Effects of Access Cavity Design and Placement Techniques on Mineral Trioxide Aggregate Obturation Quality in Simulated Immature Teeth: A Micro-Computed Tomography Study
Abstract
:1. Introduction
1.1. Necrotic Immature Permanent Teeth and Endodontic Approaches
1.2. Apexification with Calcium Hydroxide or Mineral Trioxide Aggregate
1.3. Orthograde MTA Applications: Clinical Challenges and Limitations
1.4. MTA Application Techniques
1.5. Minimal Endodontic Access Cavities
1.6. Aim of the Study
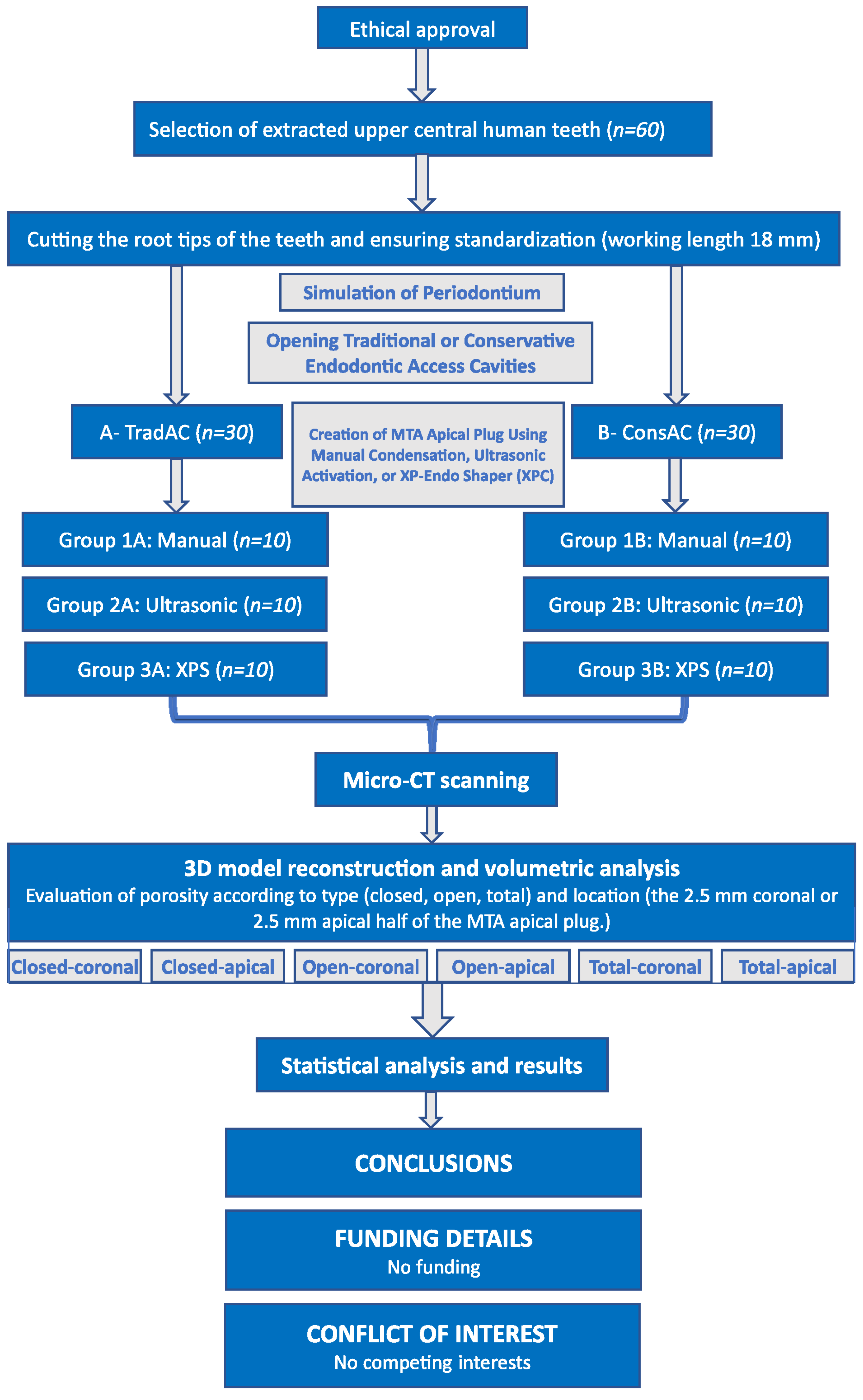
2. Materials and Methods
2.1. Ethical Approval and Sample Size Calculation
2.2. Selection and Preparation of Samples
2.3. Simulation of Periodontium
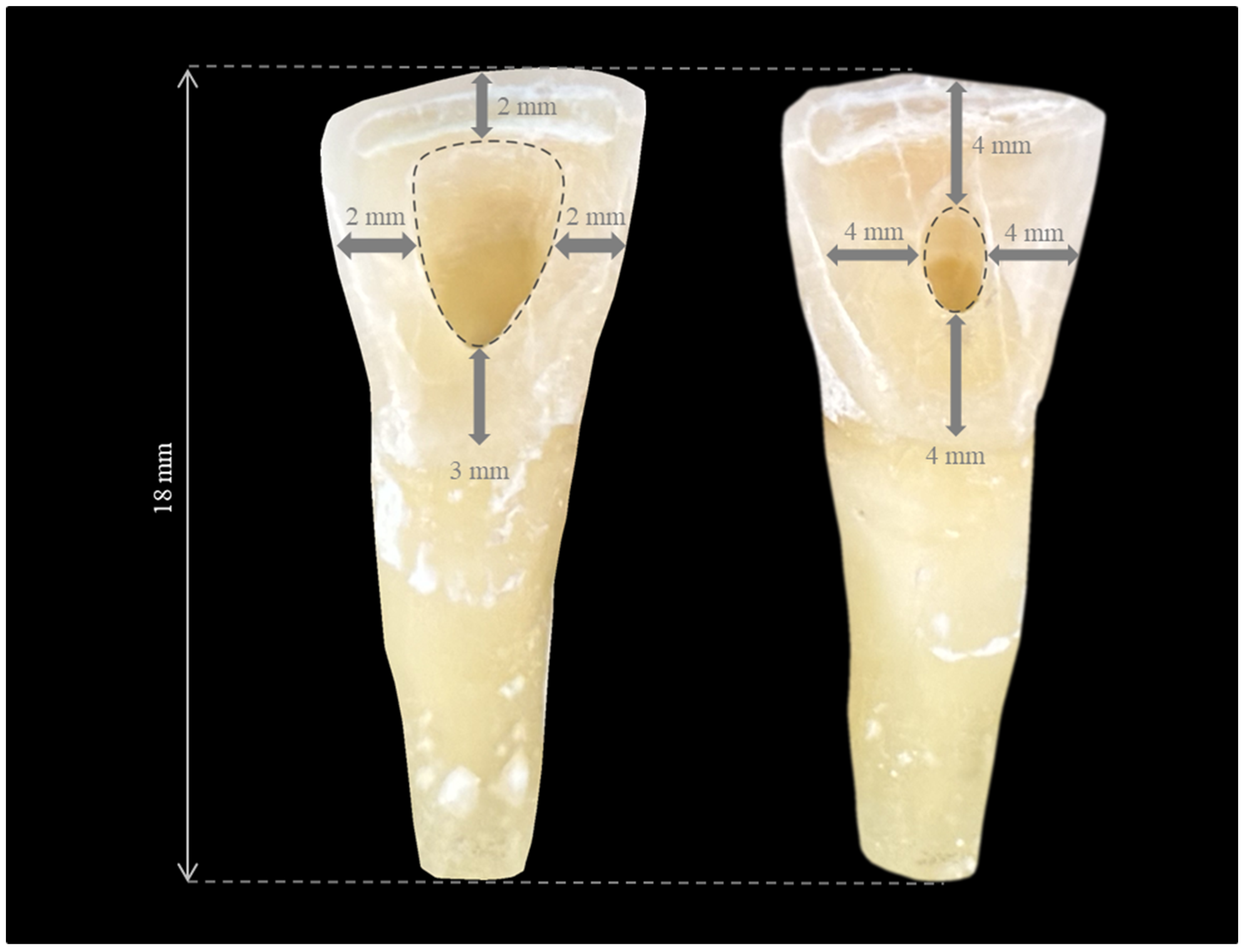
2.4. Opening Traditional Access Cavities (TradACs)
2.5. Opening Conservative Access Cavities (ConsACs)
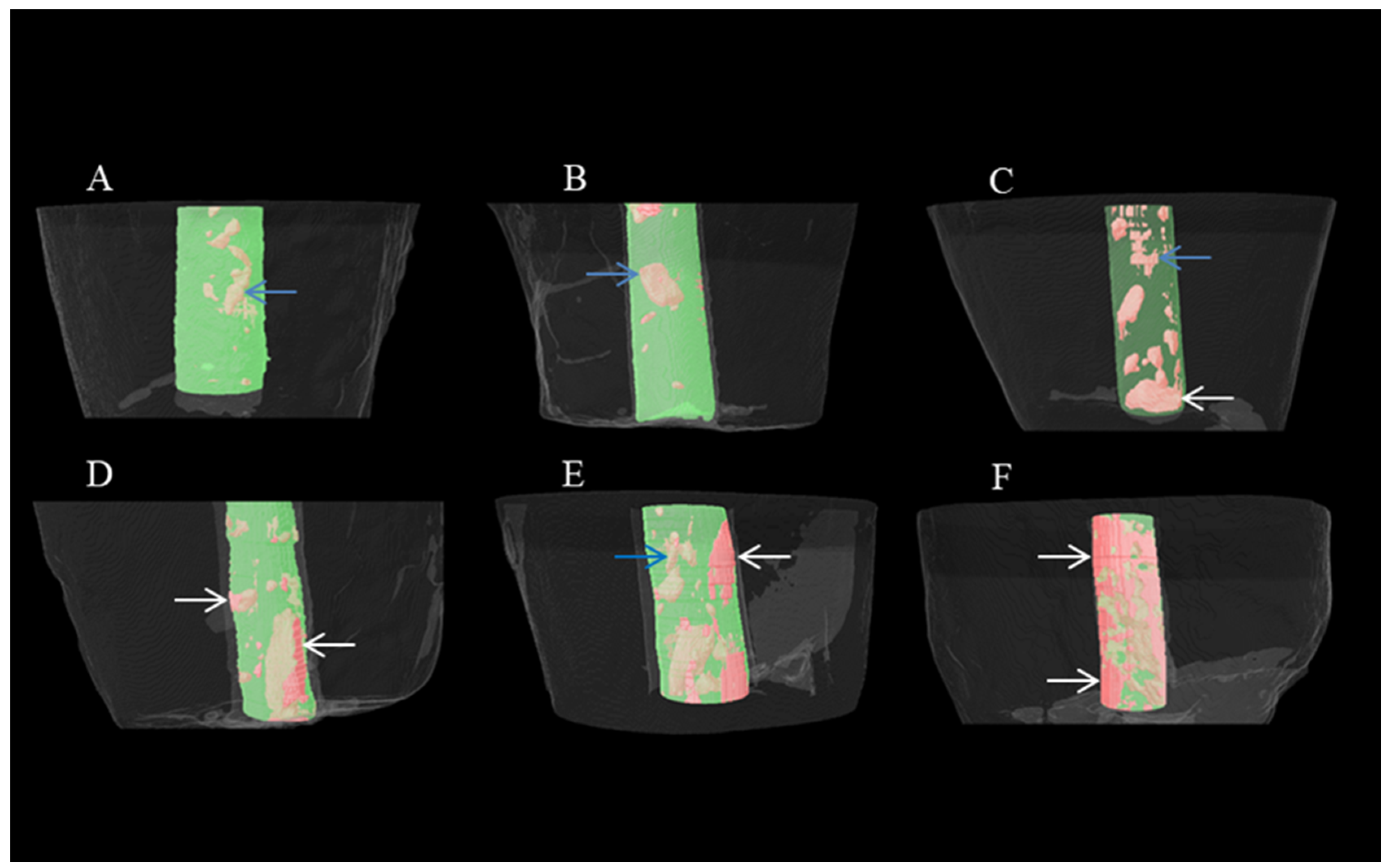
2.6. Micro-CT Scanning and Analyzing
2.7. Statistical Analysis
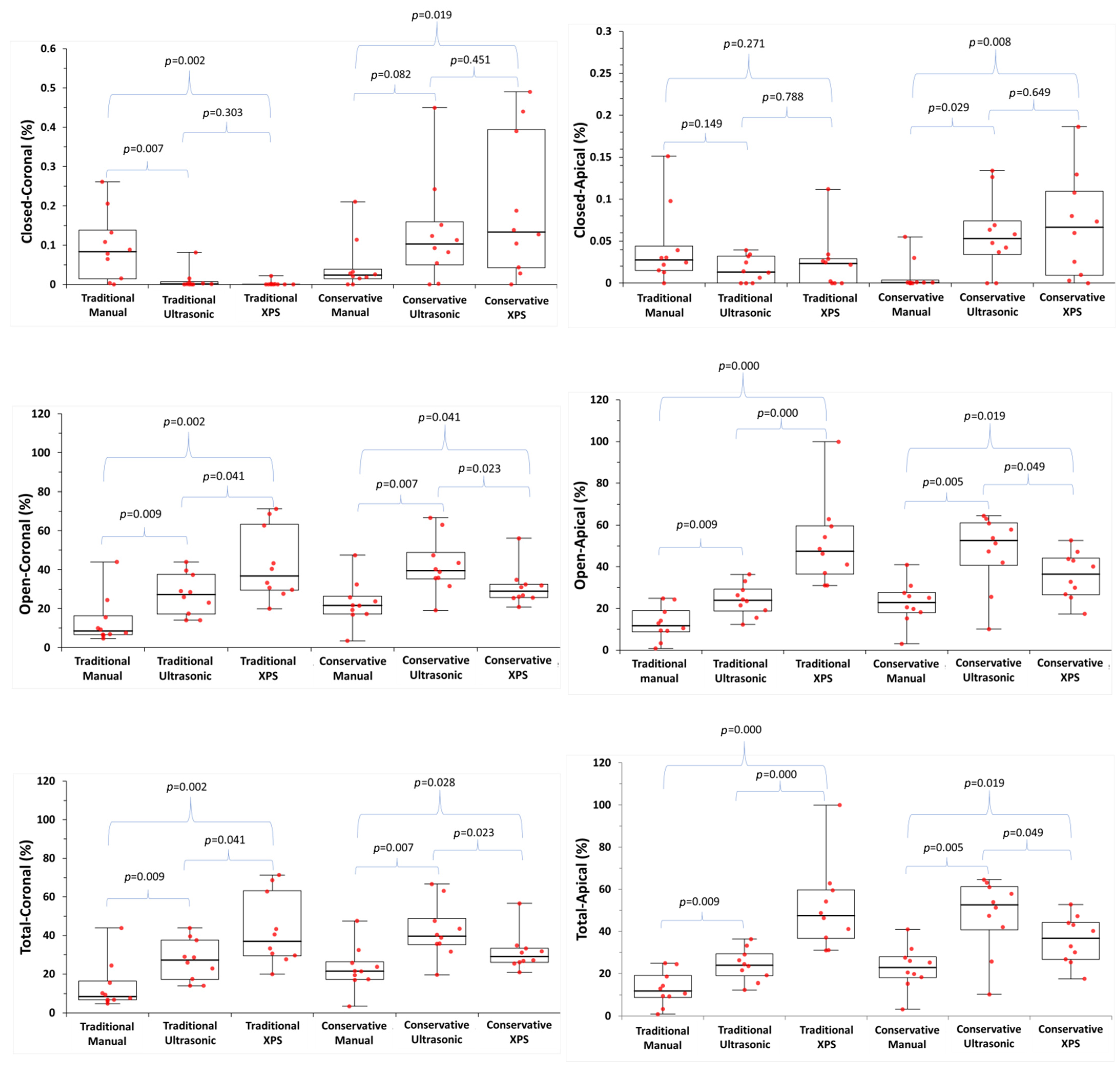
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hargreaves, K.M.; Diogenes, A.; Teixeira, F.B. Treatment options: Biological basis of regenerative endodontic procedures. J. Endod. 2013, 39, S30–S43. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E. Review of guidance for the selection of regenerative endodontics, apexogenesis, apexification, pulpotomy, and other endodontic treatments for immature permanent teeth. Int. Endod. J. 2023, 56 (Suppl. 2), 188–199. [Google Scholar] [CrossRef]
- Guerrero, F.; Mendoza, A.; Ribas, D.; Aspiazu, K. Apexification: A systematic review. J. Conserv. Dent. 2018, 21, 462–465. [Google Scholar] [CrossRef]
- Pace, R.; Giuliani, V.; Nieri, M.; Di Nasso, L.; Pagavino, G. Mineral trioxide aggregate as apical plug in teeth with necrotic pulp and immature apices: A 10-year case series. J. Endod. 2014, 40, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Shaik, I.; Dasari, B.; Kolichala, R.; Doos, M.; Qadri, F.; Arokiyasamy, J.L.; Tiwari, R.V.C. Comparison of the Success Rate of Mineral Trioxide Aggregate, Endosequence Bioceramic Root Repair Material, and Calcium Hydroxide for Apexification of Immature Permanent Teeth: Systematic Review and Meta-Analysis. J. Pharm. Bioallied Sci. 2021, 13, S43–S47. [Google Scholar] [CrossRef]
- Rafter, M. Apexification: A review. Dent. Traumatol. 2005, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.O.; Farik, B.; Munksgaard, E.C. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent. Traumatol. 2002, 18, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Purra, A.R.; Ahangar, F.A.; Chadgal, S.; Farooq, R. Mineral trioxide aggregate apexification: A novel approach. J. Conserv. Dent. 2016, 19, 377–380. [Google Scholar] [CrossRef]
- Bani, M.; Sungurtekin-Ekçi, E.; Odabaş, M.E. Efficacy of Biodentine as an Apical Plug in Nonvital Permanent Teeth with Open Apices: An In Vitro Study. Biomed. Res. Int. 2015, 2015, 359275. [Google Scholar] [CrossRef]
- Lertmalapong, P.; Jantarat, J.; Srisatjaluk, R.L.; Komoltri, C. Bacterial leakage and marginal adaptation of various bioceramics as apical plug in open apex model. J. Investig. Clin. Dent. 2019, 10, e12371. [Google Scholar] [CrossRef]
- Giuliani, V.; Baccetti, T.; Pace, R.; Pagavino, G. The use of MTA in teeth with necrotic pulps and open apices. Dent. Traumatol. 2002, 18, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M. Mineral trioxide aggregate: A comprehensive literature review--part II: Leakage and biocompatibility investigations. J. Endod. 2010, 36, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Bücher, K.; Meier, F.; Diegritz, C.; Kaaden, C.; Hickel, R.; Kühnisch, J. Long-term outcome of MTA apexification in teeth with open apices. Quintessence Int. 2016, 47, 473–482. [Google Scholar] [PubMed]
- Torabinejad, M.; Nosrat, A.; Verma, P.; Udochukwu, O. Regenerative Endodontic Treatment or Mineral Trioxide Aggregate Apical Plug in Teeth with Necrotic Pulps and Open Apices: A Systematic Review and Meta-analysis. J. Endod. 2017, 43, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.; Rosentritt, M.; Loher, H.; Kolbeck, C.; Trempler, C.; Stemplinger, B.; Kopzon, V.; Handel, G. Changes of cement properties caused by mixing errors: The therapeutic range of different cement types. Dent. Mater. 2008, 24, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Kleverlaan, C.J.; van Duinen, R.N.; Feilzer, A.J. Mechanical properties of glass ionomer cements affected by curing methods. Dent. Mater. 2004, 20, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Hachmeister, D.R.; Schindler, W.G.; Walker, W.A., 3rd; Thomas, D.D. The sealing ability and retention characteristics of mineral trioxide aggregate in a model of apexification. J. Endod. 2002, 28, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Howley, M.F.; O’Connell, A.C. Treatment of open apex teeth using two types of white mineral trioxide aggregate after initial dressing with calcium hydroxide in children. Dent. Traumatol. 2011, 27, 166–173. [Google Scholar] [CrossRef]
- Bogen, G.; Kuttler, S. Mineral trioxide aggregate obturation: A review and case series. J. Endod. 2009, 35, 777–790. [Google Scholar] [CrossRef]
- El-Ma’aita, A.M.; Qualtrough, A.J.; Watts, D.C. A micro-computed tomography evaluation of mineral trioxide aggregate root canal fillings. J. Endod. 2012, 38, 670–672. [Google Scholar] [CrossRef]
- Fridland, M.; Rosado, R. MTA solubility: A long term study. J. Endod. 2005, 31, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Basturk, F.B.; Nekoofar, M.H.; Gunday, M.; Dummer, P.M. Effect of various mixing and placement techniques on the flexural strength and porosity of mineral trioxide aggregate. J. Endod. 2014, 40, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, A.; Hanaoka, K.; Teranaka, T. Fracture toughness of resin-modified glass ionomer restorative materials: Effect of powder/liquid ratio and powder particle size reduction on fracture toughness. Dent. Mater. 2003, 19, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Sugaya, T.; Tomita, M.; Hasegawa, Y.; Miyaji, H.; Tenkumo, T.; Tanaka, S.; Motoki, Y.; Takanawa, Y.; Kawanami, M. Healing of experimental apical periodontitis after apicoectomy using different sealing materials on the resected root end. Dent. Mater. J. 2011, 30, 485–492. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, M.A.; De Bruyne, R.J.; De Moor, R.J. Capillary flow porometry to assess the seal provided by root-end filling materials in a standardized and reproducible way. J. Endod. 2006, 32, 206–209. [Google Scholar] [CrossRef]
- Keleş, A.; Alcin, H.; Kamalak, A.; Versiani, M.A. Micro-CT evaluation of root filling quality in oval-shaped canals. Int. Endod. J. 2014, 47, 1177–1184. [Google Scholar] [CrossRef]
- Aminoshariae, A.; Hartwell, G.R.; Moon, P.C. Placement of mineral trioxide aggregate using two different techniques. J. Endod. 2003, 29, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Vizgirda, P.J.; Liewehr, F.R.; Patton, W.R.; McPherson, J.C.; Buxton, T. A comparison of laterally condensed gutta-percha, thermoplasticized gutta-percha, and mineral trioxide aggregate as root canal filling materials. J. Endod. 2004, 30, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Giovarruscio, M.; Uccioli, U.; Malentacca, A.; Koller, G.; Foschi, F.; Mannocci, F. A technique for placement of apical MTA plugs using modified Thermafil carriers for the filling of canals with wide apices. Int. Endod. J. 2013, 46, 88–97. [Google Scholar] [CrossRef]
- Martin, R.L.; Monticelli, F.; Brackett, W.W.; Loushine, R.J.; Rockman, R.A.; Ferrari, M.; Pashley, D.H.; Tay, F.R. Sealing properties of mineral trioxide aggregate orthograde apical plugs and root fillings in an in vitro apexification model. J. Endod. 2007, 33, 272–275. [Google Scholar] [CrossRef]
- Yeung, P.; Liewehr, F.R.; Moon, P.C. A quantitative comparison of the fill density of MTA produced by two placement techniques. J. Endod. 2006, 32, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Lawley, G.R.; Schindler, W.G.; Walker III, W.A.; Kolodrubetz, D. Evaluation of ultrasonically placed MTA and fracture resistance with intracanal composite resin in a model of apexification. J. Endod. 2004, 30, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.S.; Shin, S.J.; Chang, S.W.; Yoo, H.M.; Oh, T.S.; Park, D.S. In vitro evaluation of bacterial leakage resistance of an ultrasonically placed mineral trioxide aggregate orthograde apical plug in teeth with wide open apexes: A preliminary study. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2009, 107, e52–e56. [Google Scholar] [CrossRef] [PubMed]
- Sisli, S.N.; Ozbas, H. Comparative Micro-computed Tomographic Evaluation of the Sealing Quality of ProRoot MTA and MTA Angelus Apical Plugs Placed with Various Techniques. J. Endod. 2017, 43, 147–151. [Google Scholar] [CrossRef]
- An, H.J.; Yoon, H.; Jung, H.I.; Shin, D.H.; Song, M. Comparison of Obturation Quality after MTA Orthograde Filling with Various Obturation Techniques. J. Clin. Med. 2021, 10, 1719. [Google Scholar] [CrossRef]
- Taneja, S. Minimally invasive endodontics: Saving the precious dentin. J. Dent. Spec. 2021, 9, 44–45. [Google Scholar] [CrossRef]
- Bürklein, S.; Schäfer, E. Minimally invasive endodontics. Quintessence Int. 2015, 46, 119–124. [Google Scholar]
- Elnawam, H.; Abdelmougod, M.; Mobarak, A.; Hussein, M.; Aboualmakarem, H.; Girgis, M.; El Backly, R. Regenerative Endodontics and Minimally Invasive Dentistry: Intertwining Paths Crossing Over Into Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 837639. [Google Scholar] [CrossRef]
- Silva, E.; De-Deus, G.; Souza, E.M.; Belladonna, F.G.; Cavalcante, D.M.; Simões-Carvalho, M.; Versiani, M.A. Present status and future directions-Minimal endodontic access cavities. Int. Endod. J. 2022, 55 (Suppl. 3), 531–587. [Google Scholar] [CrossRef]
- Lima, C.O.; Barbosa, A.F.A.; Ferreira, C.M.; Ferretti, M.A.; Aguiar, F.H.B.; Lopes, R.T.; Fidel, S.R.; Silva, E. Influence of ultraconservative access cavities on instrumentation efficacy with XP-endo Shaper and Reciproc, filling ability and load capacity of mandibular molars subjected to thermomechanical cycling. Int. Endod. J. 2021, 54, 1383–1393. [Google Scholar] [CrossRef]
- Ghasemi, N.; Janani, M.; Razi, T.; Atharmoghaddam, F. Effect of different mixing and placement methods on the quality of MTA apical plug in simulated apexification model. J. Clin. Exp. Dent. 2017, 9, e351–e355. [Google Scholar] [CrossRef] [PubMed]
- Jurado, C.A.; Amarillas-Gastelum, C.; Tonin, B.S.H.; Nielson, G.; Afrashtehfar, K.I.; Fischer, N.G. Traditional versus conservative endodontic access impact on fracture resistance of chairside CAD-CAM lithium disilicate anterior crowns: An in vitro study. J. Prosthodont. 2023, 32, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Salmani, Z.; Youssefi, N.; Heidari, B. Comparison of Microleakage of Mineral Trioxide Aggregate Apical Plug Applied by the Manual Technique and Indirect Use of Ultrasonic with Different Powers. J. Dent. 2021, 22, 290–295. [Google Scholar]
- Parashos, P.; Phoon, A.; Sathorn, C. Effect of ultrasonication on physical properties of mineral trioxide aggregate. Biomed. Res. Int. 2014, 2014, 191984. [Google Scholar] [CrossRef] [PubMed]
- Çalişkan, M.K.; Pehlivan, Y.; Sepetçioğlu, F.; Türkün, M.; Tuncer, S.Ş. Root canal morphology of human permanent teeth in a Turkish population. J. Endod. 1995, 21, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, F.M.; Kahler, B. Pulpal response after acute dental injury in the permanent dentition: Clinical implications—A review. J. Endod. 2015, 41, 299–308. [Google Scholar] [CrossRef]
- Curylofo-Zotti, F.A.; Lorencetti-Silva, F.; de Almeida Coelho, J.; Monteiro, R.M.; Watanabe, E.; Corona, S.A.M. Human teeth biobank: Microbiological analysis of the teeth storage solution. Microsc. Res. Tech. 2018, 81, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, X.; Yue, L.; Hou, B.; Yu, Q.; Fan, B.; Wei, X.; Qiu, L.; Huang, Z.; Xia, W.; et al. Experts consensus on the procedure of dental operative microscope in endodontics and operative dentistry. Int. J. Oral. Sci. 2023, 15, 43. [Google Scholar] [CrossRef]
- Swain, M.V.; Xue, J. State of the art of Micro-CT applications in dental research. Int. J. Oral. Sci. 2009, 1, 177–188. [Google Scholar] [CrossRef]
- Ørstavik, D.; Nordahl, I.; Tibballs, J.E. Dimensional change following setting of root canal sealer materials. Dent. Mater. 2001, 17, 512–519. [Google Scholar] [CrossRef]
- Torabinejad, M.; Hong, C.; McDonald, F.; Ford, T.P. Physical and chemical properties of a new root-end filling material. J. Endod. 1995, 21, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Orhan, K.; Celikten, B.; Orhan, A.I.; Tufenkci, P.; Sevimay, S. Evaluation of the sealing ability of different root canal sealers: A combined SEM and micro-CT study. J. Appl. Oral. Sci. 2018, 26, e20160584. [Google Scholar] [CrossRef]
- Keleş, A.; Torabinejad, M.; Keskin, C.; Sah, D.; Uzun, İ.; Alçin, H. Micro-CT evaluation of voids using two root filling techniques in the placement of MTA in mesial root canals of Vertucci type II configuration. Clin. Oral. Investig. 2018, 22, 1907–1913. [Google Scholar] [CrossRef]
- Jho, W.; Park, J.W.; Kim, E.; Song, M.; Seo, D.G.; Yang, D.K.; Shin, S.J. Comparison of root canal filling quality by mineral trioxide aggregate and gutta percha cones/AH plus sealer. Dent. Mater. J. 2016, 35, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Al-Kahtani, A.; Shostad, S.; Schifferle, R.; Bhambhani, S. In-vitro evaluation of microleakage of an orthograde apical plug of mineral trioxide aggregate in permanent teeth with simulated immature apices. J. Endod. 2005, 31, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Matt, G.D.; Thorpe, J.R.; Strother, J.M.; McClanahan, S.B. Comparative study of white and gray mineral trioxide aggregate (MTA) simulating a one-or two-step apical barrier technique. J. Endod. 2004, 30, 876–879. [Google Scholar] [CrossRef] [PubMed]
- de Leimburg, M.L.; Angeretti, A.; Ceruti, P.; Lendini, M.; Pasqualini, D.; Berutti, E. MTA obturation of pulpless teeth with open apices: Bacterial leakage as detected by polymerase chain reaction assay. J. Endod. 2004, 30, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.L.; Mann, V.; Gulabivala, K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: Part 1: Periapical health. Int. Endod. J. 2011, 44, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, U.; Hagglund, B.; Sundqvist, G.; Wing, K. Factors affecting the long-term results of endodontic treatment. J. Endod. 1990, 16, 498–504. [Google Scholar] [CrossRef]
- Somma, F.; Cretella, G.; Carotenuto, M.; Pecci, R.; Bedini, R.; De Biasi, M.; Angerame, D. Quality of thermoplasticized and single point root fillings assessed by micro-computed tomography. Int. Endod. J. 2011, 44, 362–369. [Google Scholar] [CrossRef]
- Dioguardi, M.; Di Gioia, G.; Illuzzi, G.; Arena, C.; Caponio, V.C.A.; Caloro, G.A.; Zhurakivska, K.; Adipietro, I.; Troiano, G.; Lo Muzio, L. Inspection of the Microbiota in Endodontic Lesions. Dent. J. 2019, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, J.L.; Fan, B. Tooth Morphology, Isolation, and Access. In Cohen’s Pathways of the Pulp, 11th ed.; Elsevier: St. Louis, MO, USA, 2016; pp. 130–208. [Google Scholar]
- Kim, S.Y.; Jang, Y.E.; Kim, B.S.; Pang, E.K.; Shim, K.; Jin, H.R.; Son, M.K.; Kim, Y. Effects of Ultrasonic Activation on Root Canal Filling Quality of Single-Cone Obturation with Calcium Silicate-Based Sealer. Materials 2021, 14, 1292. [Google Scholar] [CrossRef] [PubMed]
- Drukteinis, S.; Bilvinaite, G.; Shemesh, H.; Tusas, P.; Peciuliene, V. The Effect of Ultrasonic Agitation on the Porosity Distribution in Apically Perforated Root Canals Filled with Different Bioceramic Materials and Techniques: A Micro-CT Assessment. J. Clin. Med. 2021, 10, 4977. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alfayate, R.; Algar-Pinilla, J.; Mercade, M.; Foschi, F. Sonic activation improves bioceramic sealer’s penetration into the tubular dentin of curved root canals: A confocal laser scanning microscopy investigation. Appl. Sci. 2021, 11, 3902. [Google Scholar] [CrossRef]
- Azim, A.A.; Piasecki, L.; da Silva Neto, U.X.; Cruz, A.T.G.; Azim, K.A. XP Shaper, A Novel Adaptive Core Rotary Instrument: Micro-computed Tomographic Analysis of Its Shaping Abilities. J. Endod. 2017, 43, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.; Koch, K. In vitro radicular temperatures produced by injectable thermoplasticized gutta-percha. Int. Endod. J. 1995, 28, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Gulabivala, K.; Ng, Y.L. Factors that affect the outcomes of root canal treatment and retreatment-A reframing of the principles. Int. Endod. J. 2023, 56 (Suppl. 2), 82–115. [Google Scholar] [CrossRef]
- Billington, R.W.; Williams, J.A.; Pearson, G.J. Variation in powder/liquid ratio of a restorative glass-ionomer cement used in dental practice. Br. Dent. J. 1990, 169, 164–167. [Google Scholar] [CrossRef]
| Inclusion Criteria |
|
| Exclusion Criteria |
|
| Manual (n = 10) | Ultrasonic (n = 10) | XPS (n = 10) | (Manual-Ultrasonic) p | (Manual-XPS) p | (Ultrasonic-XPS) p | ||
|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | |||||
| Closed-coronal | TradAC | 0.08 (0.01–0.14) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.007 | 0.002 | 0.303 |
| ConsAC | 0.02 (0.01–0.03) | 0.10 (0.04–0.15) | 0.13 (0.04–0.39) | 0.082 | 0.019 | 0.451 | |
| p | 0.256 | 0.004 | 0.001 | ||||
| Closed-apical | TradAC | 0.03 (0.01–0.04) | 0.01 (0.00–0.03) | 0.02 (0.00–0.03) | 0.149 | 0.271 | 0.788 |
| ConsAC | 0.01 (0.00–0.03) | 0.05 (0.03–0.07) | 0.06 (0.01–0.11) | 0.029 | 0.008 | 0.649 | |
| p | 0.023 | 0.015 | 0.103 | ||||
| Open-coronal | TradAC | 8.4 (6.6–16.3) | 27.2 (17.1–37.5) | 36.8 (29.45–63.2) | 0.009 | 0.002 | 0.041 |
| ConsAC | 21.5 (17.2–26.3) | 39.5 (35.2–48.7) | 28.9 (25.6–32.5) | 0.007 | 0.041 | 0.023 | |
| p | 0.048 | 0.023 | 0.112 | ||||
| Open-apical | TradAC | 11.7 (8.7–18.9) | 23.9 (18.8–29.2) | 47.4 (36.5–59.6) | 0.009 | 0.000 | 0.000 |
| ConsAC | 22.8 (18.0–27.7) | 52.5 (40.6–61.0) | 36.5 (26.6–44.1) | 0.005 | 0.019 | 0.049 | |
| p | 0.019 | 0.007 | 0.046 | ||||
| Total-coronal | TradAC | 8.5 (6.7–16.3) | 27.2 (17.1–37.6) | 36.9 (29.4–63.2) | 0.009 | 0.002 | 0.041 |
| ConsAC | 21.5 (17.2–26.4) | 39.6 (35.3–48.8) | 29.0 (26–33.4) | 0.007 | 0.028 | 0.023 | |
| p | 0.045 | 0.023 | 0.131 | ||||
| Total-apical | TradAC | 11.7 (8.8–19.0) | 23.9 (18.8–29.3) | 47.4 (36.5–59.6) | 0.009 | 0.000 | 0.000 |
| ConsAC | 22.8 (18.0–27.7) | 52.5 (40.6–61.1) | 36.6 (26.6–44.2) | 0.005 | 0.019 | 0.049 | |
| p | 0.019 | 0.007 | 0.049 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odabaşı Tezer, E.; Buyuksungur, A.; Celikten, B.; Dursun, P.H.; Sevimay, F.S. Effects of Access Cavity Design and Placement Techniques on Mineral Trioxide Aggregate Obturation Quality in Simulated Immature Teeth: A Micro-Computed Tomography Study. Medicina 2024, 60, 878. https://doi.org/10.3390/medicina60060878
Odabaşı Tezer E, Buyuksungur A, Celikten B, Dursun PH, Sevimay FS. Effects of Access Cavity Design and Placement Techniques on Mineral Trioxide Aggregate Obturation Quality in Simulated Immature Teeth: A Micro-Computed Tomography Study. Medicina. 2024; 60(6):878. https://doi.org/10.3390/medicina60060878
Chicago/Turabian StyleOdabaşı Tezer, Emine, Arda Buyuksungur, Berkan Celikten, Pınar Hava Dursun, and Fatma Semra Sevimay. 2024. "Effects of Access Cavity Design and Placement Techniques on Mineral Trioxide Aggregate Obturation Quality in Simulated Immature Teeth: A Micro-Computed Tomography Study" Medicina 60, no. 6: 878. https://doi.org/10.3390/medicina60060878






