Combined Periodontal-Orthodontic Treatment with Periodontal Corticotomy Regenerative Surgery in an Adult Patient Suffering from Periodontitis and Skeletal Class II Malocclusion: A Case Report with 5-Year Longitudinal Observation
Abstract
1. Introduction
2. Case Description
2.1. Chief Complaints
2.2. History of Present Illness
2.3. History of Past Illness
2.4. Personal and Family History
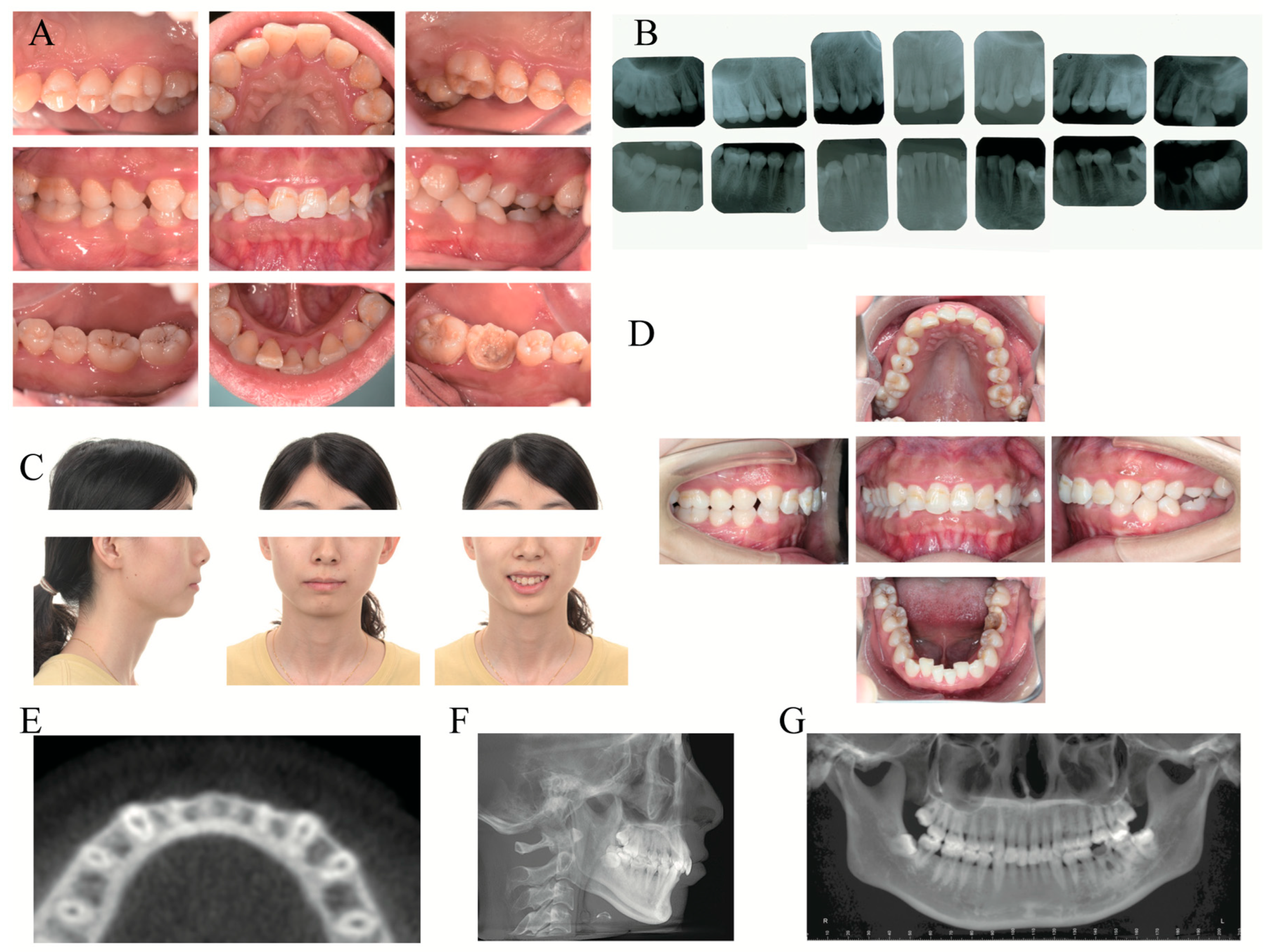
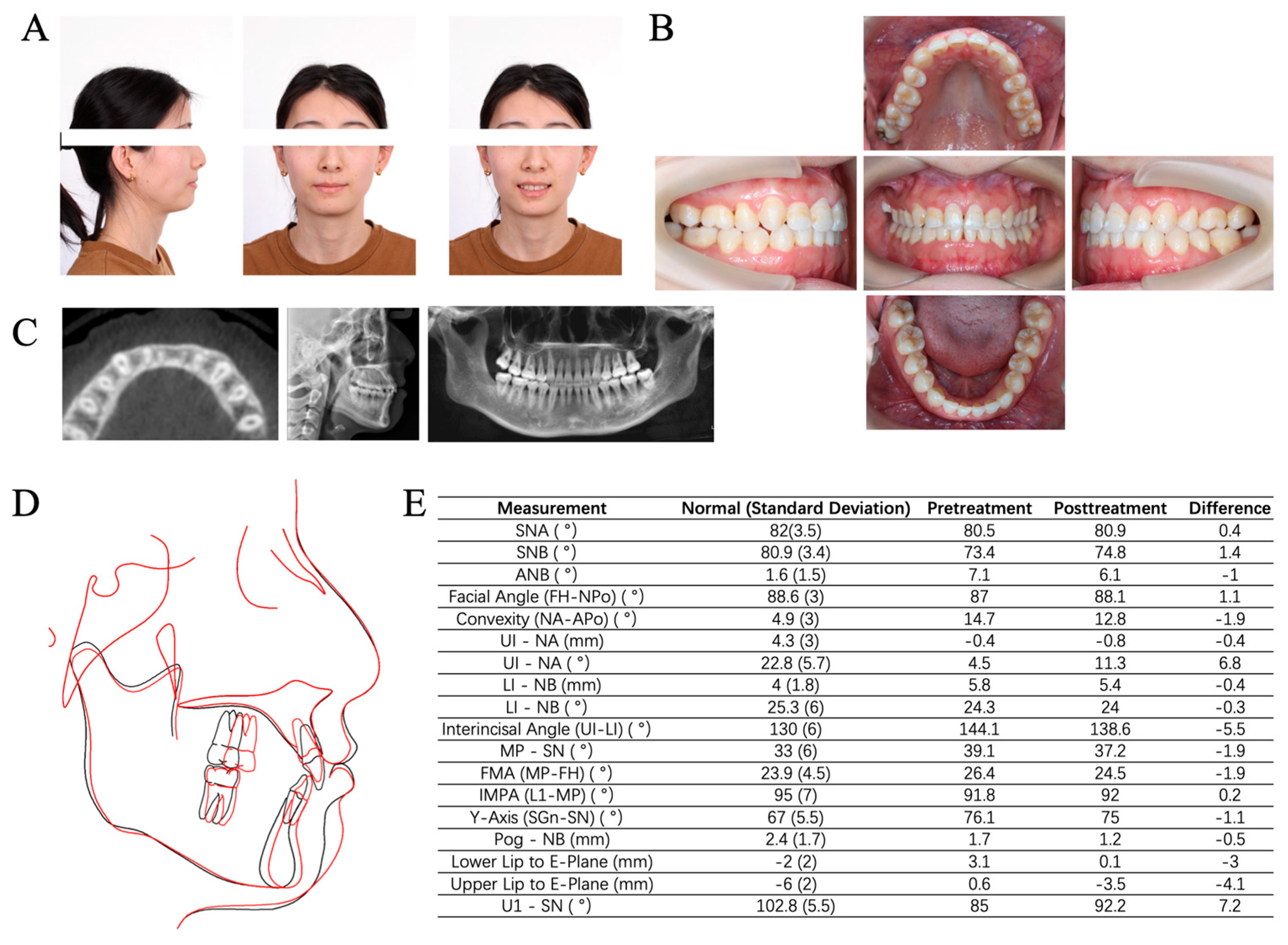
2.5. Clinical Examination
2.6. Laboratory Examinations
2.7. Imaging Examinations
2.8. Final Diagnosis and Treatment Plan
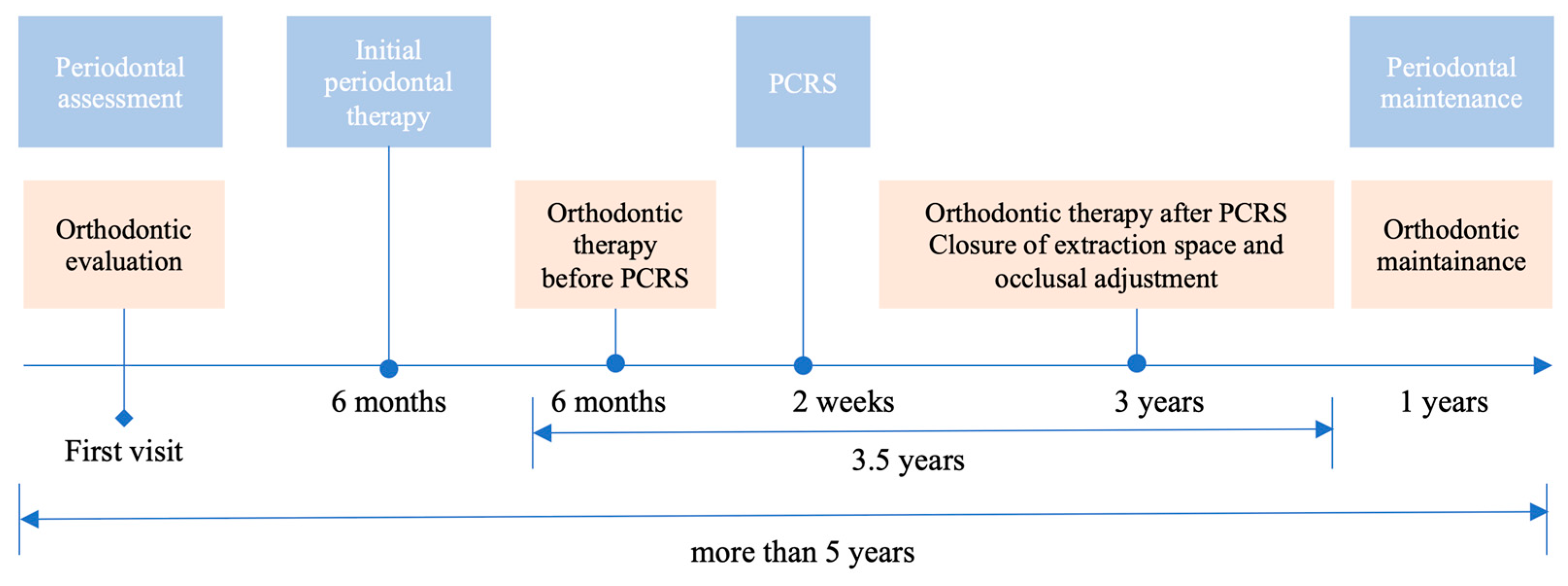
3. Treatment Process
3.1. Initial Periodontal Treatment
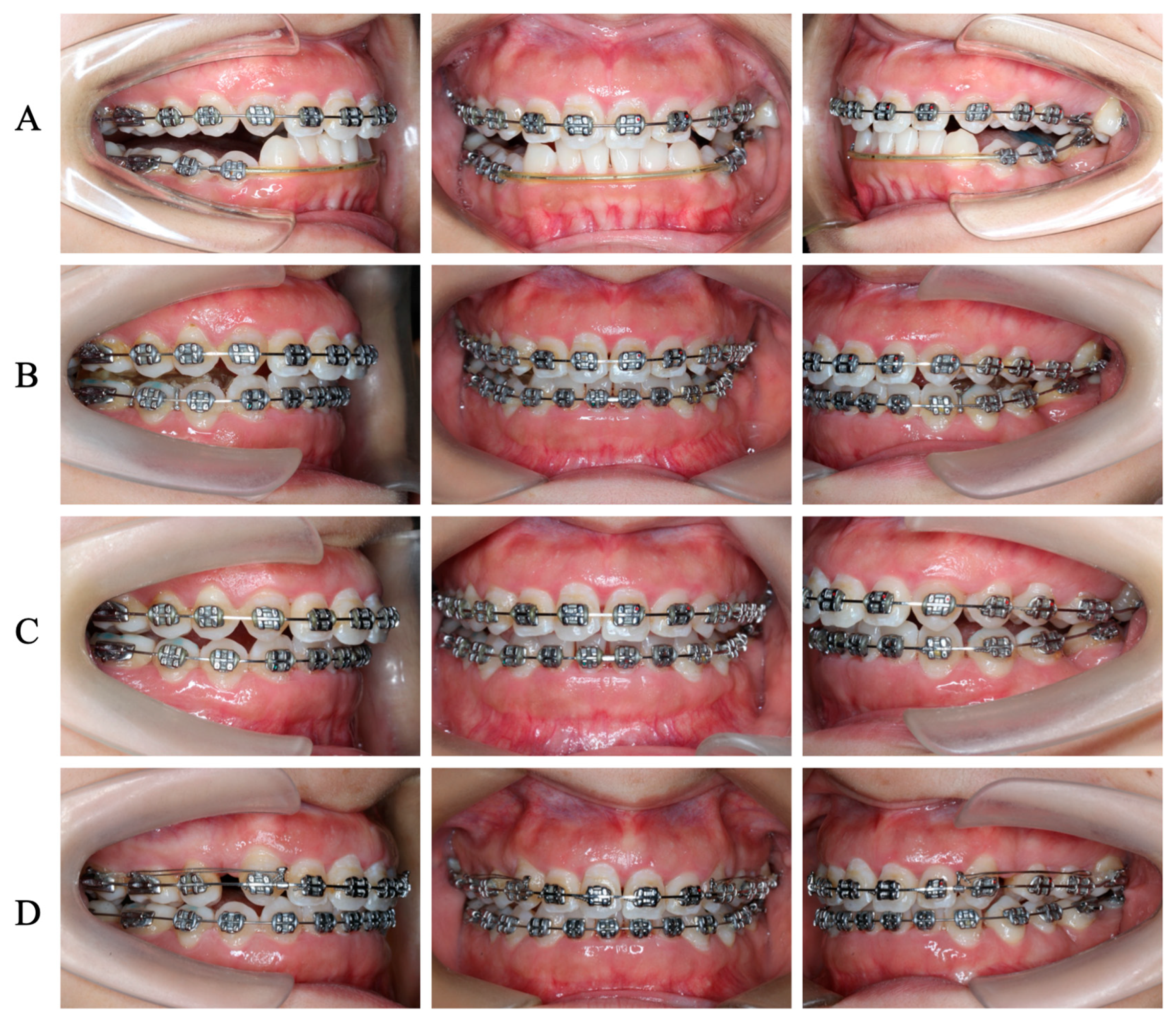
3.2. Orthodontic Treatment before PCRS
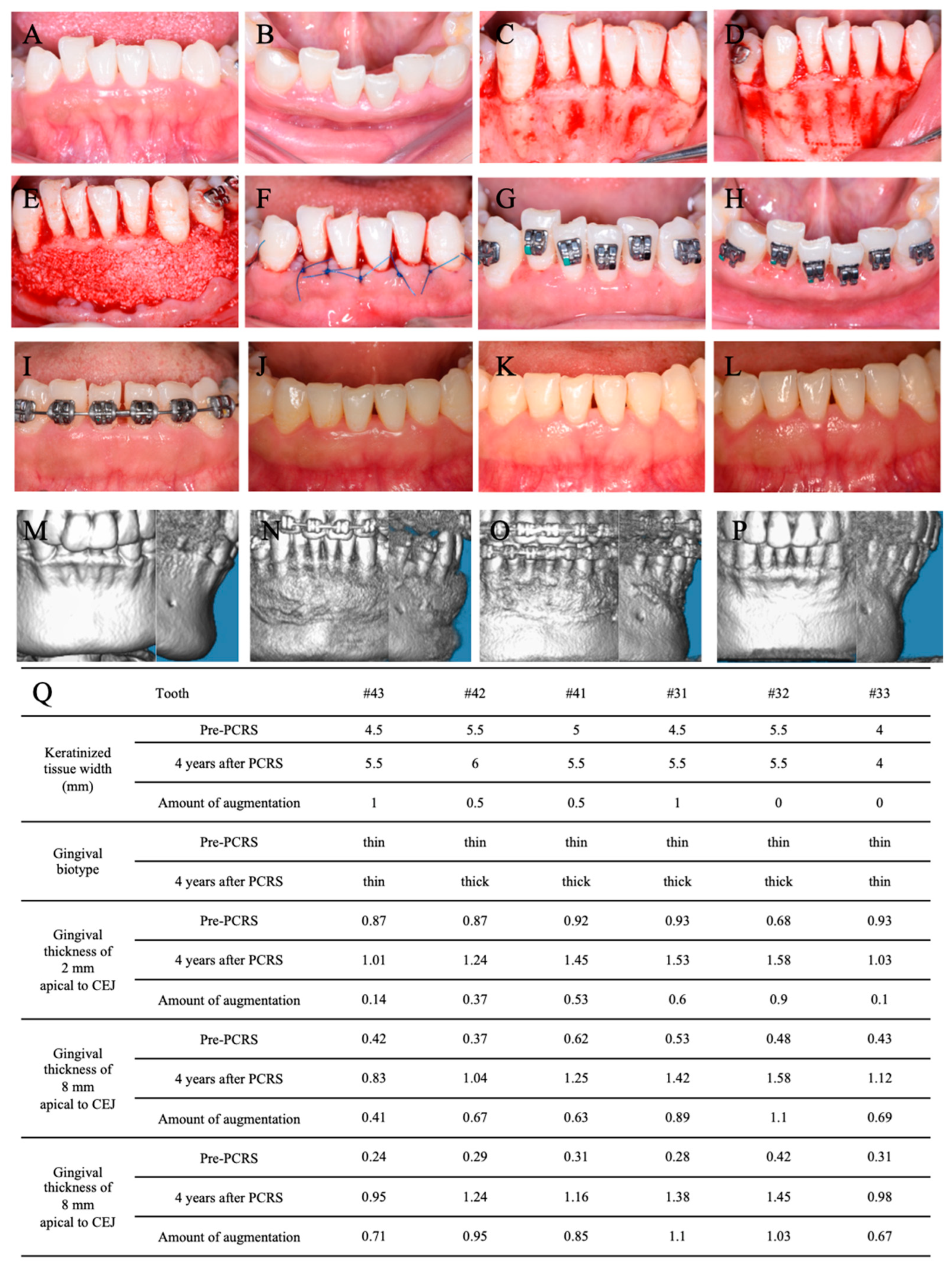
3.3. PCRS with Piezoelectric Devices
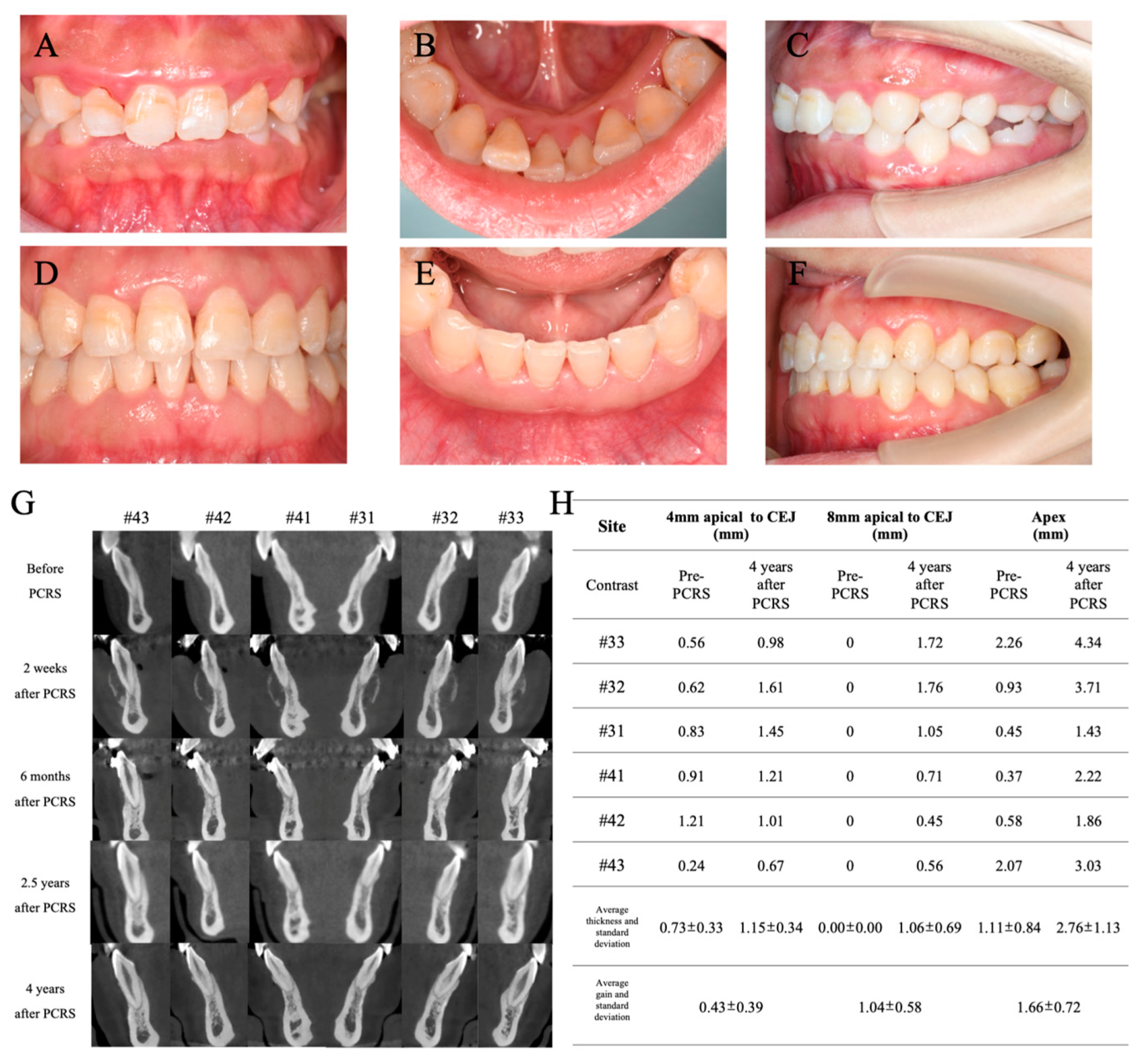
3.4. Postoperative Orthodontic Treatment and Maintenance
3.5. Periodontal Maintenance
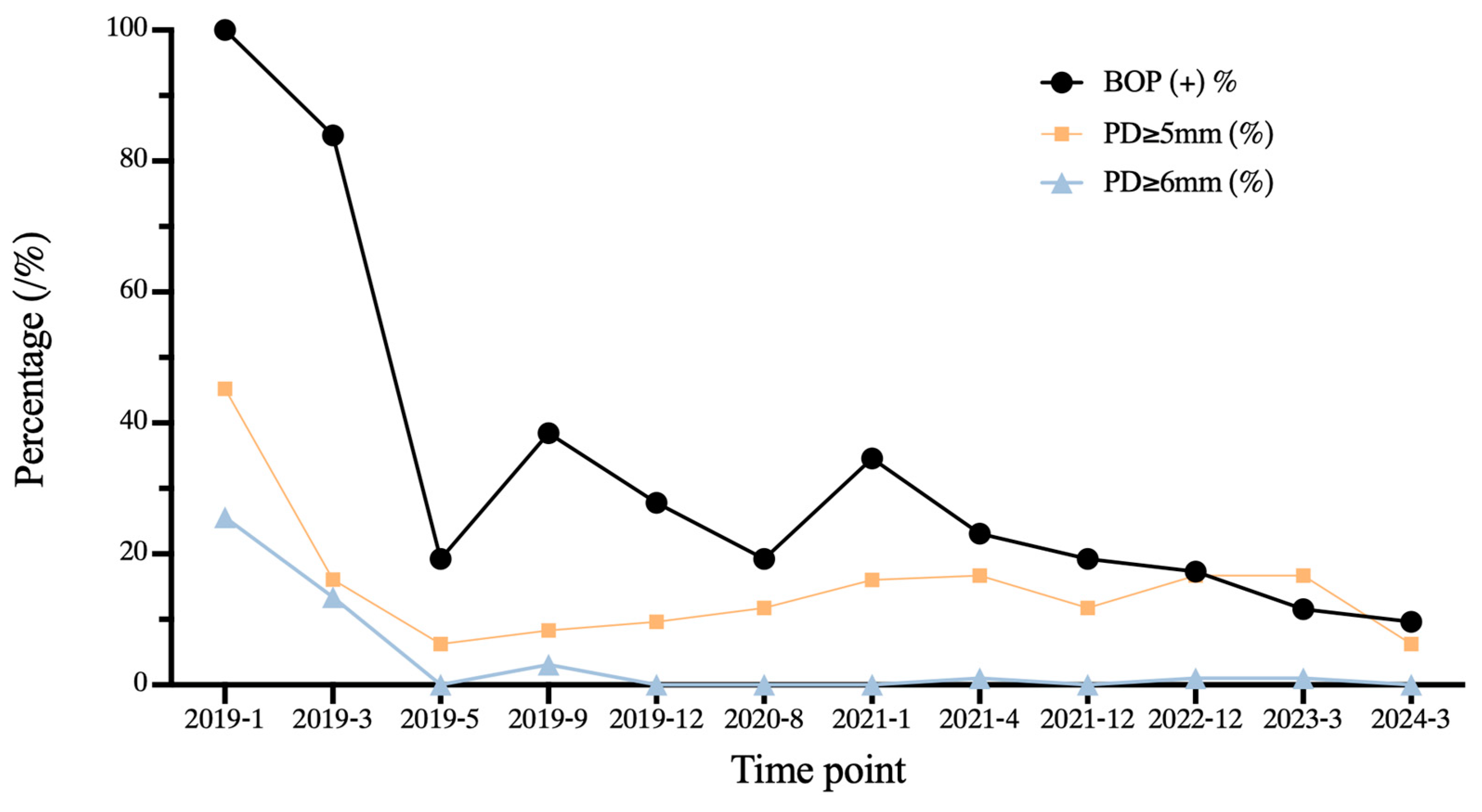
3.6. Outcome and Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- George, A.M.; Felicita, A.S.; Milling Tania, S.D.; Priyadharsini, J.V. Systematic review on the genetic factors associated with skeletal Class II malocclusion. Indian. J. Dent. Res. 2021, 32, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mei, M.; Han, G.; Luan, Q.; Zhou, Y. Effectiveness of modified periodontally accelerated osteogenic orthodontics in skeletal class II malocclusion treated by a camouflage approach. Am. J. Transl. Res. 2022, 14, 979–989. [Google Scholar] [PubMed]
- Kadkhodazadeh, M.; Amid, R.; Moscowchi, A.; Mansouri, H. Periodontal phenotype modification in orthodontic patients. J. Esthet. Restor. Dent. 2024, 36, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Gasparro, R.; Bucci, R.; De Rosa, F.; Sammartino, G.; Bucci, P.; D’Anto, V.; Marenzi, G. Effectiveness of surgical procedures in the acceleration of orthodontic tooth movement: Findings from systematic reviews and meta-analyses. Jpn. Dent. Sci. Rev. 2022, 58, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Miao, L.; Liu, J.; Xu, X.; Yue, Z.; Xu, M.; Shu, C.; Xu, L.; Hou, J. Periodontal soft tissue increase induced by periodontally accelerated osteogenic orthodontics surgery. BMC Oral Health 2022, 22, 506. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ning, R. Evaluation of root resorption in the lower incisors after orthodontic treatment of skeletal Class III malocclusion by three-dimensional volumetric measurement with cone-beam computed tomography. Angle Orthod. 2023, 93, 320–327. [Google Scholar] [CrossRef]
- Jiao, J.; Jing, W.; Si, Y.; Feng, X.; Tai, B.; Hu, D.; Lin, H.; Wang, B.; Wang, C.; Zheng, S.; et al. The prevalence and severity of periodontal disease in Mainland China: Data from the Fourth National Oral Health Survey (2015–2016). J. Clin. Periodontol. 2021, 48, 168–179. [Google Scholar] [CrossRef]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S237–S248. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.D.; Jiao, J.; Xu, L.; Hou, J.X.; Li, X.T.; Wang, X.X.; Xu, X.; Mao, M.X. Periodontal soft- and hard-tissue changes after augmented corticotomy in Chinese adult patients with skeletal Angle Class III malocclusion: A non-randomized controlled trial. J. Periodontol. 2020, 91, 1419–1428. [Google Scholar] [CrossRef]
- Ma, Z.; Zhu, Y.; Zhan, Y.; Zhang, Y.; Abdelrehem, A.; Wang, B.; Yang, C. Periosteum coverage versus collagen-membrane coverage in periodontally accelerated osteogenic orthodontics: A randomized controlled clinical trial in Class II and Class III malocclusions. BMC Oral Health 2022, 22, 439. [Google Scholar] [CrossRef]
- Rodrigues, D.M.; Petersen, R.L.; Montez, C.; de Moraes, J.R.; Januario, A.L.; Barboza, E.P. Relationship between anterior maxillary tooth sagittal root position and periodontal phenotype: A clinical and tomographic study. Clin. Oral Investig. 2022, 26, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.Q.; Liu, J.; Xu, L.; Xu, X.; Hou, J.X.; Li, X.T.; Wang, X.X. A long-term evaluation of periodontal phenotypes before and after the periodontal-orthodontic-orthognathic combined treatment of lower anterior teeth in patients with skeletal Angle class III malocclusion. Beijing Da Xue Xue Bao Yi Xue Ban 2023, 55, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Sanz, M.; Kebschull, M.; Jepsen, S.; Sculean, A.; Berglundh, T.; Papapanou, P.N.; Chapple, I.; Tonetti, M.S. Treatment of stage IV periodontitis: The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2022, 49 (Suppl. 24), 4–71. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Y.; Alkan, O.; Keskin, S. An evaluation of the gingival biotype and the width of keratinized gingiva in the mandibular anterior region of individuals with different dental malocclusion groups and levels of crowding. Korean J. Orthod. 2017, 47, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Zasciurinskiene, E.; Bulotaite, S.; Bjerklin, K.; Lodiene, G.; Sidlauskas, A.; Zaborskis, A. Knowledge, attitudes, and interest in orthodontic treatment: A cross-sectional study in adults with stage III-IV periodontitis and secondary malocclusions. BMC Oral Health 2023, 23, 853. [Google Scholar] [CrossRef]
- Wang, C.W.; Yu, S.H.; Mandelaris, G.A.; Wang, H.L. Is periodontal phenotype modification therapy beneficial for patients receiving orthodontic treatment? An American Academy of Periodontology best evidence review. J. Periodontol. 2020, 91, 299–310. [Google Scholar] [CrossRef]
- Malpartida-Carrillo, V.; Tinedo-Lopez, P.L.; Guerrero, M.E.; Amaya-Pajares, S.P.; Ozcan, M.; Rosing, C.K. Periodontal phenotype: A review of historical and current classifications evaluating different methods and characteristics. J. Esthet. Restor. Dent. 2021, 33, 432–445. [Google Scholar] [CrossRef]
- Zweers, J.; Thomas, R.Z.; Slot, D.E.; Weisgold, A.S.; Van der Weijden, F.G. Characteristics of periodontal biotype, its dimensions, associations and prevalence: A systematic review. J. Clin. Periodontol. 2014, 41, 958–971. [Google Scholar] [CrossRef]
- Xu, M.; Sun, X.Y.; Xu, J.G. Periodontally accelerated osteogenic orthodontics with platelet-rich fibrin in an adult patient with periodontal disease: A case report and review of literature. World J. Clin. Cases 2021, 9, 1367–1378. [Google Scholar] [CrossRef]
- Alsino, H.I.; Hajeer, M.Y.; Burhan, A.S.; Alkhouri, I.; Darwich, K. The Effectiveness of Periodontally Accelerated Osteogenic Orthodontics (PAOO) in Accelerating Tooth Movement and Supporting Alveolar Bone Thickness During Orthodontic Treatment: A Systematic Review. Cureus 2022, 14, e24985. [Google Scholar] [CrossRef]
- Han, Y.; Miao, L.L.; Jing, W.D.; Li, X.T.; Zhao, Y.J.; Xu, L.; Hou, J.X. [Digital evaluation of supracrestal gingival thickness induced by periodontal regenerative and corticotomy surgery in patients with skeletal class III malocclusion]. Zhonghua Kou Qiang Yi Xue Za Zhi 2020, 55, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Wilcko, M.T.; Ferguson, D.J.; Makki, L.; Wilcko, W.M. Keratinized Gingiva Height Increases After Alveolar Corticotomy and Augmentation Bone Grafting. J. Periodontol. 2015, 86, 1107–1115. [Google Scholar] [CrossRef]
- Rosetti, E.P.; Marcantonio, R.A.; Rossa, C., Jr.; Chaves, E.S.; Goissis, G.; Marcantonio, E., Jr. Treatment of gingival recession: Comparative study between subepithelial connective tissue graft and guided tissue regeneration. J. Periodontol. 2000, 71, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, G.; Tavelli, L.; McGuire, M.K.; Rasperini, G.; Feinberg, S.E.; Wang, H.L.; Giannobile, W.V. Autogenous soft tissue grafting for periodontal and peri-implant plastic surgical reconstruction. J. Periodontol. 2020, 91, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.E.; Amstad-Jossi, M. Epithelial differentiation at the mucogingival junction: A stereological comparison of the epithelia of the vestibular gingiva and alveolar mucosa. Cell Tissue Res. 1979, 202, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.F.; Wong, D.; Binkley, L.H. “Crawling Attachment” during Periodontally Accelerated Osteogenic Orthodontics Procedure. Contemp. Clin. Dent. 2021, 12, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, J.Q.; Jiang, J.H.; Liang, C.; Wang, X.E.; Jing, W.D.; Xu, L. Periodontal Effect of Periodontally Accelerated Osteogenic Orthodontics in Skeletal Angle Class III: A Nonrandomized, Controlled Trial. Int. J. Periodontics Restor. Dent. 2020, 40, e169–e177. [Google Scholar] [CrossRef]
- Vlachodimou, E.; Fragkioudakis, I.; Vouros, I. Is There an Association between the Gingival Phenotype and the Width of Keratinized Gingiva? A Systematic Review. Dent. J. 2021, 9, 34. [Google Scholar] [CrossRef]
- Kammerer, P.W.; Palarie, V.; Schiegnitz, E.; Nacu, V.; Draenert, F.G.; Al-Nawas, B. Influence of a collagen membrane and recombinant platelet-derived growth factor on vertical bone augmentation in implant-fixed deproteinized bovine bone--animal pilot study. Clin. Oral Implants Res. 2013, 24, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Mali, D.K. Periosteum eversion technique versus subpedicle connective tissue graft technique for root coverage of gingival recessions: A randomized split-mouth study. Natl. J. Maxillofac. Surg. 2020, 11, 81–88. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, P.; Yang, G.; Liu, H.; Gao, L.; Luan, Q. Combined Periodontal-Orthodontic Treatment with Periodontal Corticotomy Regenerative Surgery in an Adult Patient Suffering from Periodontitis and Skeletal Class II Malocclusion: A Case Report with 5-Year Longitudinal Observation. Medicina 2024, 60, 904. https://doi.org/10.3390/medicina60060904
Zou P, Yang G, Liu H, Gao L, Luan Q. Combined Periodontal-Orthodontic Treatment with Periodontal Corticotomy Regenerative Surgery in an Adult Patient Suffering from Periodontitis and Skeletal Class II Malocclusion: A Case Report with 5-Year Longitudinal Observation. Medicina. 2024; 60(6):904. https://doi.org/10.3390/medicina60060904
Chicago/Turabian StyleZou, Peihui, Gang Yang, Hao Liu, Li Gao, and Qingxian Luan. 2024. "Combined Periodontal-Orthodontic Treatment with Periodontal Corticotomy Regenerative Surgery in an Adult Patient Suffering from Periodontitis and Skeletal Class II Malocclusion: A Case Report with 5-Year Longitudinal Observation" Medicina 60, no. 6: 904. https://doi.org/10.3390/medicina60060904
APA StyleZou, P., Yang, G., Liu, H., Gao, L., & Luan, Q. (2024). Combined Periodontal-Orthodontic Treatment with Periodontal Corticotomy Regenerative Surgery in an Adult Patient Suffering from Periodontitis and Skeletal Class II Malocclusion: A Case Report with 5-Year Longitudinal Observation. Medicina, 60(6), 904. https://doi.org/10.3390/medicina60060904






