Healing of Periodontal Suprabony Defects following Treatment with Open Flap Debridement with or without Hyaluronic Acid (HA) Application
Abstract
1. Introduction
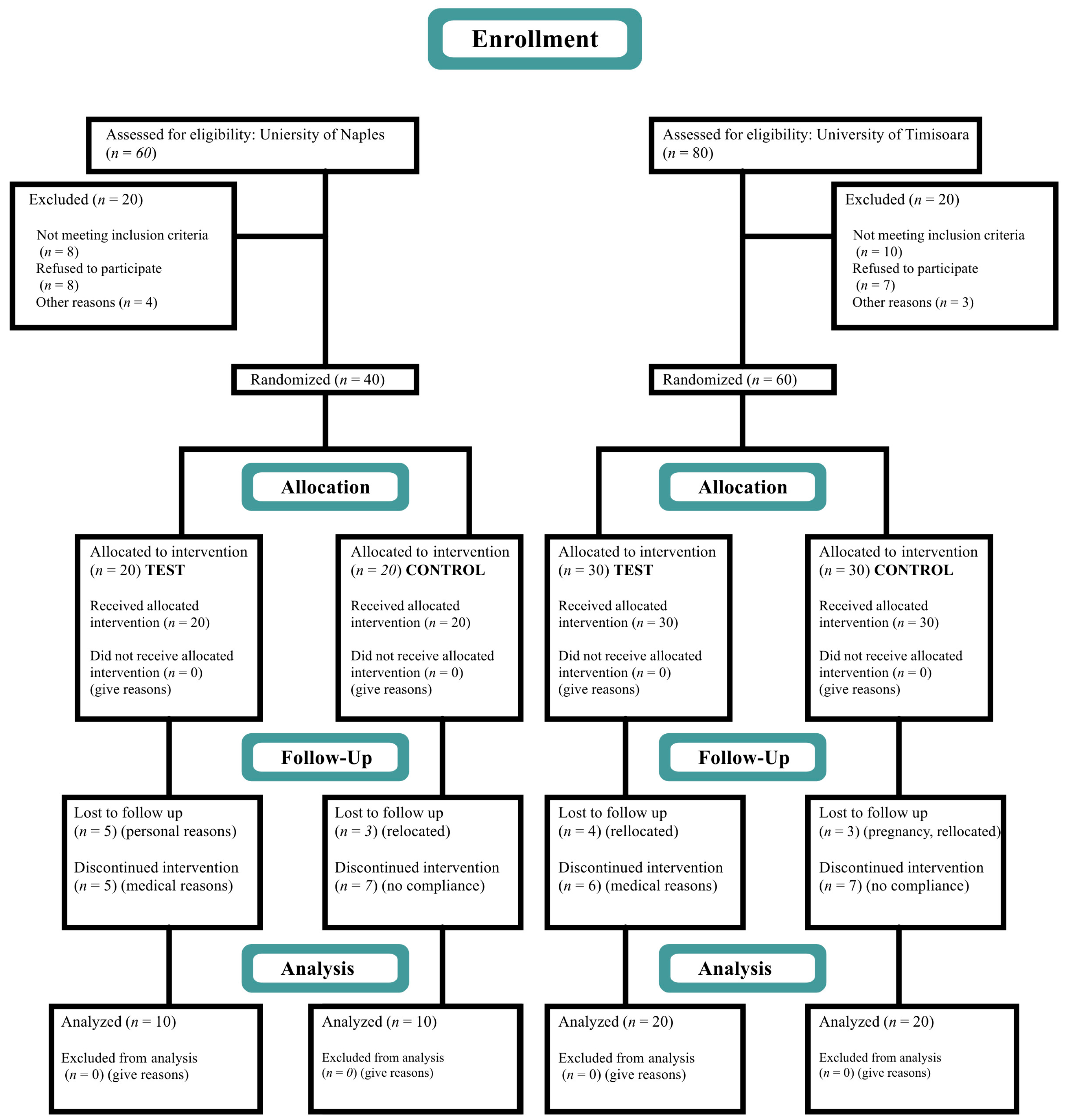
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
- Patients diagnosed with periodontitis stages III and IV, grade A/B [31].
- Age ≥ 18 years old.
- Single-rooted and multi-rooted teeth.
- Patients that did not meet the therapy targets at re-evaluation after completion of step 2 of periodontal therapy with respect to the presence of suprabony periodontal defects (i.e., defects displaying a predominantly horizontal pattern of bone loss) at a minimum of two adjacent teeth and a maximum of seven adjacent teeth in either the maxilla or the mandible, with a PPD ≥ 5 mm.
- Intrabony defect with an intraosseous component < 2 mm.
- Patients with systemic diseases.
- Prolonged antibiotic or anti-inflammatory treatment within 4 weeks before surgery.
- Pregnant or lactating.
- Tobacco smokers (≥10 cigarettes per day).
- Multi-rooted teeth with furcation involvement [34].
- Third molars or severely mispositioned teeth.
- Increased tooth mobility (grade II and III) [35].
2.3. Sample Size Calculation
Randomization
2.4. Blinding and Calibration
2.5. Clinical Measurements
2.6. Periodontal Therapy
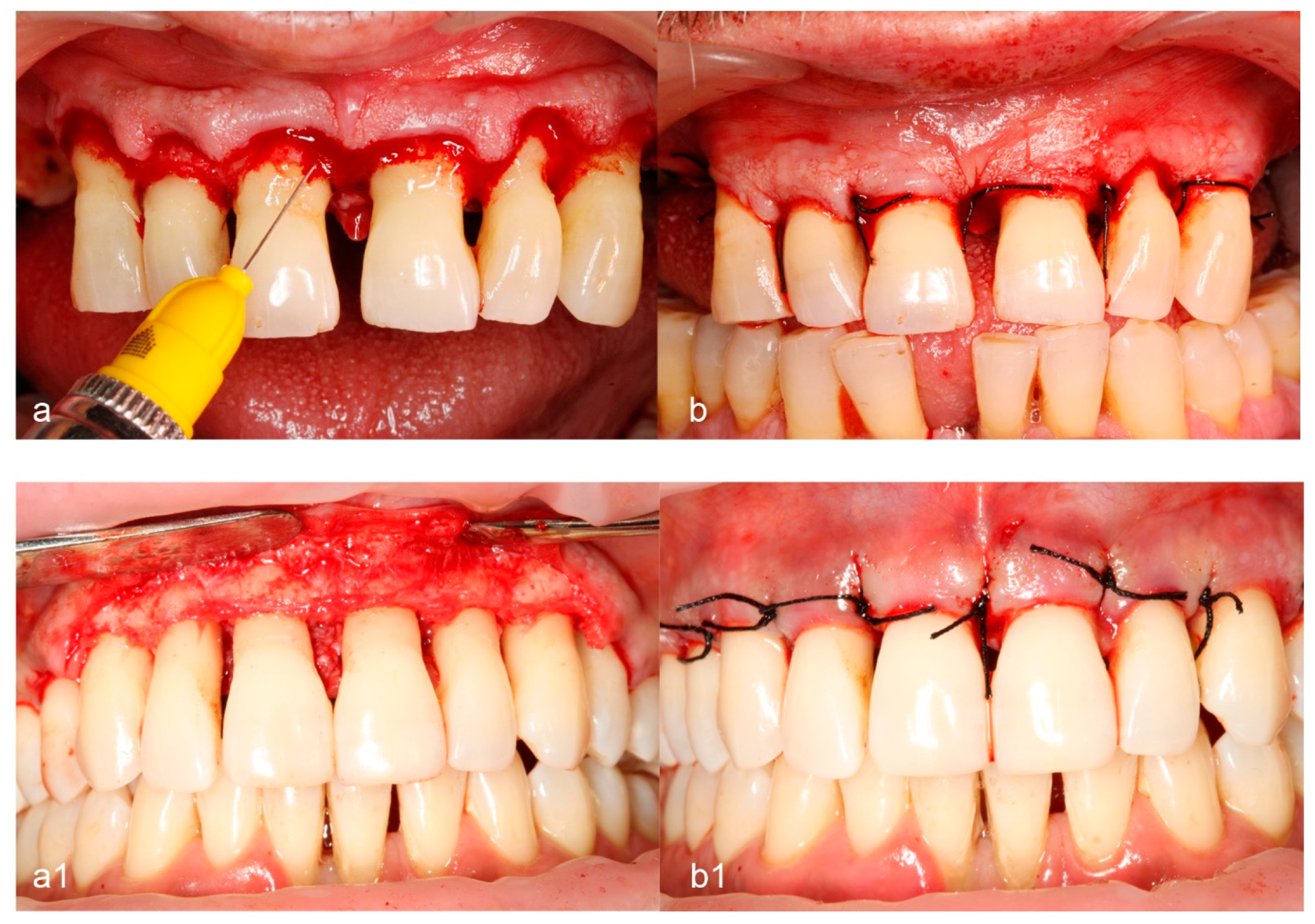
2.7. Surgical Procedure
2.8. Postoperative Care, Follow-Up, and Re-Evaluation
2.9. Outcome Measures
2.10. Statistical Analysis
3. Results
3.1. Study Population
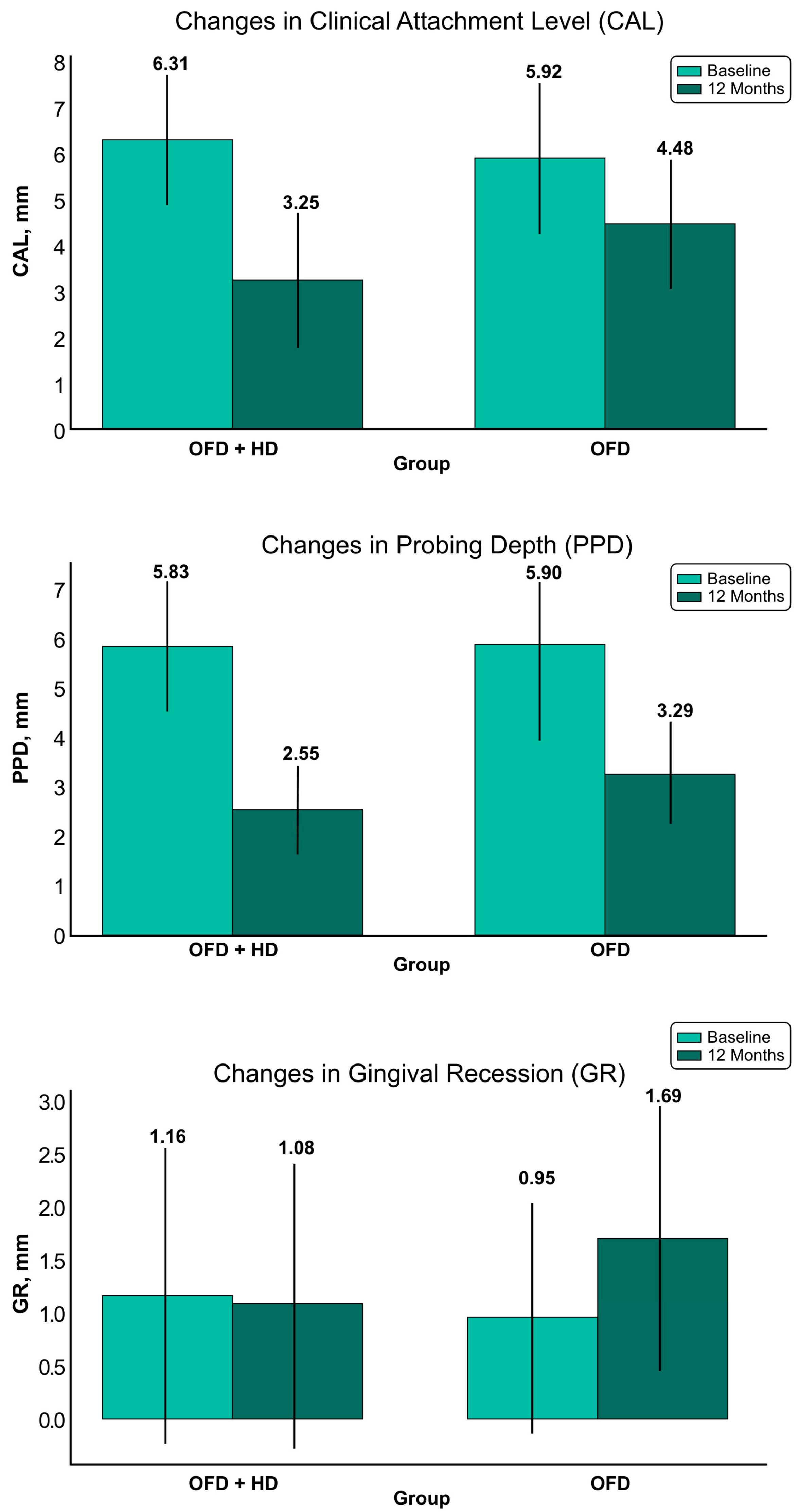
3.2. Changes in Clinical Attachment Level (CAL)
3.3. Changes in Pocket Probing Depth (PPD)
3.4. Changes in the Gingival Recession (GR)
3.5. Full-Mouth Plaque Score (FMPS) and Full-Mouth Bleeding Score (FMBS)
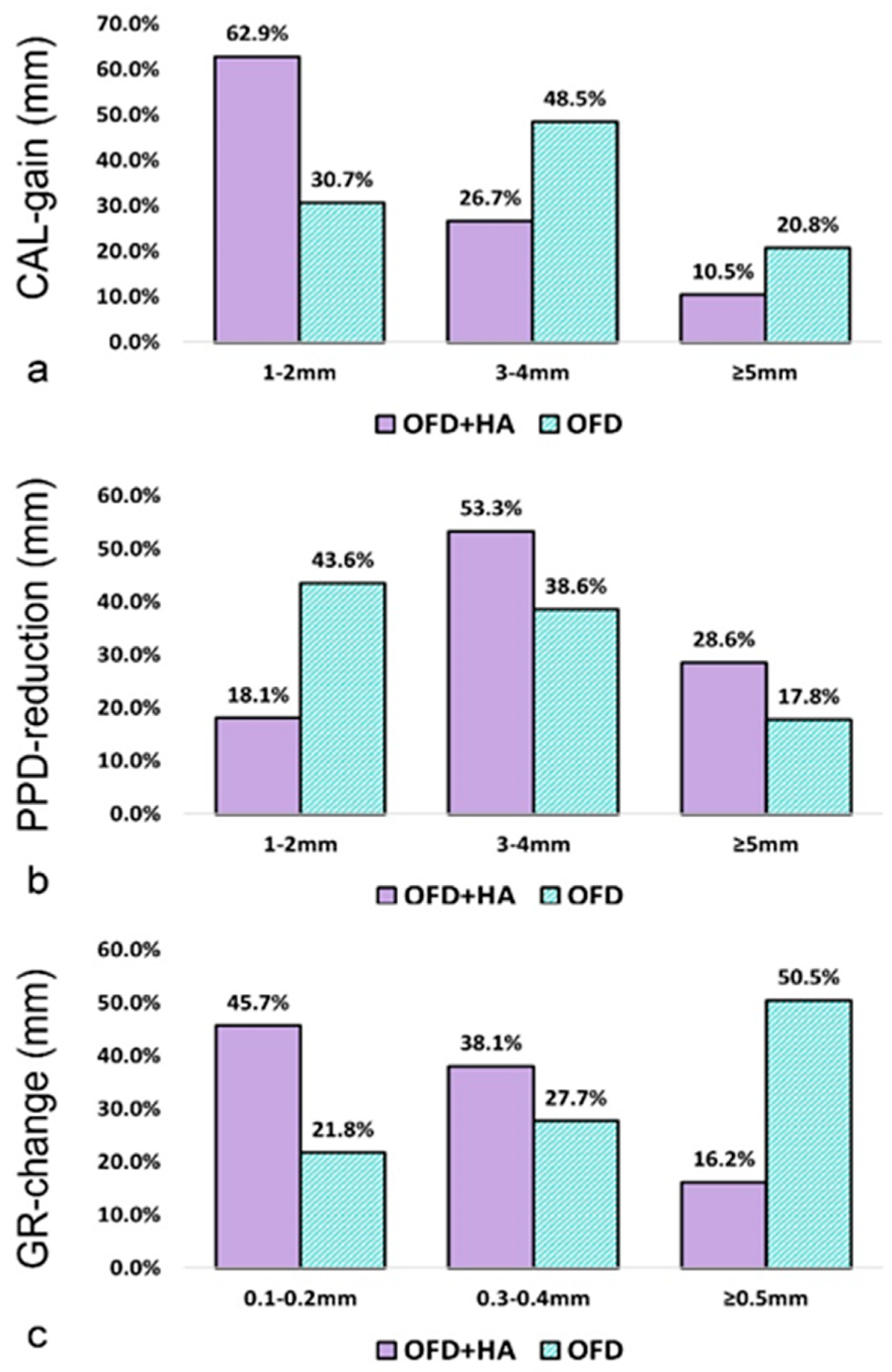
3.6. Frequency Distributions of CAL, PD, and GR Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrarotti, F.; Giraudi, M.; Citterio, F.; Fratini, A.; Gualini, G.; Piccoli, G.M.; Mariani, G.M.; Romano, F.; Aimetti, M. Pocket elimination after osseous resective surgery: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, K.; Sculean, A.; Jepsen, S. Complications and treatment errors related to regenerative periodontal surgery. Periodontology 2000 2023, 92, 120–134. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Position Paper: Periodontal Regeneration. J. Periodontol. 2005, 76, 1601–1622. [CrossRef] [PubMed]
- Wikesjö, U.M.E.; Selvig, K.A. Periodontal wound healing and regeneration. Periodontology 2000 1999, 19, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Iorio-Siciliano, V.; Blasi, A.; Stratul, S.-I.; Ramaglia, L.; Octavia, V.; Salvi, G.E.; Sculean, A. Healing of periodontal suprabony defects following treatment with open flap debridement with or without an enamel matrix derivative: A randomized controlled clinical study. Clin. Oral Investig. 2021, 25, 1019–1027. [Google Scholar] [CrossRef]
- Diehl, D.; Friedmann, A.; Liedloff, P.; Jung, R.M.; Sculean, A.; Bilhan, H. Adjunctive Application of Hyaluronic Acid in Combination with a Sodium Hypochlorite Gel for Non-Surgical Treatment of Residual Pockets Reduces the Need for Periodontal Surgery—Retrospective Analysis of a Clinical Case Series. Materials 2022, 15, 6508. [Google Scholar] [CrossRef]
- Eliezer, M.; Imber, J.-C.; Sculean, A.; Pandis, N.; Teich, S. Hyaluronic acid as adjunctive to non-surgical and surgical periodontal therapy: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 3423–3435. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Zeza, B.; Kuis, D.; Vrazic, D.; Domic, T.; Olszewska-Czyz, I.; Popova, C.; Kotsilkov, K.; Firkova, E.; Dermendzieva, Y.; et al. Treatment of Residual Periodontal Pockets Using a Hyaluronic Acid-Based Gel: A 12 Month Multicenter Randomized Triple-Blinded Clinical Trial. Antibiotics 2021, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, P.; Kamal, R. Hyaluronic acid: A boon in periodontal therapy. N. Am. J. Med. Sci. 2013, 5, 309–315. [Google Scholar] [CrossRef]
- Ialenti, A.; Rosa, M. Hyaluronic acid modulates acute and chronic inflammation. Agents Actions 1994, 43, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Ijuin, C.; Ohno, S.; Tanimoto, K.; Honda, K.; Tanne, K. Regulation of hyaluronan synthase gene expression in human periodontal ligament cells by tumour necrosis factor-α, interleukin-1β and interferon-γ. Arch. Oral Biol. 2001, 46, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Page, R.C. The effect of chronic inflammation on gingival connective tissue proteoglycans and hyaluronic acid. J. Oral Pathol. Med. 1986, 15, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P. Hyaluronan in morphogenesis. Semin. Cell Dev. Biol. 2001, 12, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.J.; Abatangelo, G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999, 7, 79–89. [Google Scholar] [CrossRef]
- Donegan, G.C.; Hunt, J.A.; Rhodes, N. Investigating the importance of flow when utilizing hyaluronan scaffolds for tissue engineering. J. Tissue Eng. Regen. Med. 2010, 4, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.C.; Yoo, M.A.; Lee, S.Y.; Lee, H.J.; Son, D.H.; Jung, J.; Noh, I.; Kim, C. Modulation of biomechanical properties of hyaluronic acid hydrogels by crosslinking agents. J. Biomed. Mater. Res. 2015, 103, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Wang, X.; Qin, L.; Zhai, M.; Yuan, J.; Chen, J.; Li, D. Effect of hyaluronic acid in bone formation and its applications in dentistry. J. Biomed. Mater. Res. 2016, 104, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Oksala, O.; Salo, T.; Tammi, R.; Häkkinen, L.; Jalkanen, M.; Inki, P.; Larjava, H. Expression of proteoglycans and hyaluronan during wound healing. J. Histochem. Cytochem. 1995, 43, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Bertl, K.; Bruckmann, C.; Isberg, P.; Klinge, B.; Gotfredsen, K.; Stavropoulos, A. Hyaluronan in non-surgical and surgical periodontal therapy: A systematic review. J. Clin. Periodontol. 2015, 42, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Guldener, K.; Lanzrein, C.; Eliezer, M.; Katsaros, C.; Stahli, A.; Sculean, A. Treatment of single mandibular recessions with the modified coronally advanced tunnel or laterally closed tunnel, hyaluronic acid, and subepithelial connective tissue graft: A report of 12 cases. Quintessence Int. 2020, 51, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, A.; Tellefsen, M.; Wikesjö, U.; Johannsen, G. Local Delivery of Hyaluronan as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis. J. Periodontol. 2009, 80, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, K.M.F.; Dahaba, M.A.; Aboul-Ela, S.; Darhous, M.S. Local application of hyaluronan gel in conjunction with periodontal surgery: A randomized controlled trial. Clin. Oral Investig. 2012, 16, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.; Fujioka-Kobayashi, M.; Mueller, H.-D.; Lussi, A.; Sculean, A.; Schmidlin, P.R.; Miron, R.J. Effect of hyaluronic acid on morphological changes to dentin surfaces and subsequent effect on periodontal ligament cell survival, attachment, and spreading. Clin. Oral Investig. 2017, 21, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Müller, H.-D.; Mueller, A.; Lussi, A.; Sculean, A.; Schmidlin, P.R.; Miron, R.J. In vitro effects of hyaluronic acid on human periodontal ligament cells. BMC Oral Health 2017, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Lanzrein, C.; Guldener, K.; Imber, J.-C.; Katsaros, C.; Stähli, A.; Sculean, A. Treatment of multiple adjacent recessions with the modified coronally advanced tunnel or laterally closed tunnel in conjunction with cross-linked hyaluronic acid and subepithelial connective tissue graft: A report of 15 cases. Quintessence Int. 2020, 51, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Schmidlin, P.R.; Sahrmann, P.; Sculean, A.; Rojas, M.A. Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: A randomized controlled clinical trial. Clin. Oral Investig. 2019, 23, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, F.; Briguglio, E.; Briguglio, R.; Cafiero, C.; Isola, G. Treatment of infrabony periodontal defects using a resorbable biopolymer of hyaluronic acid: A randomized clinical trial. Quintessence Int. 2013, 44, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Rojas, M.A.; Marini, L.; Russo, P.; Shirakata, Y.; Sculean, A.; Iacono, R. Healing of intrabony defects following regenerative surgery by means of single-flap approach in conjunction with either hyaluronic acid or an enamel matrix derivative: A 24-month randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5095–5107. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Joss, A.; Orsanic, T.; Gusberti, F.A.; Siegrist, B.E. Bleeding on probing. A predictor for the progression of periodontal disease? J. Clin. Periodontol. 1986, 13, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C. Textbook of Periodontia; Blakiston Co.: Philadelphia, PA, USA, 1950. [Google Scholar]
- Laster, L.; Laudenbach, K.W.; Stoller, N.H. An evaluation of clinical tooth mobility measurements. J. Periodontol. 1975, 46, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, H.; Purschwitz, R. A clinical study evaluating the treatment of supra-alveolar-type defects with access flap surgery with and without an enamel matrix protein derivative: A pilot study. J. Clin. Periodontol. 2008, 35, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.M.; Yekta-Michael, S.S.; Schittenhelm, F.; Reichert, S.; Kupietz, D.; Dommisch, H.; Kasaj, A.; Wied, S.; Vela, O.; Stratul, S. Comparison of three full-mouth concepts for the non-surgical treatment of stage III and IV periodontitis: A randomized controlled trial. J. Clin. Periodontol. 2021, 48, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, G.; De Boer, J. Interactively testing remote servers using the Python programming language. CWI Q. 1991, 4, 283–303. [Google Scholar]
- Asparuhova, M.B.; Kiryak, D.; Eliezer, M.; Mihov, D.; Sculean, A. Activity of two hyaluronan preparations on primary human oral fibroblasts. J. Periodontal Res. 2019, 54, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Asparuhova, M.B.; Chappuis, V.; Stahli, A.; Buser, D.; Sculean, A. Role of hyaluronan in regulating self-renewal and osteogenic differentiation of mesenchymal stromal cells and pre-osteoblasts. Clin. Oral Investig. 2020, 24, 3923–3937. [Google Scholar] [CrossRef]
- Al-Shammari, N.M.; Shafshak, S.M.; Ali, M.S. Effect of 0.8% Hyaluronic Acid in Conventional Treatment of Moderate to Severe Chronic Periodontitis. J. Contemp. Dent. Pr. 2018, 19, 527–534. [Google Scholar] [CrossRef]
- Rao, D.P.C.; Vajawat, M.; Kumar, G.S.V.; Rajeshwari, K.G.; Hareesha, M.S. Local delivery of hyaluronic acid as an adjunct to scaling and root planing in the treatment of chronic periodontitis in smokers and non-smokers: A clinical and microbiological study. J. Indian Soc. Periodontol. 2022, 26, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Rajan, P. Hyaluronic Acid as an Adjunct to Scaling and Root Planing in Chronic Periodontitis. A Randomized Clinical Trail. J. Clin. Diagn. Res. 2014, 8, ZC11–ZC14. [Google Scholar] [CrossRef] [PubMed]
- Di Tullio, M.; Femminella, B.; Pilloni, A.; Romano, L.; D’Arcangelo, C.; De Ninis, P.; Paolantonio, M. Treatment of Supra-Alveolar-Type Defects by a Simplified Papilla Preservation Technique for Access Flap Surgery With or Without Enamel Matrix Proteins. J. Periodontol. 2013, 84, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Gontiya, G.; Galgali, S.R. Effect of hyaluronan on periodontitis: A clinical and histological study. J. Indian Soc. Periodontol. 2012, 16, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Mamajiwala, A.S.; Sethi, K.S.; Raut, C.P.; Karde, P.A.; Mamajiwala, B.S. Clinical and radiographic evaluation of 0.8% hy-aluronic acid as an adjunct to open flap debridement in the treatment of periodontal intrabony defects: Randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5257–5271. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Cantore, S.; Capodiferro, S.; Grassi, F.R. Esterified Hyaluronic Acid and Autologous Bone in the Surgical Correction of the Infra-Bone Defects. Int. J. Med. Sci. 2009, 6, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Kuru, B.; Altuna-Kiraç, E. Enamel matrix proteins in the treatment of periodontal sites with horizontal type of bone loss. J. Clin. Periodontol. 2003, 30, 197–206. [Google Scholar] [CrossRef] [PubMed]
| Variable | Test Group (n = 30) | Control Group (n = 30) | p-Value |
|---|---|---|---|
| Gender (male/female) | 18 (m); 12 (f) | 16 (m); 14 (f) | 0.602 a |
| Age (mean ± SD) | 49.8 ± 7.4 | 46.9 ± 8.1 | 0.153 b |
| Tobacco smokers | 8 (26.7%) | 6 (20.0%) | 0.541 a |
| Variable (mm) | Test Group Baseline (n = 105) | Test Group 12 Months (n = 105) | Mean Difference (p-Value) | p-Value Baseline | Control Group Baseline (n = 101) | Control Group 12 Months (n = 101) | Mean Difference (p-Value) | p-Value 12 Months |
|---|---|---|---|---|---|---|---|---|
| CAL (mean ± SD) | 6.31 ± 1.44 | 3.25 ± 1.50 | 3.06 ± 1.13 (<0.0001) | 0.073 | 5.92 ± 1.66 | 4.48 ± 1.42 | 1.44 ± 1.07 (<0.0001) | <0.001 a |
| PPD (mean ± SD) | 5.83 ± 1.31 | 2.55 ± 0.94 | 3.28 ± 1.14 (<0.0001) | 0.699 | 5.90 ± 1.28 | 3.29 ± 1.05 | 2.61 ± 1.22 (<0.0001) | 0.032 a |
| GR (mean ± SD) | 1.16 ± 1.40 | 1.08 ± 1.36 | 0.08 ± 0.76 (=0.6749) | 0.234 | 0.95 ± 1.10 | 1.69 ± 1.25 | 0.74 ± 1.03 (<0.0001) | <0.001 a |
| Baseline | Baseline 95%CI | Follow-Up | Follow-Up 95%CI | Change | Change 95%CI | p-Value | |
|---|---|---|---|---|---|---|---|
| FMPS% (mean ± SD) | |||||||
| TEST | 20.3 ± 2.0 | 19.5 to 22.2 | 19.0 ± 2.0 | 18.1 to 21.0 | 1.37 ± 1.69 | 0.5 to 1.6 | 0.014 a |
| CONTROL | 20.2 ± 2.7 | 19.4 to 23.5 | 19.3 ± 2.0 | 18.5 to 21.9 | 0.93 ± 1.05 | 0.6 to 1.2 | 0.147 a |
| p-value | 0.871 | 0.563 | 0.230 | ||||
| FMBS% (mean ± SD) | |||||||
| TEST | 18.9 ± 1.8 | 18.0 to 19.5 | 16.4 ± 2.1 | 15.2 to 18.0 | 2.50 ± 1.88 | 1.1 to 3.0 | <0.001 a |
| CONTROL | 19.2 ± 2.0 | 18.2 to 21.0 | 17.7 ± 2.4 | 16.3 to 19.3 | 1.50 ± 1.42 | 0.9 to 2.2 | <0.001 a |
| p-value | 0.543 | 0.029 | 0.023 | ||||
| Variable | Test Group Baseline (n = 30) | Test Group 12 Months (n = 30) | Mean Difference (p-Value) | p-Value Baseline | Control Group Baseline (n = 30) | Control Group 12 Months (n = 30) | Mean Difference (p-Value) | p-Value 12 Months |
|---|---|---|---|---|---|---|---|---|
| FMPS% (mean ± SD) | 20.3 ± 2.0 | 19.0 ± 2.0 | 1.37 ± 1.69 (0.014) | 0.871 | 20.2 ± 2.7 | 19.3 ± 2.0 | 0.93 ± 1.05 (0.147) | 0.563 a |
| FMPS range | 0.043 | 0.116 | 0.443 b | |||||
| FMBS% (mean ± SD) | 18.9 ± 1.8 | 16.4 ± 2.1 | 2.50 ± 1.88 (<0.001) | 19.2 ± 2.0 | 17.7 ± 2.4 | 1.50 ± 1.42 (0.010) | 0.029 a | |
| FMBS range | 0.003 | 0.544 | 0.307 | 0.055 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vela, O.C.; Boariu, M.; Rusu, D.; Iorio-Siciliano, V.; Ramaglia, L.; Boia, S.; Radulescu, V.; Ilyes, I.; Stratul, S.-I. Healing of Periodontal Suprabony Defects following Treatment with Open Flap Debridement with or without Hyaluronic Acid (HA) Application. Medicina 2024, 60, 829. https://doi.org/10.3390/medicina60050829
Vela OC, Boariu M, Rusu D, Iorio-Siciliano V, Ramaglia L, Boia S, Radulescu V, Ilyes I, Stratul S-I. Healing of Periodontal Suprabony Defects following Treatment with Open Flap Debridement with or without Hyaluronic Acid (HA) Application. Medicina. 2024; 60(5):829. https://doi.org/10.3390/medicina60050829
Chicago/Turabian StyleVela, Octavia Carolina, Marius Boariu, Darian Rusu, Vincenzo Iorio-Siciliano, Luca Ramaglia, Simina Boia, Viorelia Radulescu, Ioana Ilyes, and Stefan-Ioan Stratul. 2024. "Healing of Periodontal Suprabony Defects following Treatment with Open Flap Debridement with or without Hyaluronic Acid (HA) Application" Medicina 60, no. 5: 829. https://doi.org/10.3390/medicina60050829
APA StyleVela, O. C., Boariu, M., Rusu, D., Iorio-Siciliano, V., Ramaglia, L., Boia, S., Radulescu, V., Ilyes, I., & Stratul, S.-I. (2024). Healing of Periodontal Suprabony Defects following Treatment with Open Flap Debridement with or without Hyaluronic Acid (HA) Application. Medicina, 60(5), 829. https://doi.org/10.3390/medicina60050829







