Intestinal Clear Cell Sarcoma—A Case Presentation of an Extremely Rare Tumor and Literature Review
Abstract
1. Introduction
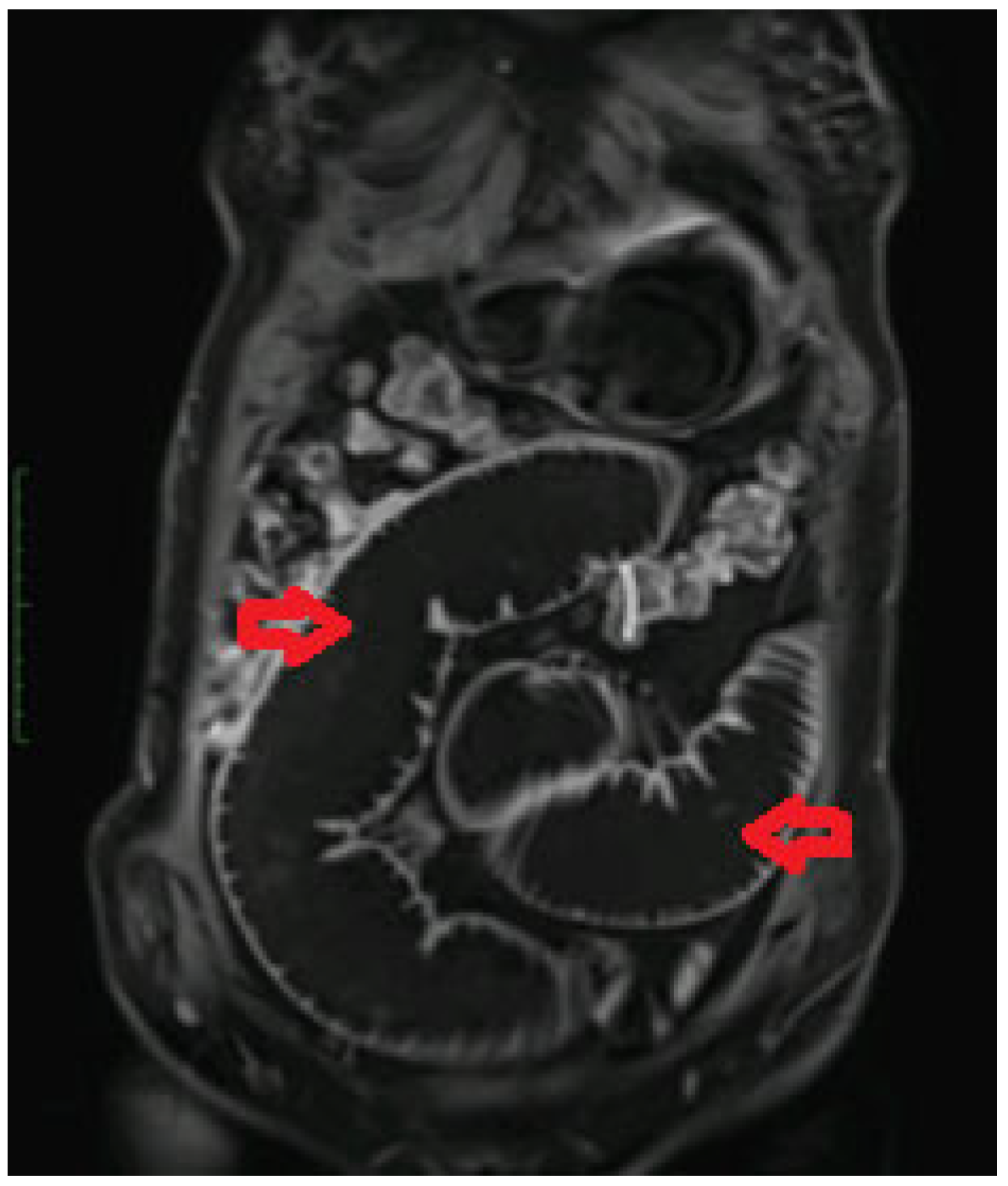
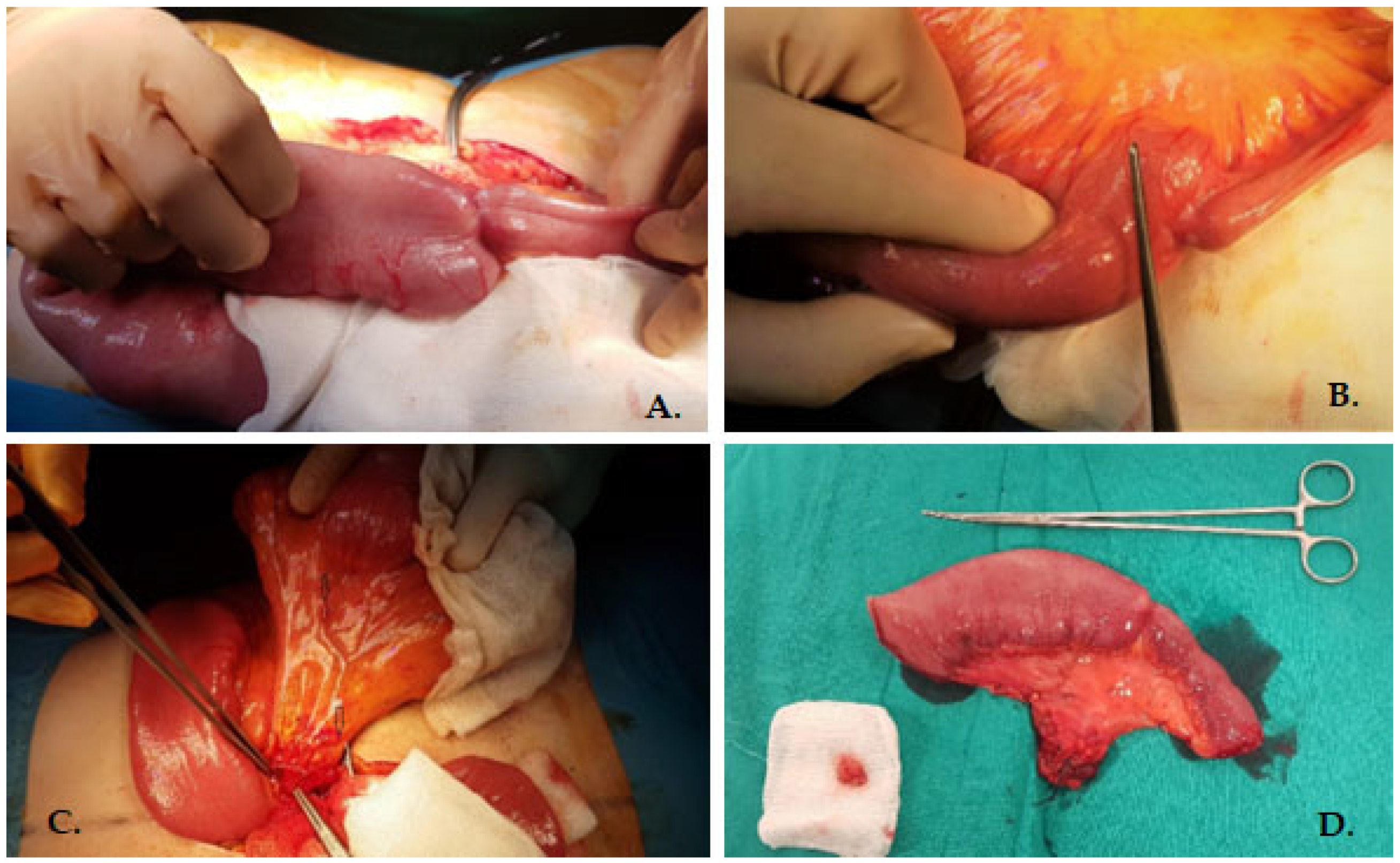
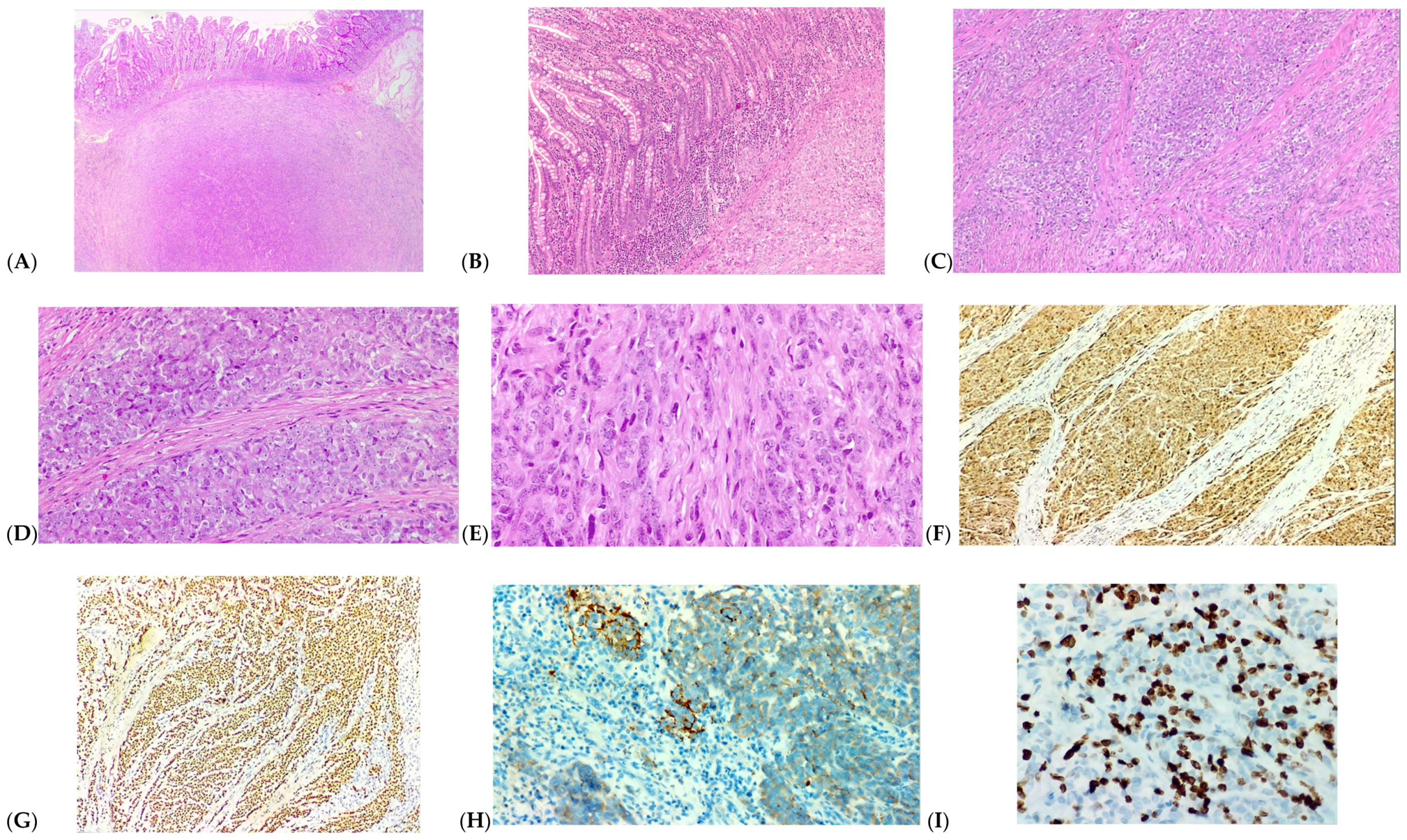
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCS | Clear cell sarcoma |
| ATF1 | Activating transcription factor 1 |
| EWSR1 | Ewing sarcoma breakpoint region 1 |
| CREB1 | cAMP responsive element binding protein 1 |
| MM | Malignant melanoma |
| HMB-45 | Human melanoma black 45 antibody |
| MiTF | Microphthalmia transcription factor |
| S100 | Calcium binding protein |
| Melan-A | Melanocyte antigen |
| FISH | Fluorescence in situ hybridization |
| RT-PCR | Real-time polymerase chain reaction |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| MPNST | Malignant peripheral nerve sheath tumor |
| GNET | Gastrointestinal neuroectodermal tumors |
| CT | Computer tomography |
| MRI | Magnetic resonance imaging |
| CA19-9 | Carbohydrate antigen 19-9 |
| CEA | Carcinoembryonic antigen |
| CA125 | Carbohydrate antigen 125 |
| CA15-3 | Carbohydrate antigen 15-3 |
| CA72-4 | Carbohydrate antigen 72-4 |
| AFP | Alpha fetoprotein |
| NSE | Neuron-specific enolase |
| SCC | Subfraction of tumor-associated antigens related to squamous cell carcinoma |
| CD99 | Cluster of differentiation 99 |
| CD15 | Cluster of differentiation 15 |
| CD56 | Cluster of differentiation 56 |
| SOX100 | Sry-related HMg-Box gene 10 |
| MART-1 | Melanocyte antigen (also called Melan-A) |
| DOG-1 gene | “Discovered on GIST 1” gene |
| CD34 | Cluster of differentiation 34 |
| CDX-2 | Caudal-type homeobox 2 |
| CKIT/CD117 | Receptor for tyrosine kinase |
| Ki67 | Proliferation index |
| HE | Hematoxylin and eosin stain |
| Ab | Antibody |
| PET-CT | Positron emission tomography |
| NGS | Next-generation sequencing |
| VUS | Genetic variants of unknown significance |
| MYC gene | Myelocytomatosis oncogene |
| STAG2 gene | Stromal antigen 2 gene |
| DFS | Disease-free survival |
| OS | Overall survival |
| ESMO | European Society of Medical Oncology |
| NCCN | National Comprehensive Cancer Network |
| MET | Mesenchymal epithelial transition—tyrosine kinase receptor |
References
- Haouimi, A. Clear cell sarcoma of soft tissue-neck. RadiopaediaOrg 2023. Available online: https://radiopaedia.org/cases/clear-cell-sarcoma-of-soft-tissue-neck (accessed on 15 May 2024). [CrossRef]
- Elousrouti, L.T.; Hammas, N.; Elmernissi, F.Z.; Elfatemi, H.; Chbani, L. Clear-Cell Sarcoma with an Unusual Presentation Mimicking Metastatic Melanoma. Cureus 2022, 14, e32010. [Google Scholar] [CrossRef]
- Cassalia, F.; Cavallin, F.; Danese, A.; Del Fiore, P.; Di Prata, C.; Rastrelli, M.; Fortina, A.B.; Mocellin, S. Soft Tissue Sarcoma Mimicking Melanoma: A Systematic Review. Cancers 2023, 15, 3584. [Google Scholar] [CrossRef]
- Kazakos, C.J.; Galanis, V.G.; Giatromanolaki, A.; Verettas, D.-A.J.; Sivridis, E. Clear cell sarcoma of the scapula. A case report and review of the literature. World J. Surg. Oncol. 2006, 4, 48. [Google Scholar] [CrossRef]
- Suehara, Y.; Yazawa, Y.; Hitachi, K.; Terakado, A. Clear cell sarcoma arising from the chest wall: A case report. J. Orthop. Sci. 2004, 9, 171–174. [Google Scholar] [CrossRef]
- Clark, M.; Johnson, M.; Thway, K.; Fisher, C.; Thomas, J.; Hayes, A. Clear cell sarcoma (melanoma of soft parts): The Royal Marsden Hospital experience. Eur. J. Surg. Oncol. 2008, 34, 800–804. [Google Scholar] [CrossRef]
- Gonzaga, M.I.; Grant, L.; Curtin, C.; Gootee, J.; Silberstein, P.; Voth, E. The epidemiology and survivorship of clear cell sarcoma: A National Cancer Database (NCDB) review. J. Cancer Res. Clin. Oncol. 2018, 144, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-B.; Jiang, B.-J.; Wang, H.-H.; Yang, Y.-S.; Zhang, X.-B.; Lan, G.-H.; Shu, W.-B. Prognostic Factors for Survival in Patients with Clear Cell Sarcoma: An Analysis of the Surveillance, Epidemiology, and End Results (SEER) Database. Med. Sci. Monit. 2019, 25, 6950–6956. [Google Scholar] [CrossRef]
- Simion, L.; Rotaru, V.; Cirimbei, C.; Gales, L.; Stefan, D.-C.; Ionescu, S.-O.; Luca, D.; Doran, H.; Chitoran, E. Inequities in Screening and HPV Vaccination Programs and Their Impact on Cervical Cancer Statistics in Romania. Diagnostics 2023, 13, 2776. [Google Scholar] [CrossRef] [PubMed]
- Simion, L.; Rotaru, V.; Cirimbei, C.; Stefan, D.-C.; Gherghe, M.; Ionescu, S.; Tanase, B.C.; Luca, D.C.; Gales, L.N.; Chitoran, E. Analysis of Efficacy-to-Safety Ratio of Angiogenesis-Inhibitors Based Therapies in Ovarian Cancer: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 1040. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martin, M.; Saez-Rodriguez, M.; Esquivel, B.; Gonzalez, R.S.; Cabrera, A.N.; Herrera, A.M. Clear cell sarcoma: A case mimicking primary cutaneous malignant melanoma. Indian J. Dermatol. 2009, 54, 168–172. [Google Scholar] [CrossRef]
- Erkizan, H.V.; Kong, Y.; Merchant, M.; Schlottmann, S.; Barber-Rotenberg, J.S.; Yuan, L.; Abaan, O.D.; Chou, T.-H.; Dakshanamurthy, S.; Brown, M.L.; et al. A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing's sarcoma. Nat. Med. 2009, 15, 750–756. [Google Scholar] [CrossRef]
- Li, Y.-J.; Zhao, X.; Vecchiarelli-Federico, L.M.; Datti, A.; Cheng, Y.; Ben-David, Y. Drug-mediated inhibition of Fli-1 for the treatment of leukemia. Blood Cancer J. 2012, 2, e54. [Google Scholar] [CrossRef]
- Davis, I.J.; McFadden, A.W.; Zhang, Y.; Coxon, A.; Burgess, T.L.; Wagner, A.J.; Fisher, D.E. Identification of the receptor tyrosine kinase c-Met and its ligand, hepatocyte growth factor, as therapeutic targets in clear cell sarcoma. Cancer Res 2010, 70, 639–645. [Google Scholar] [CrossRef]
- Davis, I.J.; Kim, J.J.; Ozsolak, F.; Widlund, H.R.; Rozenblatt-Rosen, O.; Granter, S.R.; Du, J.; Fletcher, J.A.; Denny, C.T.; Lessnick, S.L.; et al. Oncogenic MITF dysregulation in clear cell sarcoma: Defining the MiT family of human cancers. Cancer Cell 2006, 9, 473–484. [Google Scholar] [CrossRef]
- McGill, G.G.; Haq, R.; Nishimura, E.K.; Fisher, D.E. c-Met expression is regulated by Mitf in the melanocyte lineage. J. Biol. Chem. 2006, 281, 10365–10373. [Google Scholar] [CrossRef]
- Beuret, L.; Flori, E.; Denoyelle, C.; Bille, K.; Busca, R.; Picardo, M.; Bertolotto, C.; Ballotti, R. Up-regulation of MET expression by alpha-melanocyte-stimulating hormone and MITF allows hepatocyte growth factor to protect melanocytes and melanoma cells from apoptosis. J. Biol. Chem. 2007, 282, 14140–14147. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Grosso, F.; Negri, T.; Palassini, E.; Morosi, C.; Pilotti, S.; Gronchi, A.; Casali, P.G. Tumor response to sunitinib malate observed in clear-cell sarcoma. Ann. Oncol. 2010, 21, 1130–1131. [Google Scholar] [CrossRef] [PubMed]
- Tazzari, M.; Palassini, E.; Vergani, B.; Villa, A.; Rini, F.; Negri, T.; Colombo, C.; Crippa, F.; Morosi, C.; Casali, P.G.; et al. Melan-A/MART-1 immunity in a EWS-ATF1 translocated clear cell sarcoma patient treated with sunitinib: A case report. BMC Cancer 2015, 15, 58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Subbiah, V.; Holmes, O.; Gowen, K.; Spritz, D.; Amini, B.; Wang, W.-L.; Schrock, A.B.; Meric-Bernstam, F.; Zinner, R.; Piha-Paul, S.; et al. Activity of c-Met/ALK Inhibitor Crizotinib and Multi-Kinase VEGF Inhibitor Pazopanib in Metastatic Gastrointestinal Neuroectodermal Tumor Harboring EWSR1-CREB1 Fusion. Oncology 2016, 91, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Donner, L.R.; Trompler, R.A.; Dobin, S. Clear cell sarcoma of the ileum: The crucial role of cytogenetics for the diagnosis. Am. J. Surg. Pathol. 1998, 22, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Kakihara, T.; Baba, K.; Yamaki, T.; Yamaguchi, T.; Suzuki, T. Clear cell sarcoma arising in the transverse colon. Pathol. Int. 2000, 50, 412–416. [Google Scholar] [CrossRef]
- Pauwels, P.; Debiec-Rychter, M.; Sciot, R.; Vlasveld, T.; Butter, B.D.; Hagemeijer, A.; Hogendoorn, P.C.W. Clear cell sarcoma of the stomach. Histopathology 2002, 41, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, E.; Reyes-Mugica, M.; Franchi, A.; Rosai, J. An osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: Reports of 6 cases of a GIST simulator. Int. J. Surg. Pathol. 2003, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Achten, R.; Debiec-Rychter, M.; De Wever, I.; Sciot, R. An unusual case of clear cell sarcoma arising in the jejunum highlights the diagnostic value of molecular genetic techniques in establishing a correct diagnosis. Histopathology 2005, 46, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, G.; Quinn, A.M.; Williams, J.; Hammadeh, R. Clear cell sarcoma of the small bowel: A potential pitfall. APMIS 2005, 113, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Covinsky, M.; Gong, S.; Rajaram, V.; Perry, A.; Pfeifer, J. EWS-ATF1 fusion transcripts in gastrointestinal tumors previously diagnosed as malignant melanoma. Hum. Pathol. 2005, 36, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Taminelli, L.; Zaman, K.; Gengler, C.; Peloponissios, N.; Bouzourene, H.; Coindre, J.-M.; Hostein, I.; Guillou, L. Primary clear cell sarcoma of the ileum: An uncommon and misleading site. Virchows Arch. 2005, 447, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, N.; Testi, M.A.; Moiraghi, L.; Modena, P.; Paggen, E.; Plötner, A.; Wiechmann, V.; Mantovani-Löffler, L.; Merkelbach-Bruse, S.; Buettner, R.; et al. Clear cell sarcoma-like tumor with osteoclast-like giant cells in the small bowel: Further evidence for a new tumor entity. Int. J. Surg. Pathol. 2005, 13, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, X.; Li, D.; Chen, J.; Meng, K.; Wang, Y.; Lu, Z.; Zhou, X. Osteoclast-rich tumor of the gastrointestinal tract with features resembling those of clear cell sarcoma of soft parts. Virchows Arch. 2005, 448, 200–203. [Google Scholar] [CrossRef]
- Antonescu, C.R.; Nafa, K.; Segal, N.H.; Cin, P.D.; Ladanyi, M. EWS-CREB1: A Recurrent Variant Fusion in Clear Cell Sarcoma—Association with Gastrointestinal Location and Absence of Melanocytic Differentiation. Clin. Cancer Res. 2006, 12, 5356–5362. [Google Scholar] [CrossRef]
- Granville, L.; Hicks, J.; Popek, E.; Dishop, M.; Tatevian, N.; Lopez-Terrada, D. Visceral Clear Cell Sarcoma of Soft Tissue with Confirmation by EWS-ATF1 Fusion Detection. Ultrastruct. Pathol. 2006, 30, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Comin, C.E.; Novelli, L.; Tornaboni, D.; Messerini, L. Clear cell sarcoma of the ileum: Report of a case and review of literature. Virchows Arch. 2007, 451, 839–845. [Google Scholar] [CrossRef]
- Abdulkader, I.; Cameselle-Teijeiro, J.; de Alava, E.; Ruiz-Ponte, C.; Used-Aznar, M.M.; Forteza, J. Intestinal clear cell sarcoma with melanocytic differentiation and EWS [corrected] rearrangement: Report of a case. Int. J. Surg. Pathol. 2008, 16, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Lyle, P.L.; Amato, C.M.; Fitzpatrick, J.E.; Robinson, W.A. Gastrointestinal melanoma or clear cell sarcoma? Molecular evaluation of 7 cases previously diagnosed as malignant melanoma. Am. J. Surg. Pathol. 2008, 32, 858–866. [Google Scholar] [CrossRef]
- Lagmay, J.P.; Ranalli, M.; Arcila, M.; Baker, P. Clear cell sarcoma of the stomach. Pediatr. Blood Cancer 2009, 53, 214–216. [Google Scholar] [CrossRef]
- Joo, M.; Chang, S.H.; Kim, H.; Gardner, J.M.; Ro, J.Y. Primary gastrointestinal clear cell sarcoma: Report of 2 cases, one case associated with IgG4-related sclerosing disease, and review of literature. Ann. Diagn. Pathol. 2009, 13, 30–35. [Google Scholar] [CrossRef]
- Terazawa, K.; Otsuka, H.; Morita, N.; Yamashita, K.; Nishitani, H. Clear-cell sarcoma of the small intestine detected by FDG-PET/CT during comprehensive examination of an inflammatory reaction. J. Med. Investig. 2009, 56, 70–75. [Google Scholar] [CrossRef][Green Version]
- Shenjere, P.; Salman, W.D.; Singh, M.; Mangham, D.C.; Williams, A.; Eyden, B.P.; Howard, N.; Knight, B.; Banerjee, S.S. Intra-abdominal clear-cell sarcoma: A report of 3 cases, including 1 case with unusual morphological features, and review of the literature. Int. J. Surg. Pathol. 2011, 20, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Balkaransingh, P.; Saad, S.A.; Govil, S.C.; Thind, P.K.; Ballance, C.M.; Weiss, A.R. Clear cell sarcoma of the gastrointestinal tract presenting as a second malignant neoplasm following neuroblastoma in infancy. Pediatr. Blood Cancer 2011, 58, 481–482. [Google Scholar] [CrossRef]
- Yang, J.C.; Chou, A.J.; Oeffinger, K.C.; La Quaglia, M.P.; Wolden, S.L. Clear cell sarcoma of the gastrointestinal tract after very low-dose therapeutic radiation therapy: A case report. J. Pediatr. Surg. 2012, 47, 1943–1945. [Google Scholar] [CrossRef] [PubMed]
- Stockman, D.L.; Miettinen, M.; Suster, S.; Spagnolo, D.M.; Dominguez-Malagon, H.; Hornick, J.L.; Adsay, V.; Chou, P.M.; Amanuel, B.M.; VanTuinen, P.; et al. Malignant gastrointestinal neuroectodermal tumor: Clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am. J. Surg. Pathol. 2012, 36, 857–868. [Google Scholar] [CrossRef]
- Suárez-Vilela, D.; Izquierdo, F.M.; Tojo-Ramallo, S.; Riera-Velasco, J.R.; Escobar-Stein, J. Malignant gastrointestinal neuroectodermal tumor showing overlapped immunophenotype with synovial sarcoma: CD99 and SOX10 antibodies are useful in differential diagnosis. Am. J. Surg. Pathol. 2012, 36, 1905–1908. [Google Scholar] [CrossRef] [PubMed]
- D’amico, F.E.; Ruffolo, C.; Romeo, S.; Massani, M.; Tos, A.P.D.; Bassi, N. Clear cell sarcoma of the ileum: Report of a case and review of the literature. Int. J. Surg. Pathol. 2011, 20, 401–406. [Google Scholar] [CrossRef]
- Lasithiotakis, K.; Protonotarios, A.; Lazarou, V.; Tzardi, M.; Chalkiadakis, G. Clear cell sarcoma of the jejunum: A case report. World J. Surg. Oncol. 2013, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, Q.; Bu, H.; Chen, M.; Chen, H.; Lin, Y.; Zhang, H. Clear cell sarcoma of gastrointestinal tract: Clinicopathologic analyses and review of literatures. Chin. J. Clin. Exp. Pathol. 2014, 30, 383–388. [Google Scholar] [CrossRef]
- Kong, J.; Li, N.; Wu, S.; Guo, X.; Gu, C.; Feng, Z. Malignant gastrointestinal neuroectodermal tumor: A case report and review of the literature. Oncol. Lett. 2014, 8, 2687–2690. [Google Scholar] [CrossRef]
- Liu, C.; Ren, Y.; Li, X.; Cao, Y.; Chen, Y.; Cui, X.; Li, L.; Li, F. Absence of 19 known hotspot oncogenic mutations in soft tissue clear cell sarcoma: Two cases report with review of the literature. Int. J. Clin. Exp. Pathol. 2014, 7, 5242–5249. [Google Scholar] [PubMed]
- Thway, K.; Judson, I.; Fisher, C. Clear Cell Sarcoma-Like Tumor of the Gastrointestinal Tract, Presenting as a Second Malignancy after Childhood Hepatoblastoma. Case Rep. Med. 2014, 2014, 984369. [Google Scholar] [CrossRef]
- Huang, J.; Luo, R.-K.; Du, M.; Zeng, H.-Y.; Chen, L.-L.; Ji, Y. Clear cell sarcoma of the pancreas: A case report and review of literature. Int. J. Clin. Exp. Pathol. 2015, 8, 2171–2175. [Google Scholar]
- Yegen, G.; Güllüoğlu, M.; Mete, Ö.; Önder, S.; Kapran, Y. Clear cell sarcoma-like tumor of the gastrointestinal tract: A case report and review of the literature. Int. J. Surg. Pathol. 2014, 23, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Raskin, G.A.; Pozharisski, K.M.; Iyevleva, A.G.; Rikov, I.V.; Orlova, R.V.; Imyanitov, E.N. Unusual Clinical Presentation of Gastrointestinal Clear Cell Sarcoma. Gastrointest. Tumors 2015, 2, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Moslim, M.A.; Falk, G.A.; Cruise, M.; Morris-Stiff, G. Simultaneous Clear Cell Sarcomas of the Duodenum and Jejunum. Case Rep. Med. 2016, 2016, 1534029. [Google Scholar] [CrossRef] [PubMed]
- Ardakani, A.G.; Boyle, D.; Elton, C. Gastrointestinal clear cell sarcoma-like tumour of the ascending colon. Ann. R. Coll Surg. Engl. 2016, 98, e37–e39. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Liu, W.-S.; Ren, W.-H.; Wang, P.; Shi, L.; Zhou, H.-T. Multiple clear-cell sarcomas of small intestine with parotid gland metastasis: A case report. World J. Gastroenterol. 2017, 23, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Ichihara, S.; Gotoda, H.; Muraoka, S.; Kubo, T.; Sugita, S.; Hasegawa, T. Imprint cytology of clear cell sarcoma-like tumor of the gastrointestinal tract in the small intestine: A case report. Diagn. Cytopathol. 2017, 45, 1137–1141. [Google Scholar] [CrossRef]
- Askan, G.; Kombak, F.E.; Seven, I.E.; Basturk, O. Clear Cell Sarcoma-Like Tumor of the Gastrointestinal Tract. J. Gastrointest. Cancer 2018, 50, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Hirano, Y.; Ishikawa, S.; Kondo, H.; Ishii, T.; Yamaguchi, S. A long-term survivor of clear cell sarcoma-like tumor of the gastrointestinal tract with liver metastasis: A case report. Surg. Case Rep. 2020, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Zhang, T.; Bi, K.; Wu, Y.; Chen, X.; Zhang, H.; Huang, D.; Zhang, L.; Zeng, Y.; Yi, X. Primary Clear Cell Sarcoma of the Ileum: A Case Report With Next-Generation Sequencing Analysis. Int. J. Surg. Pathol. 2021, 29, 677–684. [Google Scholar] [CrossRef]
- Huang, W.-P.; Li, L.-M.; Gao, J.-B. Postoperative multiple metastasis of clear cell sarcoma-like tumor of the gastrointestinal tract in adolescent: A case report. World J. Clin. Cases 2022, 10, 6175–6183. [Google Scholar] [CrossRef]
- Njima, M.; Lahbacha, B.; Ben Jabra, S.; Moussa, A.; Bellalah, A.; Ben Abdeljelil, N.; Ben Hammouda, S.; Njim, L.; Hadhri, R.; Zakhama, A. Small Intestine Gastrointestinal Clear Cell Sarcoma: A Case Report and Review of the Literature. J. Investig. Med. High Impact Case Rep. 2024, 12, 23247096231225869. [Google Scholar] [CrossRef] [PubMed]
| Record | Year | Age | Sex | Location | S-100 | HMB-45 | Melan-A | Other IHC Findings | Genetic Findings | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Donner [21] | 1998 | 37 | M | Ileum | + | − | ND | EWSR1-ATF1 | Liver metastasis at 24 and 36 months | |
| Fukuda [22] | 2000 | 74 | M | Colon | + | + | ND | EWSR1-ATF1 | Liver metastasis at 9 months | |
| Pauwels [23] | 2002 | 30 | M | Stomach | + | − | − | + for vimentin, NSE, CD99 − for cytokeratins, EMA, CD34, CD117, SMA, desmin | EWSR1-ATF1 | LN and peritoneal metastasis at diagnosis; AWD at 18 months |
| Zambrano [24] | 2003 | 15 | F | Jejunum | + | − | − | − for CD117, CD34 | EWSR1-ATF1 | DOD 16 months |
| Achten [25] | 2005 | 57 | M | Jejunum | + | + | + | + tyrosinase − for cytokeratins, EMA, chromogranin, CD3, CD117 | EWSR1 rearrangements | NS |
| Venkataraman [26] | 2005 | 21 | F | Ileum | + | − | − | − for SMA, tyrosinase, CD34, CD117 | EWSR1-ATF1 | NS |
| Covinsky [27] | 2005 | 47 | F | Pancreas | + | + | + | EWSR1-ATF1 | NED after 24 months | |
| 85 | F | Small intestine | + | + | + | EWSR1-ATF1 | DOD at 1 month | |||
| Taminelli [28] | 2005 | 35 | M | Ileum | + | − | + | + tyrosinase − CD117, cytokeratins, EMA, SMA, desmin, CD31, CD34, chromogranin, synaptophysin | EWSR1-ATF1 | Liver metastasis at 2 months DOD 15 months |
| Friedrichs [29] | 2005 | 41 | M | Jejunum | + | − | − | + vimentin, beta-catenine, CD68, PDFG-R alfa − for CD117, CD34, desmin, SMA, chromogranin, synaptophysin, NSE | EWSR1 rearrangements | Liver metastasis at 6 months |
| Huang [30] | 2006 | 40 | M | Stomach | + | − | − | − for CD117, CD34, vimentin, SMA, synaptophysin | EWSR1-ATF1 | NS |
| Antonescu [31] | 2006 | 81 | F | Colon | + | − | − | EWSR1-CREB1 | Liver and peritoneal metastasis at 60 months | |
| 42 | F | Ileum | + | − | − | EWSR1-CREB1 | NS | |||
| 42 | F | Ileum | + | − | − | EWSR1-CREB1 | Liver and peritoneal metastasis at diagnosis | |||
| Granville [32] | 2005 | 16 | M | Ileum | + | − | ND | − for pancytokeratin, CD3, CD34, CD117, EMA, desmin, SMA | EWSR1-ATF1 | DOD 15 months |
| Comin [33] | 2007 | 31 | F | Ileum | + | − | − | − for tyrosinase, cytokeratins, EMASMA, CD34, CD31, CD117, CD99, Synaptophysin, Chromogranin A | EWSR1-ATF1 | NS |
| Abdulkader [34] | 2008 | 37 | M | Jejunum | + | + | ND | + PDGF-R alfa, EMA, NSE, vimentine − CD34, CD117 | EWSR1 rearrangement | Liver metastasis at 2 months |
| Lyle [35] | 2008 | 46 | M | Jejunum | + | + | + | EWSR1-ATF1 | NED 7 months | |
| 48 | M | Cecum | + | + | + | EWSR1-ATF1 | DOD 2 months | |||
| 60 | M | Jejunum | + | + | + | EWSR1-ATF1 | DOD 28 months | |||
| 62 | M | Ileum | + | + | + | EWSR1-ATF1 | DOD 12 months | |||
| Lagmay [36] | 2009 | 10 | F | Stomach | + | − | − | EWSR1-ATF1 | NED 4 months | |
| Joo [37] | 2009 | 60 | M | Ileum | + | − | − | EWSR1 rearrangement | NS | |
| 46 | M | Jejunum | + | − | − | EWSR1 rearrangement | NS | |||
| Terazawa [38] | 2009 | 20 | F | Ileum | + | ND | ND | EWSR1-ATF1 | NED at 24 months | |
| Shenjere [39] | 2011 | 53 | F | Ileum | + | − | − | + for vimentin, CD57, EMA, MiTF − for CD34, DOG1, CD99, SMA | EWSR1-ATF1 | Regional LN metastasis at diagnosis/NED at 7 months |
| 26 | F | Small and large bowel | + | − | − | + for EMA − for cytokeratins, CD99, chromogranin, synaptophysin, desmin, CD34 | EWSR1-CREB1 | NS | ||
| 66 | M | Small intestine | + | − | − | − for cytokeratins, chromogranin, Synaptophysin, CD56, CD34, CD117, desmin, SMA | EWSR1-CREB1 | Regional LN metastasis at diagnosis/NED | ||
| Balkaransingh [40] | 2011 | 15 | M | Ileum | ND | ND | ND | EWSR1 rearrangement | NS | |
| Yang [41] | 2012 | 15 | M | Ileum | + | ND | ND | + for vimentin | EWSR1 rearrangement | Liver metastasis at 12 months |
| Stockman [42] | 2012 | 30 | F | Jejunum | + | − | − | + for SOX10, CD56, NSE, synaptophysin | EWSR1-ATF1 | AWD at 21 months |
| 35 | M | Jejunum | + | − | − | + for SOX10, CD56, NSE, sinaptophysin | EWSR1-ATF1 | DOD at 18 months | ||
| 33 | M | Ileum | + | − | − | + for SOX10, CD56 − for synaptophysin, NSE | EWSR1-CREB1 | AWD at 1.5 months | ||
| 50 | F | Stomach | + | − | − | + for SOX10, synaptophysin − for CD56, NSE | EWSR1-ATF1 | AWD at 24 months | ||
| 20 | F | Small intestine | + | − | − | + for SOX10, CD56, NSE − for synaptophysin | EWSR1 rearrangement | NED at 20 months | ||
| 46 | M | Stomach | + | − | − | + for SOX10, CD56 − for synaptophysin, NSE | EWSR1 rearrangement | NS | ||
| 34 | F | Stomach | + | − | − | + for SOX10, CD56 − for synaptophysin, NSE | EWSR1-ATF1 | DOD at 19 months | ||
| 77 | F | Colon | + | − | − | + for SOX10, CD56, NSE, synaptophysin | EWSR1-ATF1 | DOD at 106 months | ||
| 17 | M | Small intestine | + | − | − | + for SOX10, CD56 − for synaptophysin, NSE | EWSR1 rearrangement | NS | ||
| 60 | M | Ileum | + | − | − | + for SOX10, CD56, synaptophysin − for NSE | EWSR1-CREB1 | AWD at 36 months | ||
| 60 | F | Jejunum | + | − | − | + for SOX10, CD56 − for synaptophysin | EWSR1-CREB1 | NED at 41 months | ||
| 56 | M | Stomach | + | − | − | + for SOX10, CD56 − for synaptophysin | EWSR1-CREB1 | NS | ||
| 28 | F | Small intestine | + | − | − | + for SOX10, CD56, NSE, synaptophysin | EWSR1 rearrangement | DOD at 23 months | ||
| Suárez-Vilela [43] | 2012 | 36 | F | Jejunum | + | − | − | + for CD56, vimentin, cytokeratins, EMA − for CD117, CD99, desmin, SMA, chromogranin, synaptophysin | EWSR1-ATF1 | NS |
| D’Amico [44] | 2012 | 69 | F | Ileum | + | − | ND | + for CD56 − for DOG1, EMA, SMA, CD117, desmin, myogenin | EWSR1 rearrangement | Liver metastasis at 6 months |
| Lasithiotakis [45] | 2013 | 49 | F | Jejunum | + | − | − | + for EMA, synaptophysin | EWSR1-ATF1 | NED 20 months |
| Huang [46] | 2014 | 45 | F | Colon | + | − | − | − for CD117 | EWSR1 rearrangement | NS |
| Kong [47] | 2014 | 17 | M | Stomach | + | − | − | + for vimentin − for CD34, CD117, CD99 | EWSR1 rearrangement | NED 10 months |
| Liu [48] | 76 | M | Jejunum | + | − | ND | + for CD56 − for synaptophysin | EWSR1-ATF1 | NS | |
| Thway [49] | 2014 | 36 | M | Ileum | + | − | − | + for EMA, CD56, NSE − for SMA, desmin, CD117, DOG1, chromogranin, synaptophysin, CD34 | EWSR1-CREB1 | DOD 7 months; Local recurrence + metastasis of liver, peritoneum, and regional LN at DOD |
| Huang [50] | 2015 | 36 | M | Pancreas | + | + | + | + for vimentin,MiTF − for cytokeratins, EMA, desmin, SMA, CD34, CD117, CD99, synaptophysin, chromogranin, CD56, NSE | EWSR1 rearrangement | Liver metastasis at 10 months. DOD at 10 months |
| Yegen [51] | 2015 | 25 | F | Ileum | + | − | − | + for vimentin, beta-catenin, CD56 − for CD34, CD117, SMA, desmin, chromogranin, synaptophysin | EWSR1 rearrangement | Liver metastasis at diagnosis and at 15 months; Ovarian and peritoneal metastasis at 47 months |
| Raskin [52] | 2015 | 21 | M | Small intestine | + | − | − | − for MiTF, synaptophysin, CD56 | EWSR1-ATF1 | LN metastasis at diagnosis |
| Moslim [53] | 2016 | 57 | M | Duodenum and Jejunum (2 tumors) | + | − | + | − for negative for cytokeratins, chromogranin, synaptophysin, desmin, SMA, CD34 | EWSR1 rearrangement | NED 30 months and then DOD 4 months later due to rapid metastatic progression |
| Ardakani [54] | 2016 | 22 | M | Colon | + | − | NS | − for SMA, desmin, CD34, CD117, DOG1 | EWSR1 rearrangement | NS |
| Su [55] | 2017 | 51 | M | Ileum and Jejunum (3 tumors) | + | + | + | + for vimentin, CD56 − for Synaptophysin, cytokeratins, CD34, CD117, DOG1 | EWSR1 rearrangement | NS |
| Kato [56] | 2017 | 47 | F | Colon | + | − | − | + for vimentin, SOX10 − for SMA, CD117, cytokeratin | EWSR1-CREB1 | NS |
| Aksan [57] | 2019 | 28 | M | Small intestine | + | − | NS | +for SOX10 − for CD117, DOG1, desmin | EWSR1-ATF1 | Liver and LN metastasis at diagnosis |
| Okada [58] | 2020 | 38 | F | Small intestine | + | − | − | + for CD56, synaptophysin − for desmin, chromogranin, CD34, CD117, SMA | EWSR1 rearrangement | LN metastasis at diagnosis Liver metastasis at 36 months (surgery); NED at 72 months |
| Zhu [59] | 2021 | 65 | M | Ileum | + | + | − | + for SOX10, MiTF − for cytokeratins, EMA, CD117, DOG1, CD34, SMA, desmin, synaptophysin, chromogranin | EWSR1-ATF1 | NED at 7 months |
| Huang [60] | 2022 | 16 | M | Ileum | + | − | − | + for CD34 − for cytokeratins, CD117, DOG1, desmin, NSE | EWSR1-ATF1 | DOD at 56 months Liver, lung, bone, LN, pleural and adrenal metastasis at DOD |
| Njima [61] | 2024 | 20 | F | Ileum | + | − | − | + for SOX10, synaptophysin − for CD117, DOG1, cytokeratins, CD34, SMA, desmin, chromogranin | ND * | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotaru, V.; Chitoran, E.; Mitroiu, M.N.; Ionescu, S.O.; Neicu, A.; Cirimbei, C.; Alecu, M.; Gelal, A.; Prie, A.D.; Simion, L. Intestinal Clear Cell Sarcoma—A Case Presentation of an Extremely Rare Tumor and Literature Review. Medicina 2024, 60, 847. https://doi.org/10.3390/medicina60060847
Rotaru V, Chitoran E, Mitroiu MN, Ionescu SO, Neicu A, Cirimbei C, Alecu M, Gelal A, Prie AD, Simion L. Intestinal Clear Cell Sarcoma—A Case Presentation of an Extremely Rare Tumor and Literature Review. Medicina. 2024; 60(6):847. https://doi.org/10.3390/medicina60060847
Chicago/Turabian StyleRotaru, Vlad, Elena Chitoran, Madalina Nicoleta Mitroiu, Sinziana Octavia Ionescu, Ariana Neicu, Ciprian Cirimbei, Mihnea Alecu, Aisa Gelal, Andra Delia Prie, and Laurentiu Simion. 2024. "Intestinal Clear Cell Sarcoma—A Case Presentation of an Extremely Rare Tumor and Literature Review" Medicina 60, no. 6: 847. https://doi.org/10.3390/medicina60060847
APA StyleRotaru, V., Chitoran, E., Mitroiu, M. N., Ionescu, S. O., Neicu, A., Cirimbei, C., Alecu, M., Gelal, A., Prie, A. D., & Simion, L. (2024). Intestinal Clear Cell Sarcoma—A Case Presentation of an Extremely Rare Tumor and Literature Review. Medicina, 60(6), 847. https://doi.org/10.3390/medicina60060847









