CrossRef] [PubMed]
- El Naamani, K.; Abbas, R.; Sioutas, G.S.; Tjoumakaris, S.I.; Gooch, M.R.; Herial, N.A.; Rosenwasser, R.H.; Jabbour, P.M. Endovascular Robotic Interventions. Neurosurg. Clin. N. Am. 2022, 33, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S.; Heß, A.; Werner, C.C.; Kabbert, N.; Buschfort, R. Effect on Arm Function and Cost of Robot-Assisted Group Therapy in Subacute Patients with Stroke and a Moderately to Severely Affected Arm: A Randomized Controlled Trial. Clin. Rehabil. 2014, 28, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, G.; Shin, J.-H.; You, J.H. Neuroplastic Effects of End-Effector Robotic Gait Training for Hemiparetic Stroke: A Randomised Controlled Trial. Sci. Rep. 2020, 10, 12461. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, E.; Beckwée, D.; Meeusen, R.; Baeyens, J.-P.; Kerckhofs, E. Does Robot-Assisted Gait Rehabilitation Improve Balance in Stroke Patients? A Systematic Review. Top. Stroke Rehabil. 2014, 21, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-N.; Huang, S.-W.; Kuan, Y.-C.; Chen, H.-C.; Jian, W.-S.; Lin, L.-F. Hybrid Robot-Assisted Gait Training for Motor Function in Subacute Stroke: A Single-Blind Randomized Controlled Trial. J. Neuroeng. Rehabil. 2022, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Packel, A.; Marcy, E.; Sprik, K.; Harold, E.; Xiao, R.; Esquenazi, A. Implementing Robotic-Assisted Gait Training in Acute Inpatient Stroke Rehabilitation: A Quality Improvement Initiative. J. Int. Soc. Phys. Rehabil. Med. 2021, 4, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Kayabinar, B.; Alemdaroğlu-Gürbüz, İ.; Yilmaz, Ö. The Effects of Virtual Reality Augmented Robot-Assisted Gait Training on Dual-Task Performance and Functional Measures in Chronic Stroke: A Randomized Controlled Single-Blind Trial. Eur. J. Phys. Rehabil. Med. 2021, 57, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Pérez, I.; González-González, N.; Peinado-Rubia, A.B.; Nieto-Escamez, F.A.; Obrero-Gaitán, E.; García-López, H. Efficacy of Robot-Assisted Gait Therapy Compared to Conventional Therapy or Treadmill Training in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis. Sensors 2022, 22, 9910. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Chun, M.H.; Lee, J.; Kim, J.W.; Lee, J.Y. Intensity Control of Robot-Assisted Gait Training Based on Biometric Data: Preliminary Study. Medicine 2022, 101, e30818. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.Y.; Lee, S.H.; Kim, J.H.; Kim, D.Y.; Lim, K.-B.; Yoo, J. End-Effector Lower Limb Robot-Assisted Gait Training Effects in Subacute Stroke Patients: A Randomized Controlled Pilot Trial. Medicine 2023, 102, e35568. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chun, M.H.; Seo, Y.J.; Lee, A.; Choi, J.; Son, C. Effects of a Lower Limb Rehabilitation Robot with Various Training Modes in Patients with Stroke: A Randomized Controlled Trial. Medicine 2022, 101, e31590. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Shin, J.-H.; Yang, S.P.; Shin, M.A.; Lee, S.H. Robot-Assisted Gait Training for Balance and Lower Extremity Function in Patients with Infratentorial Stroke: A Single-Blinded Randomized Controlled Trial. J. Neuroeng. Rehabil. 2019, 16, 99. [Google Scholar] [CrossRef]
- Belas Dos Santos, M.; Barros de Oliveira, C.; Dos Santos, A.; Garabello Pires, C.; Dylewski, V.; Arida, R.M. A Comparative Study of Conventional Physiotherapy versus Robot-Assisted Gait Training Associated to Physiotherapy in Individuals with Ataxia after Stroke. Behav. Neurol. 2018, 2018, 2892065. [Google Scholar] [CrossRef] [PubMed]
- Tamburella, F.; Moreno, J.C.; Herrera Valenzuela, D.S.; Pisotta, I.; Iosa, M.; Cincotti, F.; Mattia, D.; Pons, J.L.; Molinari, M. Influences of the Biofeedback Content on Robotic Post-Stroke Gait Rehabilitation: Electromyographic vs Joint Torque Biofeedback. J. Neuroeng. Rehabil. 2019, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Talaty, M.; Esquenazi, A. Feasibility and Outcomes of Supplemental Gait Training by Robotic and Conventional Means in Acute Stroke Rehabilitation. J. Neuroeng. Rehabil. 2023, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Mustafaoglu, R.; Erhan, B.; Yeldan, I.; Gunduz, B.; Tarakci, E. Does Robot-Assisted Gait Training Improve Mobility, Activities of Daily Living and Quality of Life in Stroke? A Single-Blinded, Randomized Controlled Trial. Acta Neurol. Belg. 2020, 120, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Choi, W. Effects of Robot-Assisted Gait Training with Body Weight Support on Gait and Balance in Stroke Patients. Int. J. Environ. Res. Public Health 2022, 19, 5814. [Google Scholar] [CrossRef] [PubMed]
- Pournajaf, S.; Calabrò, R.S.; Naro, A.; Goffredo, M.; Aprile, I.; Tamburella, F.; Filoni, S.; Waldner, A.; Mazzoleni, S.; Focacci, A.; et al. Robotic versus Conventional Overground Gait Training in Subacute Stroke Survivors: A Multicenter Controlled Clinical Trial. J. Clin. Med. 2023, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Aprile, I.; Conte, C.; Cruciani, A.; Pecchioli, C.; Castelli, L.; Insalaco, S.; Germanotta, M.; Iacovelli, C. Efficacy of Robot-Assisted Gait Training Combined with Robotic Balance Training in Subacute Stroke Patients: A Randomized Clinical Trial. J. Clin. Med. 2022, 11, 5162. [Google Scholar] [CrossRef]
- Seo, J.S.; Yang, H.S.; Jung, S.; Kang, C.S.; Jang, S.; Kim, D.H. Effect of Reducing Assistance during Robot-Assisted Gait Training on Step Length Asymmetry in Patients with Hemiplegic Stroke. Medicine 2018, 97, e11792. [Google Scholar] [CrossRef] [PubMed]
- Yokota, C.; Tanaka, K.; Omae, K.; Kamada, M.; Nishikawa, H.; Koga, M.; Ihara, M.; Fujimoto, Y.; Sankai, Y.; Nakajima, T.; et al. Effect of Cyborg-Type Robot Hybrid Assistive Limb on Patients with Severe Walking Disability in Acute Stroke: A Randomized Controlled Study. J. Stroke Cerebrovasc. Dis. 2023, 32, 107020. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, M.; Möller, M.C.; Björklund, M.; Borg, J.; Palmcrantz, S. The Impact of Visuospatial and Executive Function on Activity Performance and Outcome after Robotic or Conventional Gait Training, Long-Term after Stroke—As Part of a Randomized Controlled Trial. PLoS ONE 2023, 18, e0281212. [Google Scholar] [CrossRef] [PubMed]
- Yeung, L.-F.; Lau, C.C.Y.; Lai, C.W.K.; Soo, Y.O.Y.; Chan, M.-L.; Tong, R.K.Y. Effects of Wearable Ankle Robotics for Stair and Over-Ground Training on Sub-Acute Stroke: A Randomized Controlled Trial. J. Neuroeng. Rehabil. 2021, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Louie, D.R.; Mortenson, W.B.; Durocher, M.; Teasell, R.; Yao, J.; Eng, J.J. Exoskeleton for Post-Stroke Recovery of Ambulation (ExStRA): Study Protocol for a Mixed-Methods Study Investigating the Efficacy and Acceptance of an Exoskeleton-Based Physical Therapy Program during Stroke Inpatient Rehabilitation. BMC Neurol. 2020, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Stone, K.; Martinelli, L.; Fryer, S.; Smith, G.; Lambrick, D.; Stoner, L.; Jobson, S.; Faulkner, J. Effect of Combined Home-Based, Overground Robotic-Assisted Gait Training and Usual Physiotherapy on Clinical Functional Outcomes in People with Chronic Stroke: A Randomized Controlled Trial. Clin. Rehabil. 2021, 35, 882–893. [Google Scholar] [CrossRef]
- Alingh, J.F.; Fleerkotte, B.M.; Groen, B.E.; Rietman, J.S.; Weerdesteyn, V.; van Asseldonk, E.H.F.; Geurts, A.C.H.; Buurke, J.H. Effect of Assist-as-Needed Robotic Gait Training on the Gait Pattern Post Stroke: A Randomized Controlled Trial. J. Neuroeng. Rehabil. 2021, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Yang, Z.; Lei, L.; Chaoming, N.; Ming, W. Robot-Assisted Gait Training Plan for Patients in Poststroke Recovery Period: A Single Blind Randomized Controlled Trial. Biomed. Res. Int. 2021, 2021, 5820304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Gong, Y.; Wu, J.; Chen, J.; Chen, W.; Pei, Z.; Zhang, W.; Dai, L.; Shu, X.; et al. Three-Dimensional Gait Analysis and sEMG Measures for Robotic-Assisted Gait Training in Subacute Stroke: A Randomized Controlled Trial. Biomed. Res. Int. 2023, 2023, 7563802. [Google Scholar] [CrossRef]
- Kang, C.J.; Chun, M.H.; Lee, J.; Lee, J.Y. Effects of Robot (SUBAR)-Assisted Gait Training in Patients with Chronic Stroke: Randomized Controlled Trial. Medicine 2021, 100, e27974. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Ma, X.; Chen, P.; Xu, S.; Li, M.; Zhao, Y.; Jin, A.; Liu, X. Effect of Early Integrated Robot-Assisted Gait Training on Motor and Balance in Patients with Acute Ischemic Stroke: A Single-Blinded Randomized Controlled Trial. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221123195. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, D.; Matsushima, A.; Maruyama, Y.; Mizukami, N.; Tetsuya, M.; Hashimoto, M.; Yoshida, K. Gait Training with a Wearable Powered Robot during Stroke Rehabilitation: A Randomized Parallel-Group Trial. J. Neuroeng. Rehabil. 2023, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Palmcrantz, S.; Wall, A.; Vreede, K.S.; Lindberg, P.; Danielsson, A.; Sunnerhagen, K.S.; Häger, C.K.; Borg, J. Impact of Intensive Gait Training with and without Electromechanical Assistance in the Chronic Phase after Stroke—A Multi-Arm Randomized Controlled Trial with a 6 and 12 Months Follow Up. Front. Neurosci. 2021, 15, 660726. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.; Paludan-Müller, A.S.; Laursen, D.R.T.; Savović, J.; Boutron, I.; Sterne, J.A.C.; Higgins, J.P.T.; Hróbjartsson, A. Evaluation of the Cochrane Tool for Assessing Risk of Bias in Randomized Clinical Trials: Overview of Published Comments and Analysis of User Practice in Cochrane and Non-Cochrane Reviews. Syst. Rev. 2016, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Schubauer-Berigan, M.K.; Vermeulen, R.; Lunn, R.M.; Straif, K.; Zahm, S.; Stewart, P.; Arroyave, W.D.; Mehta, S.S.; Pearce, N. Risk of Bias Assessments and Evidence Syntheses for Observational Epidemiologic Studies of Environmental and Occupational Exposures: Strengths and Limitations. Environ. Health Perspect. 2020, 128, 095002. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.F.; Weiss, P.L.; Keshner, E.A. Emergence of Virtual Reality as a Tool for Upper Limb Rehabilitation: Incorporation of Motor Control and Motor Learning Principles. Phys. Ther. 2015, 95, 415–425. [Google Scholar] [CrossRef]
| Robot Type | Study | Applied Robot | Intervention Periods | Assessment Period | Outcomes |
|---|---|---|---|---|---|
| End-effector | Lee (2023) [23] | End-effector RAGT | 4 weeks | BF, AF | Significant improvements in all outcome measures; robot group improved more in FAC. |
| Kim (2022) [22] | Morning walk | 6 weeks | BF, AF | Significant improvements in FAC, MBI, BBS, TUG, 10MWT in both groups; no significant differences between groups. | |
| Aprile (2022) [32] | End-effector system | 1 month | BF, AF | Improvement in balance ability in both groups; significant improvements in lower limb muscle strength and muscle tone in GTG group. | |
| Kim (2020) [16] | G-EO system evolution | 4 weeks | BF, AF | Increased activation in primary sensorimotor cortex, supplementary motor area, premotor cortex; significantly better FMA scores in E RAGT group. | |
| Exoskeleton (fix) | Kim (2019) [25] | Lokomat® PRO | 4 weeks | BF, AF | Significant differences in outcomes between groups; significantly greater improvements in FMA-LE and SARA in RAGT + CPT group. |
| Belas (2018) [26] | Lokomat® 5.0 | 5 months | BF, AF | Statistically significant improvements in balance, functional independence, and general ataxia symptoms in both groups; no significant between-group differences. | |
| Tamburella (2019) [27] | Lokomat | 4 weeks | BF, AF | Significant improvements in gait/daily living activity independence and trunk control; EMGb more effective in reducing spasticity and improving muscle force. | |
| Alingh (2021) [39] | AANmDOF Robotic (LOPESII) | 6 weeks | BF, AF, FU | Improvements in gait parameters and functional gait tasks; no significant group differences except for paretic knee flexion improvement in AANmDOF group. | |
| Seo (2018) [33] | Walkbot | 10 weeks | BF, AF, FU | Clinical measurements improved in both groups; significant improvements in step length asymmetry ratio and hip maximal extension moment in group 1, and dorsiflexion angle in group 2. | |
| Yu (2021) [40] | G-EO system evolution | 14 consecutive days | BF, AF, FU | Significant effect on changes in space parameters and FMA scores in RT group; no significant differences between groups. | |
| Zhang (2023) [41] | MANBUZHEKANGFU (GR-A1) | 4 weeks | BF, AF | Experimental group significantly outperformed control group in various measures; significant improvement in co-contraction index of the knee in experimental group. | |
| Choi (2022) [30] | Lokomat® PRO | 6 weeks | BF, AF | Robot groups showed significantly better 10MWT results and shorter TUG than non-robot group; significant improvement in BBS scores for robot group A. | |
| Lee (2022) [24] | Morning walk | 4 weeks | BF, AF | Significant improvements in BBS, TUG, MI-Lower in pelvic off group; greater improvement in TUG and BBS in pelvic on group, and in 10MWT and MI-Lower in CIMT group. | |
| Kang (2021) [42] | SUBAR | 3 weeks | BF, AF | Significant improvements in MAS and step length in SUBAR group; control group showed significant improvements in BBS, MAS, and stride length. | |
| Talaty (2023) [28] | Lokomat | 3 weeks | BF, AF, FU | Both groups showed significant improvements in several measures. CGT group had 45% more supplemental sessions than the Lokomat group. Both groups showed greater FIM improvement scores than a reference group with no supplemental therapy. | |
| Mustafaoglu (2020) [29] | Lokomat | 6 weeks | BF, AF | Significant improvements in BI, 6MWT, SS-QOL, and SCT for primary outcomes and FMA-LE, CWT, RPE for secondary outcomes, except FWT. Group 1 showed significant improvement compared to group 2 and 3. | |
| Kayabinar (2021) [20] | RoboGait | 6 weeks | BF, AF | Increase in single and dual-task gait speeds and cognitive dual-task performance in the study group. No significant difference between groups in all assessments after treatment. | |
| Meng (2022) [43] | Walkbot | 4 weeks | BF, AF | Significant improvements in 6MWT, FAC, TUG, DTW, Tinetti’s test, BI, SS-QOL, and gait. RAGT group performed better in several measures compared to ELLT and CRT groups. | |
| Exoskeleton (wearable) | Miyagawa (2023) [44] | Curara | 15 days | BF, AF, FU | No significant difference in main outcomes between groups at the end of gait training. Significant intragroup improvements in gait speed, stride length, stride duration, and cadence. |
| Yokota (2023) [34] | Hybrid assistive limb | 20 sessions (5~6 day) | BF, AF, FU | No significant differences in primary outcomes. Apathy scale showed a decreasing trend in HAL group and a slight increasing trend in CPT group. | |
| Bergqvist (2023) [35] | Hybrid assistive limb | 6 weeks | BF, AF, FU | No significant associations between MoCA Vis/Ex and 6MWT in robotic gait training group. | |
| Yeung (2021) [36] | Exoskeleton ankle robot (PAAR, SCAR) | 20 sessions | BF, AF | Statistically significant improvements in functional ambulatory category and walking speed for SCAR and PAAR, respectively. | |
| Palmcrantz (2021) [45] | Hybrid assistive limb | 6 weeks | BF, AF, FU | HAL group walked twice as far as conventional group during intervention. Post-intervention, only the conventional group showed significant improvement compared group. | |
| Wright (2021) [38] | AlterG Bionic Leg | 10 weeks | BF, AF | Significant increases in walking distance, FAC, DGI, and BBS for over-ground robotic-assisted gait training. Improvements maintained at 22 weeks. | |
| Exoskeleton(fix) and End-effector | Pournajaf (2022) [31] | G-EO (End-effector) | 20 sessions | BF, AF | Robotic Group showed significant benefits in 10 MWT, 6 MWT, TUG, and MBI. Robot in gait speed, endurance, balance, and ADL. RobotEND-group improved walking speed more than RobotEXO-group. |
| Study | D1a | D1b | D2 | D3 | D4 | D5 | Overall | Study | D1a | D1b | D2 | D3 | D4 | D5 | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim (2022) [22] | H | L | Sc | Sc | Sc | Sc | Sc | Meng (2022) [43] | Sc | L | Sc | L | Sc | L | Sc |
| Aprile (2022) [31] | Sc | L | L | L | L | L | Sc | Miyagawa (2023) [44] | Sc | Sc | L | Sc | Sc | Sc | Sc |
| Kim (2020) [16] | Sc | L | Sc | L | L | Sc | Sc | Yokota (2023) [34] | Sc | L | L | Sc | Sc | Sc | Sc |
| Kim (2019) [25] | Sc | L | L | L | L | L | Sc | Bergqvist (2023) [35] | Sc | L | Sc | Sc | L | L | Sc |
| Belas (2018) [26] | Sc | L | L | L | L | L | Sc | Yeung (2021) [36] | Sc | L | L | L | L | L | Sc |
| Tamburella (2019) [27] | Sc | L | L | L | Sc | L | Sc | Palmcrantz (2021) [45] | L | L | L | L | L | L | L |
| Alingh (2021) [39] | Sc | L | L | Sc | L | L | Sc | Wright (2021) [38] | Sc | L | L | L | L | L | Sc |
| Yu (2021) [40] | Sc | L | L | Sc | L | L | Sc | Lee (2023) [23] | Sc | L | Sc | Sc | L | L | Sc |
| Zhang (2023) [41] | Sc | L | Sc | Sc | Sc | Sc | Sc | Seo (2018) [33] | Sc | L | L | H | L | H | H |
| Lee (2022) [24] | Sc | L | Sc | L | Sc | L | Sc | Choi (2022) [30] | Sc | L | L | L | Sc | L | Sc |
| Kang (2021) [42] | H | L | Sc | L | Sc | L | H | Kayabinar (2021) [20] | Sc | L | L | Sc | L | L | Sc |
| Talaty (2023) [28] | Sc | L | L | L | L | L | Sc | Pournajaf (2023) [31] | Sc | L | L | H | H | L | H |
| Mustafaoglu (2020) [29] | Sc | L | L | L | L | L | Sc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-H.; Lee, D.-H.; Lee, J.-H. A Comprehensive Review: Robot-Assisted Treatments for Gait Rehabilitation in Stroke Patients. Medicina 2024, 60, 620. https://doi.org/10.3390/medicina60040620
Park Y-H, Lee D-H, Lee J-H. A Comprehensive Review: Robot-Assisted Treatments for Gait Rehabilitation in Stroke Patients. Medicina. 2024; 60(4):620. https://doi.org/10.3390/medicina60040620
Chicago/Turabian StylePark, Yong-Hwa, Dae-Hwan Lee, and Jung-Ho Lee. 2024. "A Comprehensive Review: Robot-Assisted Treatments for Gait Rehabilitation in Stroke Patients" Medicina 60, no. 4: 620. https://doi.org/10.3390/medicina60040620
APA StylePark, Y.-H., Lee, D.-H., & Lee, J.-H. (2024). A Comprehensive Review: Robot-Assisted Treatments for Gait Rehabilitation in Stroke Patients. Medicina, 60(4), 620. https://doi.org/10.3390/medicina60040620






