Impact of Intraoperative Nefopam on Postoperative Pain, Opioid Use, and Recovery Quality with Parietal Pain Block in Single-Port Robotic Cholecystectomy: A Prospective Randomized Controlled Trial
Abstract
1. Introduction
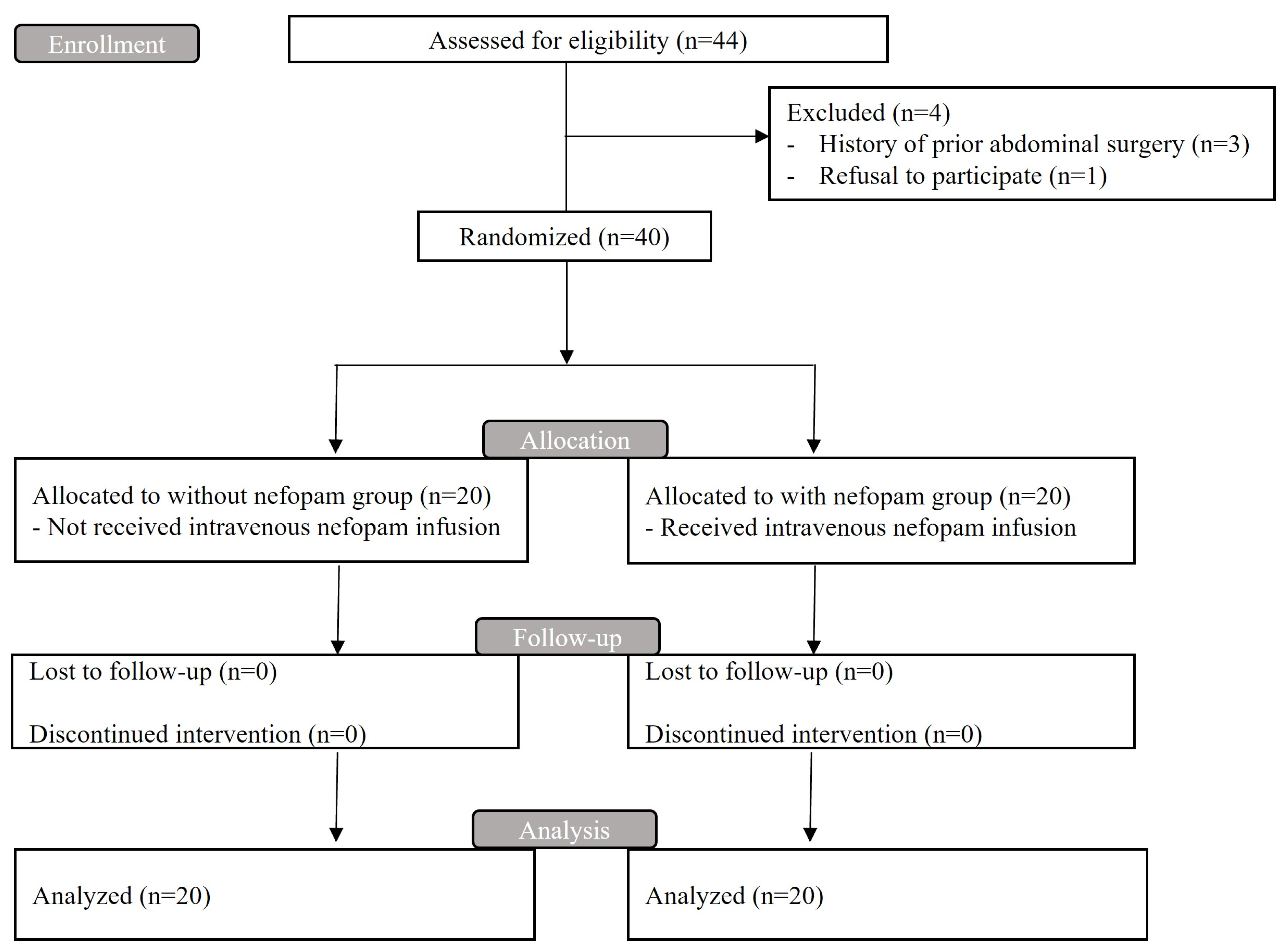
2. Patients and Methods
2.1. Ethical Considerations
2.2. Study Population
2.3. Randomization and Blinding
2.4. Study Protocol
2.4.1. Surgery and General Anesthesia
2.4.2. Nefopam Administration
2.4.3. Performance of RSB for Parietal Pain Relief
2.4.4. Postoperative Pain Assessment
2.5. Clinical Variables
2.5.1. Pre- and Intraoperative Variables
2.5.2. Postoperative Quality of Recovery Assessment
2.5.3. Postoperative Complications
2.6. Sample Size and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Strasberg, S.M. Clinical practice. Acute calculous cholecystitis. N. Engl. J. Med. 2008, 358, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Csikesz, N.G.; Tseng, J.F.; Shah, S.A. Trends in surgical management for acute cholecystitis. Surgery 2008, 144, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Toro, J.P.; Lin, E.; Patel, A.D. Review of robotics in foregut and bariatric surgery. Surg. Endosc. 2015, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sucandy, I.; Jabbar, F.; Syblis, C.; Crespo, K.; App, S.; Ross, S.; Rosemurgy, A. Robotic Versus Open Extrahepatic Biliary Reconstruction for Iatrogenic Bile Duct Injury. Am. Surg. 2022, 88, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Rudiman, R.; Hanafi, R.V.; Almawijaya, A. Single-site robotic cholecystectomy versus single-incision laparoscopic cholecystectomy: A systematic review and meta-analysis. Ann. Gastroenterol. Surg. 2023, 7, 709–718. [Google Scholar] [CrossRef]
- Muaddi, H.; Hafid, M.E.; Choi, W.J.; Lillie, E.; de Mestral, C.; Nathens, A.; Stukel, T.A.; Karanicolas, P.J. Clinical Outcomes of Robotic Surgery Compared to Conventional Surgical Approaches (Laparoscopic or Open): A Systematic Overview of Reviews. Ann. Surg. 2021, 273, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, T.; Klarskov, B.; Rosenberg, J.; Kehlet, H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain 2001, 90, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ure, B.M.; Troidl, H.; Spangenberger, W.; Dietrich, A.; Lefering, R.; Neugebauer, E. Pain after laparoscopic cholecystectomy. Intensity and localization of pain and analysis of predictors in preoperative symptoms and intraoperative events. Surg. Endosc. 1994, 8, 90–96. [Google Scholar] [CrossRef]
- Rosero, E.B.; Joshi, G.P. Hospital readmission after ambulatory laparoscopic cholecystectomy: Incidence and predictors. J. Surg. Res. 2017, 219, 108–115. [Google Scholar] [CrossRef]
- Park, S.E.; Hong, T.H. The effectiveness of extremely low-pressure pneumoperitoneum on pain reduction after robot-assisted cholecystectomy. Asian J. Surg. 2023, 46, 539–544. [Google Scholar] [CrossRef]
- Shim, J.W.; Ko, J.; Bae, J.H.; Park, J.; Lee, H.M.; Kim, Y.S.; Moon, Y.E.; Hong, S.H.; Chae, M.S. Pre-emptive multimodal analgesic bundle with transversus abdominis plane block enhances early recovery after laparoscopic cholecystectomy. Asian J. Surg. 2022, 45, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, J.; Li, X.; Li, Y.; Su, S. Comparative outcomes of single-incision laparoscopic, mini-laparoscopic, four-port laparoscopic, three-port laparoscopic, and single-incision robotic cholecystectomy: A systematic review and network meta-analysis. Updates Surg. 2023, 75, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Hootsmans, N.; Parmiter, S.; Connors, K.; Badve, S.B.; Snyder, E.; Turcotte, J.J.; Jayaraman, S.S.; Zahiri, H.R. Outcomes of an enhanced recovery after surgery (ERAS) program to limit perioperative opioid use in outpatient minimally invasive GI and hernia surgeries. Surg. Endosc. 2023, 37, 7192–7198. [Google Scholar] [CrossRef] [PubMed]
- Girard, P.; Chauvin, M.; Verleye, M. Nefopam analgesia and its role in multimodal analgesia: A review of preclinical and clinical studies. Clin. Exp. Pharmacol. Physiol. 2016, 43, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Verleye, M.; André, N.; Heulard, I.; Gillardin, J.M. Nefopam blocks voltage-sensitive sodium channels and modulates glutamatergic transmission in rodents. Brain Res. 2004, 1013, 249–255. [Google Scholar] [CrossRef]
- Mimoz, O.; Incagnoli, P.; Josse, C.; Gillon, M.C.; Kuhlman, L.; Mirand, A.; Soilleux, H.; Fletcher, D. Analgesic efficacy and safety of nefopam vs. propacetamol following hepatic resection. Anaesthesia 2001, 56, 520–525. [Google Scholar] [CrossRef] [PubMed]
- McLintock, T.T.; Kenny, G.N.; Howie, J.C.; McArdle, C.S.; Lawrie, S.; Aitken, H. Assessment of the analgesic efficacy of nefopam hydrochloride after upper abdominal surgery: A study using patient controlled analgesia. Br. J. Surg. 1988, 75, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, A.; Laska, E. Nefopam and morphine in man. Clin. Pharmacol. Ther. 1975, 18, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Ok, Y.M.; Cheon, J.H.; Choi, E.J.; Chang, E.J.; Lee, H.M.; Kim, K.H. Nefopam Reduces Dysesthesia after Percutaneous Endoscopic Lumbar Discectomy. Korean J. Pain. 2016, 29, 40–47. [Google Scholar] [CrossRef]
- Kim, K.H.; Abdi, S. Rediscovery of nefopam for the treatment of neuropathic pain. Korean J. Pain. 2014, 27, 103–111. [Google Scholar] [CrossRef]
- Evans, M.S.; Lysakowski, C.; Tramèr, M.R. Nefopam for the prevention of postoperative pain: Quantitative systematic review. Br. J. Anaesth. 2008, 101, 610–617. [Google Scholar] [CrossRef]
- Du Manoir, B.; Aubrun, F.; Langlois, M.; Le Guern, M.E.; Alquier, C.; Chauvin, M.; Fletcher, D. Randomized prospective study of the analgesic effect of nefopam after orthopaedic surgery. Br. J. Anaesth. 2003, 91, 836–841. [Google Scholar] [CrossRef]
- Guindon, J.; Walczak, J.S.; Beaulieu, P. Recent advances in the pharmacological management of pain. Drugs 2007, 67, 2121–2133. [Google Scholar] [CrossRef]
- Novelli, A.; Díaz-Trelles, R.; Groppetti, A.; Fernández-Sánchez, M.T. Nefopam inhibits calcium influx, cGMP formation, and NMDA receptor-dependent neurotoxicity following activation of voltage sensitive calcium channels. Amino Acids 2005, 28, 183–191. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Lim, B.G.; Kim, H.; Kong, M.H.; Lee, I.O.; Kim, N.S. The analgesic effect of nefopam combined with low dose remifentanil in patients undergoing middle ear surgery under desflurane anesthesia: A randomized controlled trial. Korean J. Anesthesiol. 2015, 68, 43–49. [Google Scholar] [CrossRef]
- Lee, J.H.; Ki, M.; Choi, S.; Woo, C.J.; Kim, D.; Lim, H.; Kim, D.C. Validity and reliability of the Korean version of the Quality of Recovery-15 questionnaire. Korean J. Anesthesiol. 2021, 74, 142–149. [Google Scholar] [CrossRef]
- Tae, N.Y.; Yi, J.W.; Jung, J.K.; Lee, J.; Jo, S.; Kim, H. A Randomized Comparison of Multimodal Analgesia and Fentanyl-Based Patient-Controlled Analgesia in Women Undergoing Robot-Assisted Bilateral Axillary Breast Approach Thyroidectomy. J. Clin. Med. 2024, 13, 702. [Google Scholar] [CrossRef] [PubMed]
- In, C.B.; Jeon, Y.T.; Oh, A.Y.; Jin, S.J.; Park, B.S.; Choi, E.S. Effects of Intraoperative Nefopam on Catheter-Related Bladder Discomfort in Patients Undergoing Robotic Nephrectomy: A Randomized Double-Blind Study. J. Clin. Med. 2019, 8, 519. [Google Scholar] [CrossRef]
- Kim, B.G.; Moon, J.Y.; Choi, J.Y.; Park, I.S.; Oh, A.Y.; Jeon, Y.T.; Hwang, J.W.; Ryu, J.H. The Effect of Intraoperative Nefopam Administration on Acute Postoperative Pain and Chronic Discomfort After Robotic or Endoscopic Assisted Thyroidectomy: A Randomized Clinical Trial. World J. Surg. 2018, 42, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Bolaños, Á.; Armas-Domínguez, A.; Valencia, L.; Jiménez-Marrero, P.; López-Ruiz, S.; Rodríguez-Pérez, A. Pain Prevalence and Satisfaction with Pain Management in Inpatients: A Cross-Sectional Study. Healthcare 2023, 11, 3191. [Google Scholar] [CrossRef]
- Zeeni, C.A.; Kaddoum, R.N.; Aouad, M.T.; Shebbo, F.M.; Ramadan, J.G.; Habli, Y.A.; Hassani, J.R.; Siddik-Sayyid, S.M. The effect of the addition of nefopam to intraoperative ketoprofen and acetaminophen on postoperative morphine requirements after laparoscopic cholecystectomy: A randomized controlled trial. Minerva Anestesiol. 2024, 90, 31–40. [Google Scholar] [CrossRef]
- Choi, E.; Karm, M.H.; So, E.; Choi, Y.J.; Park, S.; Oh, Y.; Yun, H.J.; Kim, H.J.; Seo, K.S. Effects on postoperative nausea and vomiting of nefopam versus fentanyl following bimaxillary orthognathic surgery: A prospective double-blind randomized controlled trial. J. Dent. Anesth. Pain. Med. 2019, 19, 55–66. [Google Scholar] [CrossRef]
- Zhao, T.; Shen, Z.; Sheng, S. The efficacy and safety of nefopam for pain relief during laparoscopic cholecystectomy: A meta-analysis. Medicine 2018, 97, e0089. [Google Scholar] [CrossRef]
- Oh, C.S.; Jung, E.; Lee, S.J.; Kim, S.H. Effect of nefopam- versus fentanyl-based patient-controlled analgesia on postoperative nausea and vomiting in patients undergoing gynecological laparoscopic surgery: A prospective double-blind randomized controlled trial. Curr. Med. Res. Opin. 2015, 31, 1599–1607. [Google Scholar] [CrossRef]
- Heel, R.C.; Brogden, R.N.; Pakes, G.E.; Speight, T.M.; Avery, G.S. Nefopam: A review of its pharmacological properties and therapeutic efficacy. Drugs 1980, 19, 249–267. [Google Scholar] [CrossRef]
- Tigerstedt, I.; Tammisto, T.; Leander, P. Comparison of the analgesic dose-effect relationships of nefopam and oxycodone in postoperative pain. Acta Anaesthesiol. Scand. 1979, 23, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, P.; Bordeianou, L. Implementation of an ERAS Pathway in Colorectal Surgery. Clin. Colon. Rectal Surg. 2019, 32, 102–108. [Google Scholar] [PubMed]
| Group | Without Nefopam | With Nefopam |
|---|---|---|
| n | 20 | 20 |
| Preoperative variables | ||
| Sex (female) | 11 (55.0%) | 13 (65.0%) |
| Age (years) | 47.8 ± 9.2 | 48.9 ± 12.3 |
| Body mass index (kg/m2) | 27.2 ± 4.1 | 25.2 ± 4.0 |
| ASA physical status classification | ||
| I | 6 (30.0%) | 8 (40.0%) |
| II | 14 (70.0%) | 12 (60.0%) |
| Diabetes mellitus | 3 (15.0%) | 2 (10.0%) |
| Hypertension | 8 (40.0%) | 3 (15.0%) |
| Laboratory variables | ||
| Hemoglobin (g/dL) | 13.9 ± 1.4 | 13.4 ± 1.5 |
| White blood cell count (×109/L) | 7.3 ± 1.9 | 7.0 ± 2.7 |
| Aspartate aminotransferase (IU/L) | 21.5 (16.3–24.8) | 26.0 (19.5–33.0) |
| Alanine aminotransferase (IU/L) | 24.5 (13.5–38.8) | 24.5 (14.8–35.0) |
| Intraoperative variables | ||
| Operation time (min) | 65.6 ± 13.6 | 69.6 ± 17.4 |
| Rescue ephedrine dose (mg) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Group | Without Nefopam | With Nefopam | p |
|---|---|---|---|
| n | 20 | 20 | |
| Peak NRS score in the PACU | |||
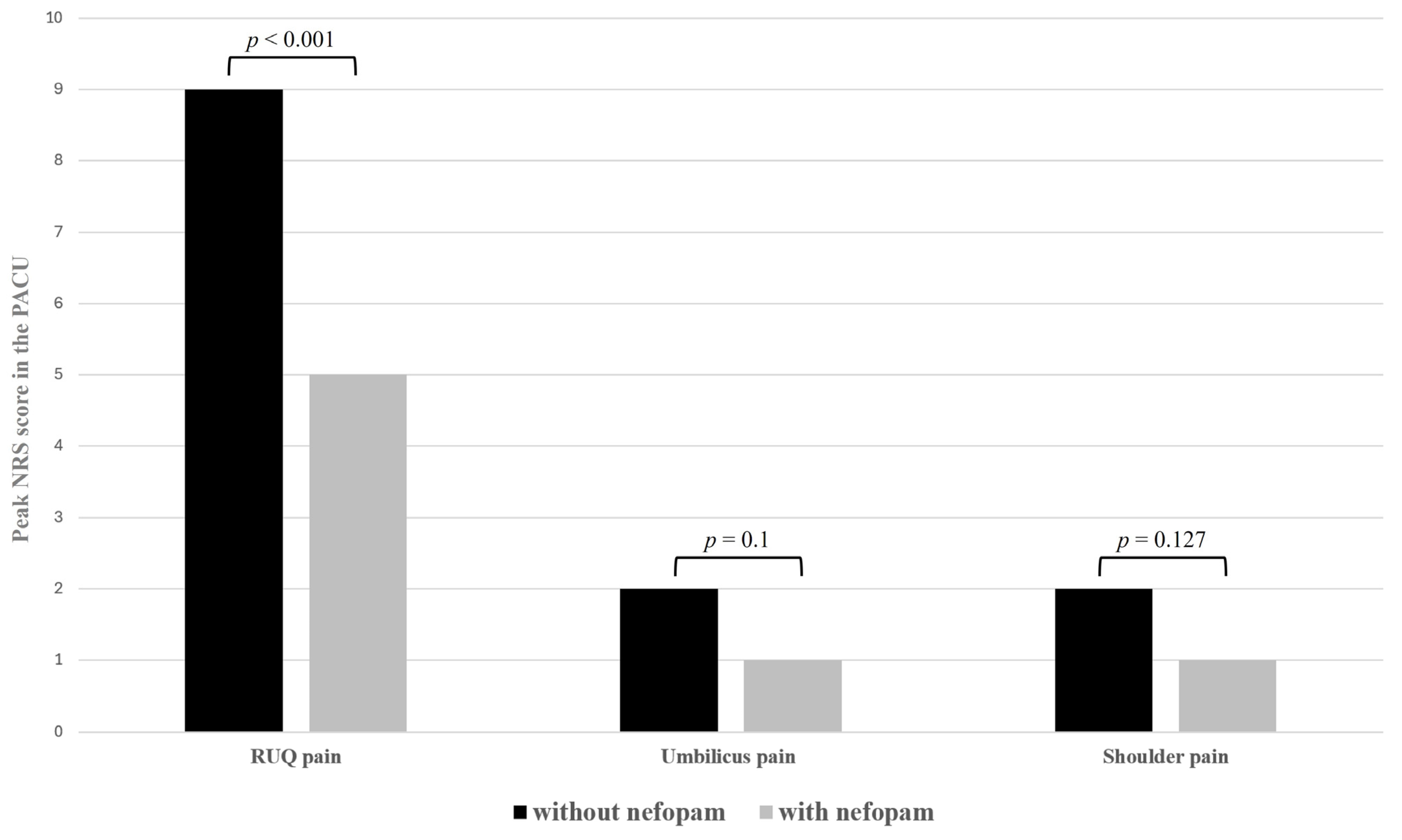
| RUQ pain | 9 (8–10) | 5 (3–6) | <0.001 |
| Umbilicus pain | 2 (1–5) | 1 (1–2) | 0.1 |
| Shoulder pain | 2 (1–3) | 1 (1–2) | 0.127 |
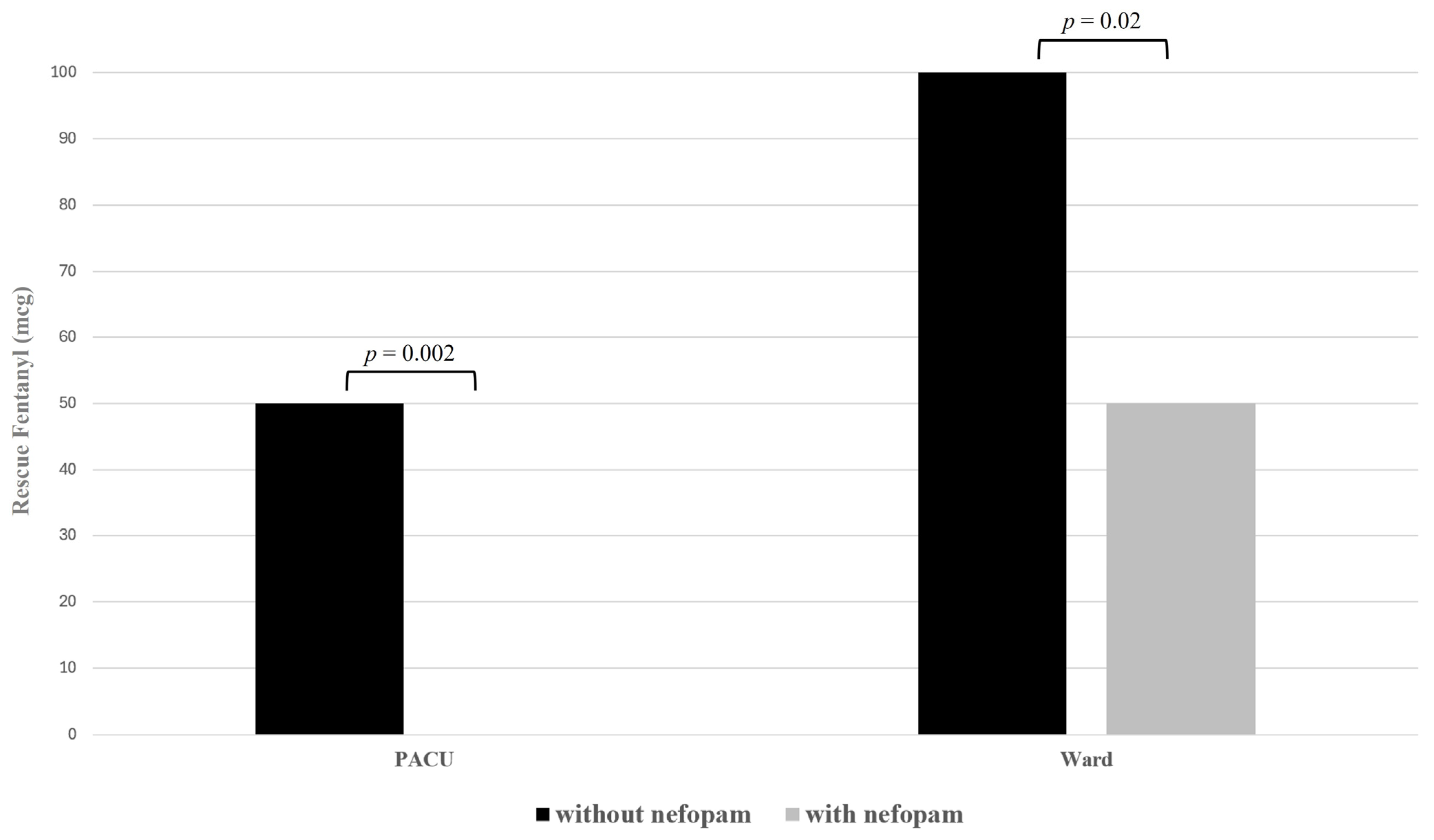
| Rescue fentanyl in the PACU (mcg) | 50 (50–100) | 0 (0–50) | 0.002 |
| Rescue fentanyl in the ward (mcg) | 100 (50–100) | 50 (25–94) | 0.02 |
| Group | Without Nefopam | With Nefopam | p |
|---|---|---|---|
| n | 20 | 20 | |
| Total QoR-15 score (points) | 93 (72–119) | 106 (69–134) | 0.482 |
| 1 Able to breathe easy | 9 (5–10) | 7 (5–10) | 0.813 |
| 2 Been able to enjoy food | 5 (0–9) | 5 (1–10) | 0.564 |
| 3 Feeling rested | 6 (3–9) | 8 (5–10) | 0.434 |
| 4 Have had a good sleep | 6 (2–9) | 7 (2–10) | 0.87 |
| 5 Able to look after personal toilet and hygiene unaided | 10 (4–10) | 10 (4–10) | 0.977 |
| 6 Able to communicate with family or friends | 10 (5–10) | 10 (4–10) | 0.678 |
| 7 Getting support from hospital doctors and nurses | 10 (9–10) | 10 (9–10) | 0.633 |
| 8 Able to return to work or usual home activities | 5 (2–10) | 6 (2–10) | 0.858 |
| 9 Feeling comfortable and in control | 7 (4–9) | 8 (5–10) | 0.373 |
| 10 Having a feeling of general well-being | 6 (3–9) | 7 (4–10) | 0.228 |
| 11 Moderate pain | 4 (2–6) | 4 (2–6) | 0.681 |
| 12 Severe pain | 4 (2–5) | 4 (2–10) | 0.337 |
| 13 Nausea or vomiting | 10 (7–10) | 10 (10–10) | 0.006 |
| 14 Feeling worried or anxious | 7 (3–10) | 9 (5–10) | 0.23 |
| 15 Feeling sad or depressed | 7 (5–10) | 10 (4–10) | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.Y.; Kim, D.H.; Park, J.H.; Chae, M.S. Impact of Intraoperative Nefopam on Postoperative Pain, Opioid Use, and Recovery Quality with Parietal Pain Block in Single-Port Robotic Cholecystectomy: A Prospective Randomized Controlled Trial. Medicina 2024, 60, 848. https://doi.org/10.3390/medicina60060848
Lee SY, Kim DH, Park JH, Chae MS. Impact of Intraoperative Nefopam on Postoperative Pain, Opioid Use, and Recovery Quality with Parietal Pain Block in Single-Port Robotic Cholecystectomy: A Prospective Randomized Controlled Trial. Medicina. 2024; 60(6):848. https://doi.org/10.3390/medicina60060848
Chicago/Turabian StyleLee, So Yeon, Dong Hyun Kim, Jung Hyun Park, and Min Suk Chae. 2024. "Impact of Intraoperative Nefopam on Postoperative Pain, Opioid Use, and Recovery Quality with Parietal Pain Block in Single-Port Robotic Cholecystectomy: A Prospective Randomized Controlled Trial" Medicina 60, no. 6: 848. https://doi.org/10.3390/medicina60060848
APA StyleLee, S. Y., Kim, D. H., Park, J. H., & Chae, M. S. (2024). Impact of Intraoperative Nefopam on Postoperative Pain, Opioid Use, and Recovery Quality with Parietal Pain Block in Single-Port Robotic Cholecystectomy: A Prospective Randomized Controlled Trial. Medicina, 60(6), 848. https://doi.org/10.3390/medicina60060848







