Assessing the Feasibility and Efficacy of Pre-Sleep Dim Light Therapy for Adults with Insomnia: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Biochemical Analyses
2.3. Actigraphy
2.4. Sleep-Related Questionnaire
2.5. Assessment of Salivary Melatonin
2.6. Real-Time Reverse Transcription (RT) Polymerase Chain Reaction (PCR) Analysis of Clock Genes
2.7. Treatment
2.8. Statistical Analysis
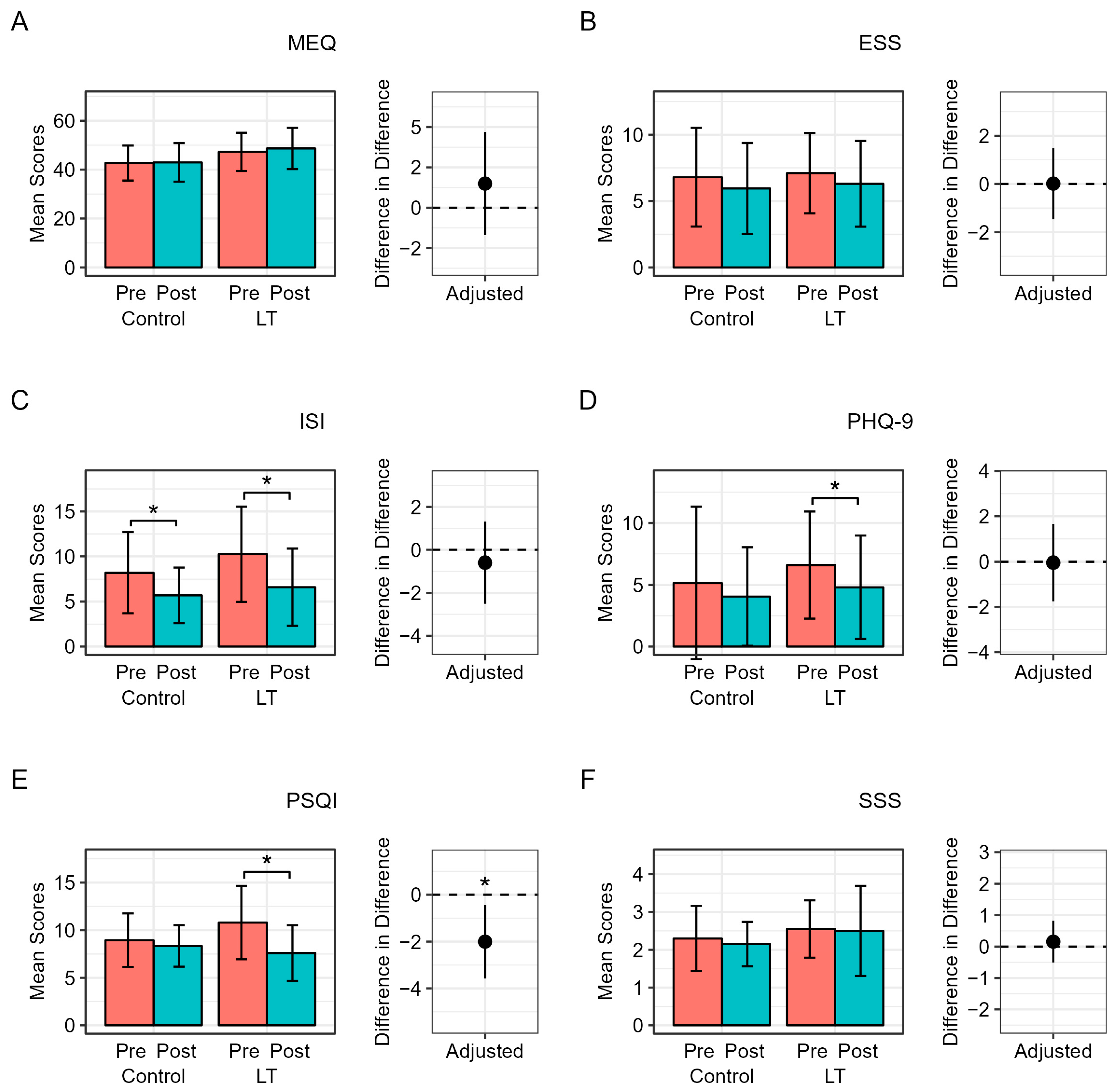
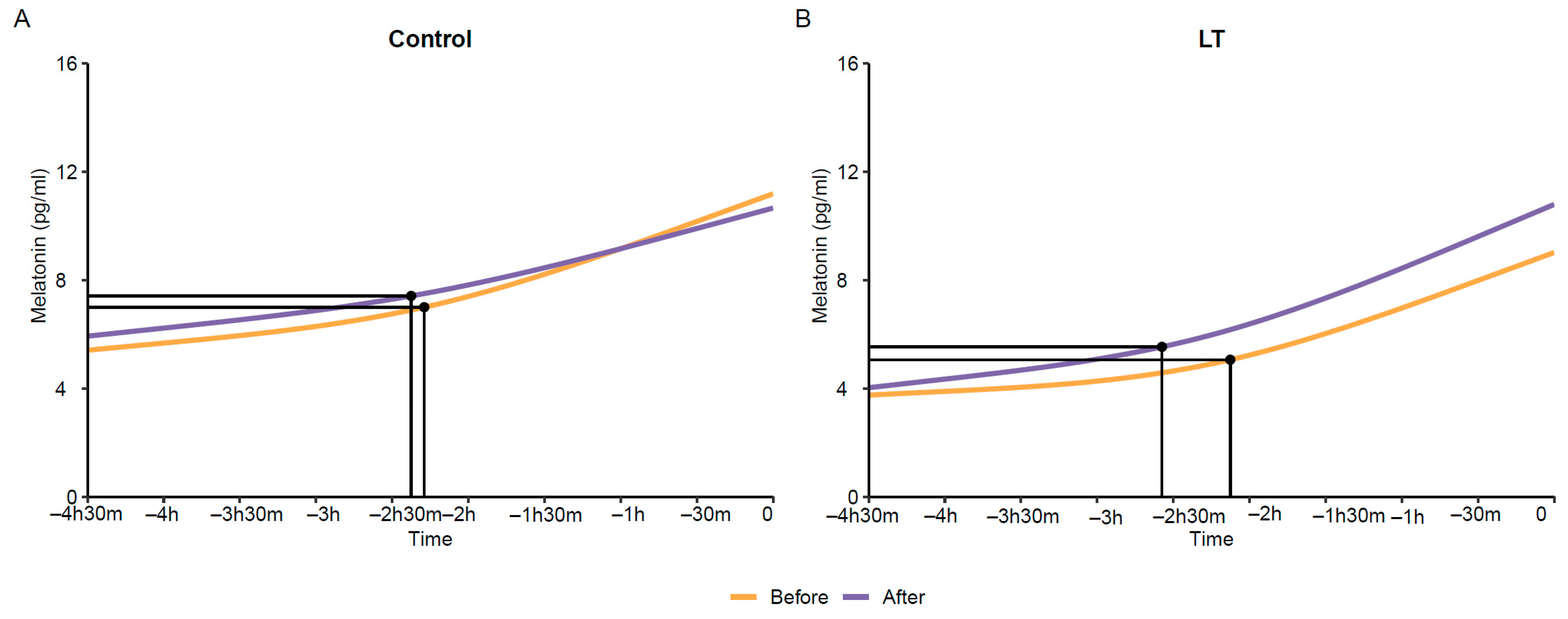
3. Results
Compliance and Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morin, C.M.; Drake, C.L.; Harvey, A.G.; Krystal, A.D.; Manber, R.; Riemann, D.; Spiegelhalder, K. Insomnia disorder. Nat. Rev. Dis. Primers 2015, 1, 15026. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Shaikh, A.; Yasmin, F.; Sughra, F.; Sheikh, A.; Owais, R.; Raheel, H.; Virk, H.U.H.; Mustapha, J.A. Incidence of adverse cardiovascular events in patients with insomnia: A systematic review and meta-analysis of real-world data. PLoS ONE 2023, 18, e0291859. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef]
- van Maanen, A.; Meijer, A.M.; van der Heijden, K.B.; Oort, F.J. The effects of light therapy on sleep problems: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 29, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Emeklİ, R.; İsmaİloğullari, S.; Bayram, A.; Akalin, H.; Tuncel, G.; Dündar, M. Comparing expression levels of period genes per1, per2 and per3 in chronic insomnia patients and medical staff working in the night shift. Sleep Med. 2020, 73, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Steiner, R.; Oelhafen, P.; Lang, D.; Götz, T.; Krebs, J.; Cajochen, C. Acute exposure to evening blue-enriched light impacts on human sleep. J. Sleep Res. 2013, 22, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.P., Jr.; McHill, A.W.; Birks, B.R.; Griffin, B.R.; Rusterholz, T.; Chinoy, E.D. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 2013, 23, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Stothard, E.R.; McHill, A.W.; Depner, C.M.; Birks, B.R.; Moehlman, T.M.; Ritchie, H.K.; Guzzetti, J.R.; Chinoy, E.D.; LeBourgeois, M.K.; Axelsson, J.; et al. Circadian entrainment to the natural light-dark cycle across seasons and the weekend. Curr. Biol. 2017, 27, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J.; Chua, E.C. Diurnal regulation of lipid metabolism and applications of circadian lipidomics. J. Genet. Genom. 2014, 41, 231–250. [Google Scholar] [CrossRef]
- Namkung, J.; Kim, H.; Park, S. Peripheral serotonin: A new player in systemic energy homeostasis. Mol. Cells 2015, 38, 1023–1028. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging roles for serotonin in regulating metabolism: New implications for an ancient molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; LeBlanc, M.; Bélanger, L.; Ivers, H.; Mérette, C.; Savard, J. Prevalence of insomnia and its treatment in canada. Can. J. Psychiatry 2011, 56, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.; Coulouvrat, C.; Hajak, G.; Lakoma, M.D.; Sampson, N.A.; Shahly, V.; Shillington, A.C.; Stephenson, J.J.; Walsh, J.K.; Kessler, R.C. Prevalence and perceived health associated with insomnia based on dsm-iv-tr; international statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classification of sleep disorders, second edition criteria: Results from the america insomnia survey. Biol. Psychiatry 2011, 69, 592–600. [Google Scholar] [PubMed]
- Ohayon, M.M.; Hong, S.C. Prevalence of insomnia and associated factors in south korea. J. Psychosom. Res. 2002, 53, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Heo, S.; Lee, H.; Sul, E.; Han, T.; Kwon, Y.J. Feasibility and efficacy of morning light therapy for adults with insomnia: A pilot, randomized, open-label, two-arm study. Medicina 2023, 59, 1066. [Google Scholar] [CrossRef]
- Chambe, J.; Reynaud, E.; Maruani, J.; Fraih, E.; Geoffroy, P.A.; Bourgin, P. Light therapy in insomnia disorder: A systematic review and meta-analysis. J. Sleep Res. 2023, 32, e13895. [Google Scholar] [CrossRef]
- Figueiro, M.G.; Plitnick, B.; Rea, M.S. The effects of chronotype, sleep schedule and light/dark pattern exposures on circadian phase. Sleep Med. 2014, 15, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, K.V.; Hommes, V. Influence of artificial dusk on sleep. Sleep Biol. Rhythm. 2016, 14, 47–53. [Google Scholar] [CrossRef]
- Fontana Gasio, P.; Kräuchi, K.; Cajochen, C.; Someren, E.; Amrhein, I.; Pache, M.; Savaskan, E.; Wirz-Justice, A. Dawn-dusk simulation light therapy of disturbed circadian rest-activity cycles in demented elderly. Exp. Gerontol. 2003, 38, 207–216. [Google Scholar] [CrossRef]
- Santhi, N.; Thorne, H.C.; van der Veen, D.R.; Johnsen, S.; Mills, S.L.; Hommes, V.; Schlangen, L.J.; Archer, S.N.; Dijk, D.J. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J. Pineal Res. 2012, 53, 47–59. [Google Scholar] [CrossRef]
- Canazei, M.; Weninger, J.; Pohl, W.; Marksteiner, J.; Weiss, E.M. Effects of dynamic bedroom lighting on measures of sleep and circadian rest-activity rhythm in inpatients with major depressive disorder. Sci. Rep. 2022, 12, 6137. [Google Scholar] [CrossRef] [PubMed]
- Terman, M.; Schlager, D.; Fairhurst, S.; Perlman, B. Dawn and dusk simulation as a therapeutic intervention. Biol. Psychiatry 1989, 25, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The phq-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Yun, C.H.; Ahn, J.H.; Suh, S.; Cho, H.J.; Lee, S.K.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Choi, K.M.; et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J. Clin. Endocrinol. Metab. 2015, 100, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- MacLean, A.W.; Fekken, G.C.; Saskin, P.; Knowles, J.B. Psychometric evaluation of the stanford sleepiness scale. J. Sleep Res. 1992, 1, 35–39. [Google Scholar] [CrossRef]
- Lapin, B.R.; Bena, J.F.; Walia, H.K.; Moul, D.E. The epworth sleepiness scale: Validation of one-dimensional factor structure in a large clinical sample. J. Clin. Sleep Med. 2018, 14, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Smits, M.; Spence, W.; Srinivasan, V.; Cardinali, D.P.; Lowe, A.D.; Kayumov, L. Dim light melatonin onset (dlmo): A tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 1–11. [Google Scholar] [CrossRef]
- Figueiro, M.G.; Wood, B.; Plitnick, B.; Rea, M.S. The impact of light from computer monitors on melatonin levels in college students. Neuroendocrinol. Lett. 2011, 32, 158–163. [Google Scholar]
- Cajochen, C.; Munch, M.; Kobialka, S.; Krauchi, K.; Steiner, R.; Oelhafen, P.; Orgul, S.; Wirz-Justice, A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef]
- Scott, J.; Langsrud, K.; Goulding, I.R.; Kallestad, H. Let there be blue-depleted light: In-patient dark therapy, circadian rhythms and length of stay. BJPsych Adv. 2021, 27, 73–84. [Google Scholar] [CrossRef]
- Vethe, D.; Scott, J.; Engstrøm, M.; Salvesen, Ø.; Sand, T.; Olsen, A.; Morken, G.; Heglum, H.S.; Kjørstad, K.; Faaland, P.M. The evening light environment in hospitals can be designed to produce less disruptive effects on the circadian system and improve sleep. Sleep 2021, 44, zsaa194. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J.; Chamberlain, K.; Smith, K.A.; Khalsa, S.B.S.; Rajaratnam, S.M.; Van Reen, E.; Zeitzer, J.M.; Czeisler, C.A.; Lockley, S.W. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 2011, 96, E463–E472. [Google Scholar] [CrossRef] [PubMed]
- Kronauer, R.E.; Forger, D.B.; Jewett, M.E. Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the phototopic range. J. Biol. Rhythm. 1999, 14, 501–516. [Google Scholar] [CrossRef]
- Gronfier, C.; Wright, K.P., Jr.; Kronauer, R.E.; Jewett, M.E.; Czeisler, C.A. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am. J. Physiol.-Endocrinol. Metab. 2004, 287, E174–E181. [Google Scholar] [CrossRef]
- Rimmer, D.W.; Boivin, D.B.; Shanahan, T.L.; Kronauer, R.E.; Duffy, J.F.; Czeisler, C.A. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1574–R1579. [Google Scholar] [CrossRef]
- Dardente, H.; Menet, J.S.; Poirel, V.-J.; Streicher, D.; Gauer, F.; Vivien-Roels, B.; Klosen, P.; Pevet, P.; Masson-Pevet, M. Melatonin induces cry1 expression in the pars tuberalis of the rat. Mol. Brain Res. 2003, 114, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Von Gall, C.; Weaver, D.R.; Moek, J.; Jilg, A.; Stehle, J.H.; KORF, H.W. Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann. N. Y. Acad. Sci. 2005, 1040, 508–511. [Google Scholar] [CrossRef]
- James, F.O.; Boivin, D.B.; Charbonneau, S.; Bélanger, V.; Cermakian, N. Expression of clock genes in human peripheral blood mononuclear cells throughout the sleep/wake and circadian cycles. Chronobiol. Int. 2007, 24, 1009–1034. [Google Scholar] [CrossRef]


| Variable | Overall (n = 40) | Light Therapy Group (n = 20) | Control Group (n = 20) | p-Value |
|---|---|---|---|---|
| Age (year) | 40.0 ± 10.2 | 42.0 ± 9.7 | 37.9 ± 10.5 | 0.207 |
| Sex | 0.235 | |||
| Women | 32 (80.0%) | 18 (90.0%) | 14 (70.0%) | |
| Men | 8 (20.0%) | 2 (10.0%) | 6 (30.0%) | |
| SBP, mmHg | 124.3 ± 14.0 | 123.2 ± 11.6 | 125.3 ± 16.3 | 0.641 |
| DBP, mmHg | 75.5 ± 11.1 | 75.3 ± 10.2 | 75.8 ± 12.2 | 0.900 |
| BMI, kg/m2 | 23.5 ± 3.4 | 24.8 ± 3.5 | 22.2 ± 2.8 | 0.013 |
| Smoking, n (%) | >0.999 | |||
| Yes | 11 (27.5%) | 6 (30.0%) | 5 (25.0%) | |
| No | 29 (72.5%) | 14 (70.0%) | 15 (75.0%) | |
| Drinking *, n (%) | >0.999 | |||
| Yes | 37 (92.5%) | 18 (90.0%) | 19 (95.0%) | |
| No | 3 (7.5%) | 2 (10.0%) | 1 (5.0%) | |
| Exercise †, n (%) | 0.451 | |||
| Yes | 31 (77.5%) | 14 (70.0%) | 17 (85.0%) | |
| No | 9 (22.5%) | 6 (30.0%) | 3 (15.0%) | |
| DM, n (%) | >0.999 | |||
| Yes | 1 (2.5%) | 1 (5.0%) | 0 (0.0%) | |
| No | 39 (97.5%) | 19 (95.0%) | 20 (100.0%) | |
| HTN, n (%) | >0.999 | |||
| Yes | 1 (2.5%) | 0 (0.0%) | 1 (5.0%) | |
| No | 39 (97.5%) | 20 (100.0%) | 19 (95.0%) | |
| Dyslipidemia, n (%) | 0.091 | |||
| Yes | 7 (17.5%) | 6 (30.0%) | 1 (5.0%) | |
| No | 33 (82.5%) | 14 (70.0%) | 19 (95.0%) |
| Variable | Light Therapy Group (n = 20) | Control Group (n = 20) | Difference in Difference (95% CI) † | p-Value † | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Diff | p-Value * | Pre | Post | Diff | p-Value * | |||
| WBC, 103/μL | 6.93 ± 2.2 | 6.5 ± 1.7 | −0.43 ± 1.64 | 0.252 | 5.8 ± 1.4 | 6.2 ± 1.6 | 0.37 ± 1.37 | 0.241 | −0.27 (−1.29, 0.74) | 0.587 |
| Glucose, mg/dL | 97.1 ± 9.3 | 97.2 ± 9.9 | 0.05 ± 11.59 | 0.985 | 92.5 ± 9.5 | 92.9 ± 9.4 | 0.35 ± 13.61 | 0.910 | 4.66 (−1.31, 10.64) | 0.122 |
| Insulin, IU/L | 15.76 ± 18.61 | 15.82 ± 15.19 | 0.06 ± 20.01 | 0.989 | 8.14 ± 3.44 | 9.90 ± 6.15 | 1.77 ± 3.84 | 0.054 | 4.78 (−2.92, 12.47) | 0.216 |
| HOMA−IR | 3.97 ± 5.12 | 4.06 ± 4.53 | 0.09 ± 5.99 | 0.946 | 1.89 ± 0.96 | 2.26 ± 1.33 | 0.37 ± 0.79 | 0.050 | 1.73 (−0.53, 4.00) | 0.130 |
| TC, mg/dL | 182.7 ± 33.2 | 175.6 ± 32.9 | −7.05 ± 19.47 | 0.122 | 178.9 ± 23.2 | 181.0 ± 29.8 | 2.10 ± 19.93 | 0.643 | −13.59 (−28.33, 1.14) | 0.069 |
| TG, mg/dL | 158.4 ± 132.6 | 115.0 ± 38.8 | −43.35 ± 121.42 | 0.127 | 103.0 ± 57.8 | 102.5 ± 52.6 | −0.50 ± 38.58 | 0.954 | −1.95 (−31.69, 27.79) | 0.895 |
| HDL−C, mg/dL | 60.3 ± 14.3 | 60.8 ± 12.0 | 0.50 ± 7.56 | 0.771 | 62.8 ± 12.4 | 64.2 ± 11.4 | 1.40 ± 6.29 | 0.332 | −1.26 (−5.99, 3.47) | 0.590 |
| LDL−C | 109.9 ± 33.8 | 107.4 ± 32.8 | −2.50 ± 19.95 | 0.582 | 110.6 ± 24.5 | 113.6 ± 28.3 | 3.00 ± 18.11 | 0.468 | −11.25 (−24.97, 2.48) | 0.105 |
| CRP, mg/dL | 1.11 ± 1.38 | 0.82 ± 0.46 | −0.29 ± 1.43 | 0.368 | 1.00 ± 1.14 | 1.98 ± 6.05 | 0.98 ± 6.13 | 0.484 | −1.71 (−5.03, 1.61) | 0.303 |
| Cortisol, µg/dL | 9.3 ± 3.6 | 11.6 ± 5.8 | 2.4 ± 5.4 | 0.065 | 10.2 ± 4.1 | 11.1 ± 4.0 | 0.85 ± 4.39 | 0.395 | 2.35 (−1.11, 5.81) | 0.177 |
| ACTH, pg/mL | 23.1 ± 14.8 | 27.9 ± 26.3 | 4.7 ± 29.2 | 0.479 | 23.8 ± 12.5 | 27.3 ± 12.1 | 3.54 ± 13.55 | 0.257 | 0.07 (−14.93, 15.07) | 0.993 |
| Serotonin, ng/mL | 117.0 ± 67.8 | 123.6 ± 63.6 | 4.09 ± 47.85 | 0.746 | 171.4 ± 68.6 | 191.0 ± 106.4 | 19.64 ± 62.25 | 0.174 | −33.05 (−83.63, 17.52) | 0.192 |
| Variable | Light Therapy Group | Control Group | Difference (95% CI) † | p-Value † |
|---|---|---|---|---|
| Sleep efficiency | 84.98 ± 8.47 | 82.11 ± 12.57 | 3.89 (0.52, 7.26) | 0.032 |
| Time in bed | 443.04 ± 105.79 | 454.49 ± 139.25 | −5.62 (−46.05, 34.82) | 0.790 |
| TST | 375.01 ± 94.93 | 368.39 ± 102.27 | 14.15 (−20.94, 49.25) | 0.441 |
| WASO | 61.69 ± 44.39 | 76.91 ± 86.16 | −17.94 (−38.12, 2.25) | 0.095 |
| Awakenings | 19.55 ± 9.46 | 19.84 ± 11.36 | 0.26 (−4.31, 4.82) | 0.915 |
| Average awakenings | 3.27 ± 2.54 | 3.92 ± 3.71 | −0.89 (−1.74, −0.05) | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.; Heo, S.-J.; Lee, H.; Sul, E.-G.; Han, T.; Kwon, Y.-J. Assessing the Feasibility and Efficacy of Pre-Sleep Dim Light Therapy for Adults with Insomnia: A Pilot Study. Medicina 2024, 60, 632. https://doi.org/10.3390/medicina60040632
Yoon J, Heo S-J, Lee H, Sul E-G, Han T, Kwon Y-J. Assessing the Feasibility and Efficacy of Pre-Sleep Dim Light Therapy for Adults with Insomnia: A Pilot Study. Medicina. 2024; 60(4):632. https://doi.org/10.3390/medicina60040632
Chicago/Turabian StyleYoon, Jihyun, Seok-Jae Heo, Hyangkyu Lee, Eun-Gyeong Sul, Taehwa Han, and Yu-Jin Kwon. 2024. "Assessing the Feasibility and Efficacy of Pre-Sleep Dim Light Therapy for Adults with Insomnia: A Pilot Study" Medicina 60, no. 4: 632. https://doi.org/10.3390/medicina60040632
APA StyleYoon, J., Heo, S.-J., Lee, H., Sul, E.-G., Han, T., & Kwon, Y.-J. (2024). Assessing the Feasibility and Efficacy of Pre-Sleep Dim Light Therapy for Adults with Insomnia: A Pilot Study. Medicina, 60(4), 632. https://doi.org/10.3390/medicina60040632





