Is It Still Time for Safety Walkaround? Pilot Project Proposing a New Model and a Review of the Methodology
Abstract
:1. Introduction
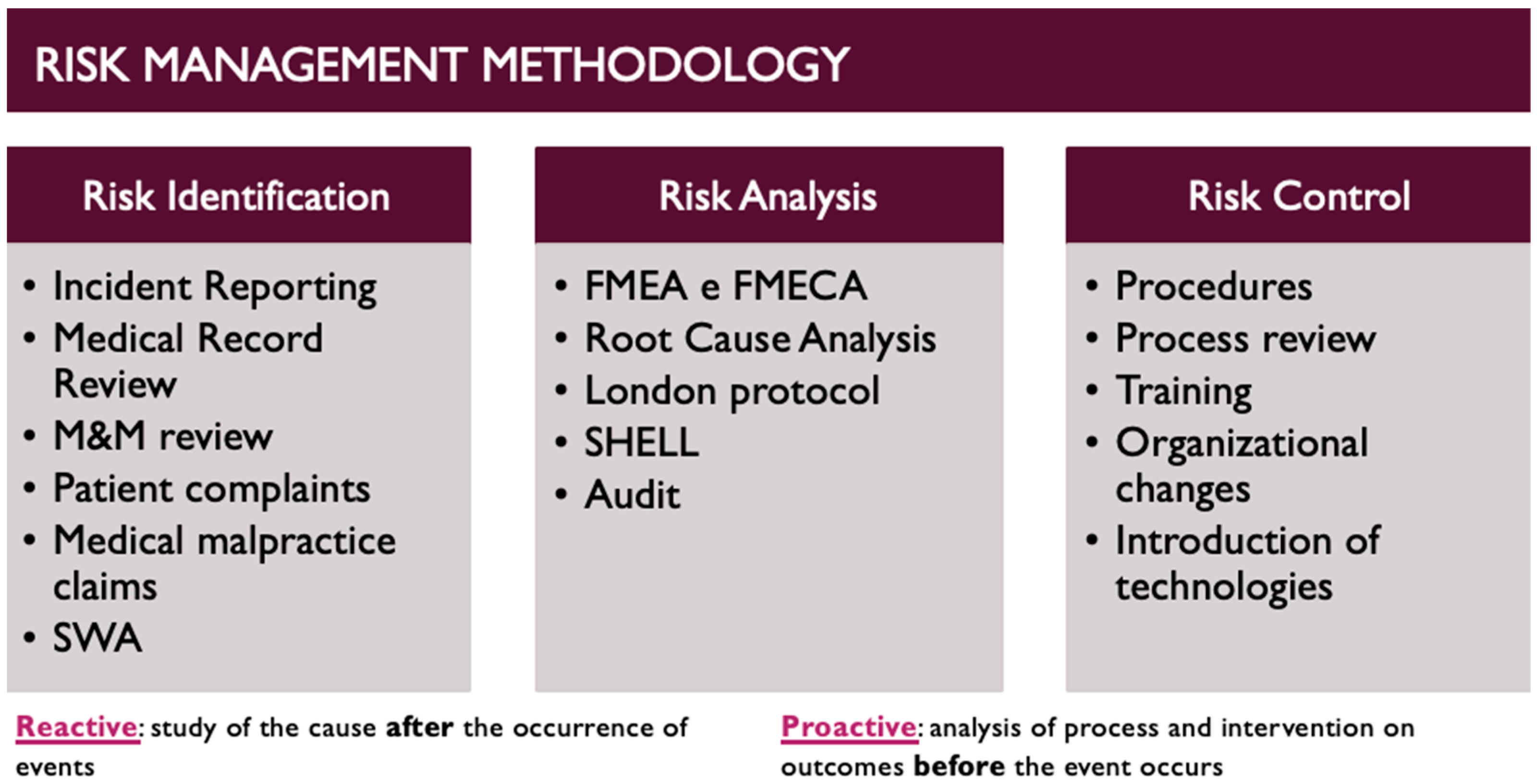
2. Materials and Methods
- -
- A physician director from the healthcare (HC) management and the clinical risk manager of the hospital as group coordinators;
- -
- A specialist in forensic medicine to evaluate issues relating to informed consent and the correct compilation of health documentation;
- -
- An external expert with expertise in medical error research for operational support;
- -
- Nursing staff for the assessment of nursing activities;
- -
- A pharmacist for the examination of potentially dangerous practices inherent to the process of distribution, storage, and administration of drugs;
- -
- The occupational doctor of the company for the prevention of workplace risks;
- -
- A psychologist expert in burnout assessment for the analysis of behavioral and emotional variables;
- -
- A patient representative proposed by patient associations, involved in measuring the perception of the patient population.
3. Results
- -
- The analysis of medical records showed incomplete compilation of the medical records;
- -
- The study of forms related to informed consent within the medical records indicated that insufficient information given to patients and insufficient completion of the forms were present;
- -
- The evaluation of the application of the procedures relating to the 19 recommendations of the Italian Ministry of Health showed sufficient compliance with EBM practices;
- -
- The specialist psychological evaluation concluded a not critical level of burnout despite operating volumes;
- -
- The CARE questionnaire revealed that all (100% of 50 patients) of the interviewees measured a level of satisfaction no lower than “Very Good” and “Excellent” in both departments, confirming HCW dedication for willingness to dialogue with patients and optimal level of empathy in the relationship with the patient.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Expenditure on Health: Public Spending on the Rise? Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2021.
- Davies, H.T.; Nutley, S.M.; Mannion, R. Organisational culture and quality of health care. Qual. Health Care 2000, 9, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Reason, J. Human Error; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar] [CrossRef]
- Kohn, L.T.; Corrigan, J.M.; Donaldson, M.S. (Eds.) To Err Is Human: Building a Safer Health System; National Academies Press: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Tasker, A. Blaming, naming and a just culture. BMJ Lead. 2023, 7, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Brown, G.D.; Hicks, L.L. From a blame culture to a just culture in health care. Health Care Manag. Rev. 2009, 34, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.gazzettaufficiale.it/eli/id/2017/03/17/17G00041/sg (accessed on 2 April 2024).
- Frankel, A.; Graydon-Baker, E.; Neppl, C.; Simmonds, T.; Gustafson, M.; Gandhi, T.K. Patient Safety Leadership WalkRounds. Jt. Comm. J. Qual. Saf. 2003, 29, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Klimmeck, S.; Sexton, J.B.; Schwendimann, R. Changes in Safety and Teamwork Climate After Adding Structured Observations to Patient Safety WalkRounds. Jt. Comm. J. Qual. Patient Saf. 2021, 47, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Sexton, J.B.; Adair, K.C.; Profit, J.; Bae, J.; Rehder, K.J.; Gosselin, T.; Milne, J.; Leonard, M.; Frankel, A. Safety Culture and Workforce Well-Being Associations with Positive Leadership WalkRounds. Jt. Comm. J. Qual. Patient Saf. 2021, 47, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, M.; Boecker, M.; Forkmann, T.; Neumann, M. Evaluation of the “Consultation and Relational Empathy” (CARE) measure by means of Rasch-analysis at the example of cancer patients. Patient Educ. Couns. 2011, 82, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Sablone, S.; Spagnolo, L.; Macorano, E.; Ciavarella, M.C.; Pascale, N.; Strisciullo, G.; Introna, F.; Di Fazio, A. “Freedom and Dignity Are Worth More than Life”: The Dramatic Suicide of an Anti-Vax Man. Healthcare 2022, 10, 2141. [Google Scholar] [CrossRef] [PubMed]
- Mercer, S.W.; Maxwell, M.; Heaney, D.; Watt, G.C. The consultation and relational empathy (CARE) measure: Development and preliminary validation and reliability of an empathy-based consultation process measure. Fam. Pract. 2004, 21, 699–705. [Google Scholar] [CrossRef]
- Aven, T. Risk assessment and risk management: Review of recent advances on their foundation. Eur. J. Oper. Res. 2016, 253, 1–13. [Google Scholar] [CrossRef]
- Liu, H.C.; Zhang, L.J.; Ping, Y.J.; Wang, L. Failure mode and effects analysis for proactive healthcare risk evaluation: A systematic literature review. J. Eval. Clin. Pract. 2020, 26, 1320–1337. [Google Scholar] [CrossRef] [PubMed]
- World Alliance for Patient Safety. WHO Draft Guidelines for Adverse Event Reporting and Learning Systems: From Information to Action; WHO REFERENCE NUMBER: WHO/EIP/SPO/QPS/05.3; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Carlfjord, S.; Öhrn, A.; Gunnarsson, A. Experiences from ten years of incident reporting in health care: A qualitative study among department managers and coordinators. BMC Health Serv. Res. 2018, 18, 113. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.B.-A.; Sheldon, T.A.; Cracknell, A.; Turnbull, A. Sensitivity of routine system for reporting patient safety incidents in an NHS hospital: Retrospective patient case note review. BMJ 2007, 334, 79. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.W.; Garrubba, M.L.; Melder, A.M. Informing best practice for conducting morbidity and mortality reviews: A literature review. Aust. Health Rev. 2018, 42, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Esclassan, R.; Valera, M.-C.; Bergia, J.M.; Canceill, T.; Mendes, L.C.; Bailleul-Forestier, I.; Gardette, V.; Vaysse, F.; Gurgel-Georgelin, M.; Noirrit, E. Morbidity and Mortality Review in a University Dental Hospital: A Necessary Tool to Improve Quality of Care. Eur. J. Dent. 2021, 15, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; Bertozzi, G.; Volonnino, G.; Di Fazio, A.; Di Fazio, N.; Arcangeli, M.; La Russa, R.; Frati, P. Learning from the Past to Improve the Future-Vaccine Hesitancy Determinants in the Italian Population: A Systematic Review. Vaccines 2023, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, V.; Gerardi, D.; Giugliano, G.; Di Fazio, A.; Stabile, E. Focus on Prevention: Peripheral Arterial Disease and the Central Role of the Cardiologist. J. Clin. Med. 2023, 12, 4338. [Google Scholar] [CrossRef]
- Albano, G.D.; Bertozzi, G.; Maglietta, F.; Montana, A.; Di Mizio, G.; Esposito, M.; Mazzeo, P.; D’Errico, S.; Salerno, M. Medical Records Quality as Prevention Tool for Healthcare-Associated Infections (HAIs) Related Litigation: A Case Series. Curr. Pharm. Biotechnol. 2019, 20, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.A.; Localio, A.R.; Leape, L.L.; Laird, N.M.; Peterson, L.; Hiatt, H.H.; Barnes, B.A. Identification of adverse events occurring during hospitalization. A cross-sectional study of litigation, quality assurance, and medical records at two teaching hospitals. Ann. Intern. Med. 1990, 112, 221–226. [Google Scholar] [CrossRef]
- Available online: https://qualityindicators.ahrq.gov/measures/psi_resources (accessed on 1 April 2024).
- Havranek, M.M.; Rüter, F.; Bilger, S.; Dahlem, Y.; Oliveira, L.; Ehbrecht, D.; Moos, R.M.; Westerhoff, C.; Beck, T.; Le Pogam, M.-A. Validity of 16 AHRQ Patient Safety Indicators to identify in-hospital complications: A medical record review across nine Swiss hospitals. Int. J. Qual. Health Care 2023, 35. [Google Scholar] [CrossRef]
- Bertozzi, G.; Maglietta, F.; Baldari, B.; Besi, L.; Torsello, A.; Di Gioia, C.R.T.; Sessa, F.; Aromatario, M.; Cipolloni, L. Mistrial or Misdiagnosis: The Importance of Autopsy and Histopathological Examination in Cases of Sudden Infant Bronchiolitis-Related Death. Front. Pediatr. 2020, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Garon-Sayegh, P. Analysis of medical malpractice claims to improve quality of care: Cautionary remarks. J. Eval. Clin. Pract. 2019, 25, 744–750. [Google Scholar] [CrossRef]
- Shojania, K.G. The Elephant of Patient Safety: What You See Depends on How You Look. Jt. Comm. J. Qual. Patient Saf. 2010, 36, 399–401, AP1–AP3. [Google Scholar] [CrossRef]
- Levtzion-Korach, O.; Frankel, A.; Alcalai, H.; Keohane, C.; Orav, J.; Graydon-Baker, E.; Barnes, J.; Gordon, K.; Puopolo, A.L.; Tomov, E.I.; et al. Integrating Incident Data from Five Reporting Systems to Assess Patient Safety: Making Sense of the Elephant. Jt. Comm. J. Qual. Patient Saf. 2010, 36, 402–410, AP1–AP18. [Google Scholar] [CrossRef] [PubMed]
- Carayon, P.; Wetterneck, T.B.; Rivera-Rodriguez, A.J.; Hundt, A.S.; Hoonakker, P.; Holden, R.; Gurses, A.P. Human factors systems approach to healthcare quality and patient safety. Appl. Ergon. 2014, 45, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Global Patient Safety Action Plan 2021–2030: Towards Eliminating Avoidable Harm in Health Care; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2021.
- Liepelt, S.; Sundal, H.; Kirchhoff, R. Team experiences of the root cause analysis process after a sentinel event: A qualitative case study. BMC Health Serv. Res. 2023, 23, 1224. [Google Scholar] [CrossRef]
- Nicolini, D.; Waring, J.; Mengis, J. Policy and practice in the use of root cause analysis to investigate clinical adverse events: Mind the gap. Soc. Sci. Med. 2011, 73, 217–225. [Google Scholar] [CrossRef]
- Cronin, C.M.G. Five years of learning from analysis of clinical occurrences in pediatric care using the London Protocol. Healthc. Q. 2006, 9, 16–21. [Google Scholar] [CrossRef] [PubMed]
- La Russa, R.; Fazio, V.; Ferrara, M.; Di Fazio, N.; Viola, R.V.; Piras, G.; Ciano, G.; Micheletta, F.; Frati, P. Proactive Risk Assessment Through Failure Mode and Effect Analysis (FMEA) for Haemodialysis Facilities: A Pilot Project. Front. Public Health 2022, 10, 823680. [Google Scholar] [CrossRef]
- Micheletta, F.; Ferrara, M.; Bertozzi, G.; Volonnino, G.; Nasso, M.; La Russa, R. Proactive Risk Assessment through Failure Mode and Effect Analysis (FMEA) for Perioperative Management Model of Oral Anticoagulant Therapy: A Pilot Project. Int. J. Environ. Res. Public Health 2022, 19, 16430. [Google Scholar] [CrossRef]
- Molloy, G.J.; O’Boyle, C.A. The SHEL model: A useful tool for analyzing and teaching the contribution of Human Factors to medical error. Acad. Med. 2005, 80, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Cacciabue, P.C.; Vella, G. Human factors engineering in healthcare systems: The problem of human error and accident management. Int. J. Med. Inform. 2010, 79, e1–e17. [Google Scholar] [CrossRef]
- Esposito, P.; Dal Canton, A. Clinical audit, a valuable tool to improve quality of care: General methodology and applications in nephrology. World J. Nephrol. 2014, 3, 249–255. [Google Scholar] [CrossRef]
- Bowie, P.; McNaughton, E.; Bruce, D.; Holly, D.; Forrest, E.; Macleod, M.; Kennedy, S.; Power, A.; Toppin, D.; Black, I.; et al. Enhancing the Effectiveness of Significant Event Analysis: Exploring Personal Impact and Applying Systems Thinking in Primary Care. J. Contin. Educ. Health Prof. 2016, 36, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; Bertozzi, G.; Di Fazio, N.; Aquila, I.; Di Fazio, A.; Maiese, A.; Volonnino, G.; Frati, P.; La Russa, R. Risk Management and Patient Safety in the Artificial Intelligence Era: A Systematic Review. Healthcare 2024, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Evans, L.E.; Rhodes, A. The Surviving Sepsis Campaign Bundle: 2018 Update. Crit. Care Med. 2018, 46, 925–928. [Google Scholar] [CrossRef]
- Smith, K.M.; Valenta, A.L. Safety I to Safety II: A Paradigm Shift or More Work as Imagined? Comment on “False Dawns and New Horizons in Patient Safety Research and Practice”. Int. J. Health Policy Manag. 2018, 7, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Mannion, R.; Braithwaite, J. False Dawns and New Horizons in Patient Safety Research and Practice. Int. J. Health Policy Manag. 2017, 6, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, M.J.; de Vos, M.S.; Sujan, M.; Hamming, J.F. The problem with making Safety-II work in healthcare. BMJ Qual. Saf. 2022, 31, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nisio, A.; Magrì, F. L’ERM nel settore sanitario italiano: Proposta di un modello. In Proceedings of the 5th Management Control Journal Workshop, Rimini, Italy, 23–24 June 2016. [Google Scholar] [CrossRef]
- Available online: https://www.erm-academy.org/risk-management-knowledge/coso-erm-framework/ (accessed on 1 April 2024).
- Etges, A.P.; de Souza, J.; Kliemann, F.; Felix, E. A proposed enterprise risk management model for health organizations A proposed enterprise risk management model for health organizations. J. Risk Res. 2018, 22, 1422780. [Google Scholar] [CrossRef]
- Burgess, N.; Radnor, Z. Evaluating Lean in healthcare. Int. J. Health Care Qual. Assur. 2013, 26, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.A.; Tortorella, G.; Rossini, M.; Portioli-Staudacher, A. Lean implementation in healthcare supply chain: A scoping review. J. Health Organ. Manag. 2019, 33, 304–322. [Google Scholar] [CrossRef] [PubMed]
- Womack, J.; Jones, D. Lean Thinking: Banish Waste and Create Wealth in Your Corporation; Simon & Schuster: New York, NY, USA, 1996; Volume 48. [Google Scholar]
- Pakdil, F.; Harwood, T.N.; Isin, F.B. Implementing Lean Principles in the Healthcare Industry: A Theoretical and Practical Overview. In Delivering Superior Health and Wellness Management with IoT and Analytics; Wickramasinghe, N., Bodendorf, F., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 383–413. ISBN 978-3-030-17347-0. [Google Scholar]
- Poksinska, B. The current state of Lean implementation in health care: Literature review. Qual. Manag. Health Care 2010, 19, 319–329. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, P.; Kreckler, S.; New, S.; Sheena, Y.; Handa, A.; Catchpole, K. Effect of a “Lean” intervention to improve safety processes and outcomes on a surgical emergency unit. BMJ 2010, 341, c5469. [Google Scholar] [CrossRef] [PubMed]
- Raab, S.S.; Andrew-Jaja, C.; Grzybicki, D.M.; Vrbin, C.M.; Chesin, C.M.; Fisch, J.M.; Dabbs, D.J.; Sommer, D.L.; Blaser, S.M. Dissemination of Lean methods to improve Pap testing quality and patient safety. J. Low. Genit. Tract Dis. 2008, 12, 103–110. [Google Scholar] [CrossRef]
- Smith, M.L.; Wilkerson, T.; Grzybicki, D.M.; Raab, S.S. The effect of a Lean quality improvement implementation program on surgical pathology specimen accessioning and gross preparation error frequency. Am. J. Clin. Pathol. 2012, 138, 367–373. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, M.; Pascale, N.; Ciavarella, M.; Bertozzi, G.; Bellettieri, A.P.; Di Fazio, A. Is It Still Time for Safety Walkaround? Pilot Project Proposing a New Model and a Review of the Methodology. Medicina 2024, 60, 903. https://doi.org/10.3390/medicina60060903
Ferrara M, Pascale N, Ciavarella M, Bertozzi G, Bellettieri AP, Di Fazio A. Is It Still Time for Safety Walkaround? Pilot Project Proposing a New Model and a Review of the Methodology. Medicina. 2024; 60(6):903. https://doi.org/10.3390/medicina60060903
Chicago/Turabian StyleFerrara, Michela, Natascha Pascale, Mauro Ciavarella, Giuseppe Bertozzi, Angela Pia Bellettieri, and Aldo Di Fazio. 2024. "Is It Still Time for Safety Walkaround? Pilot Project Proposing a New Model and a Review of the Methodology" Medicina 60, no. 6: 903. https://doi.org/10.3390/medicina60060903






