Cranial Nerve Palsy and Risk of Kidney Cancer: A Nationwide Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Study Population
2.2. Definitions of Kidney Cancer, Ocular Motor CNP, and Confounders
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
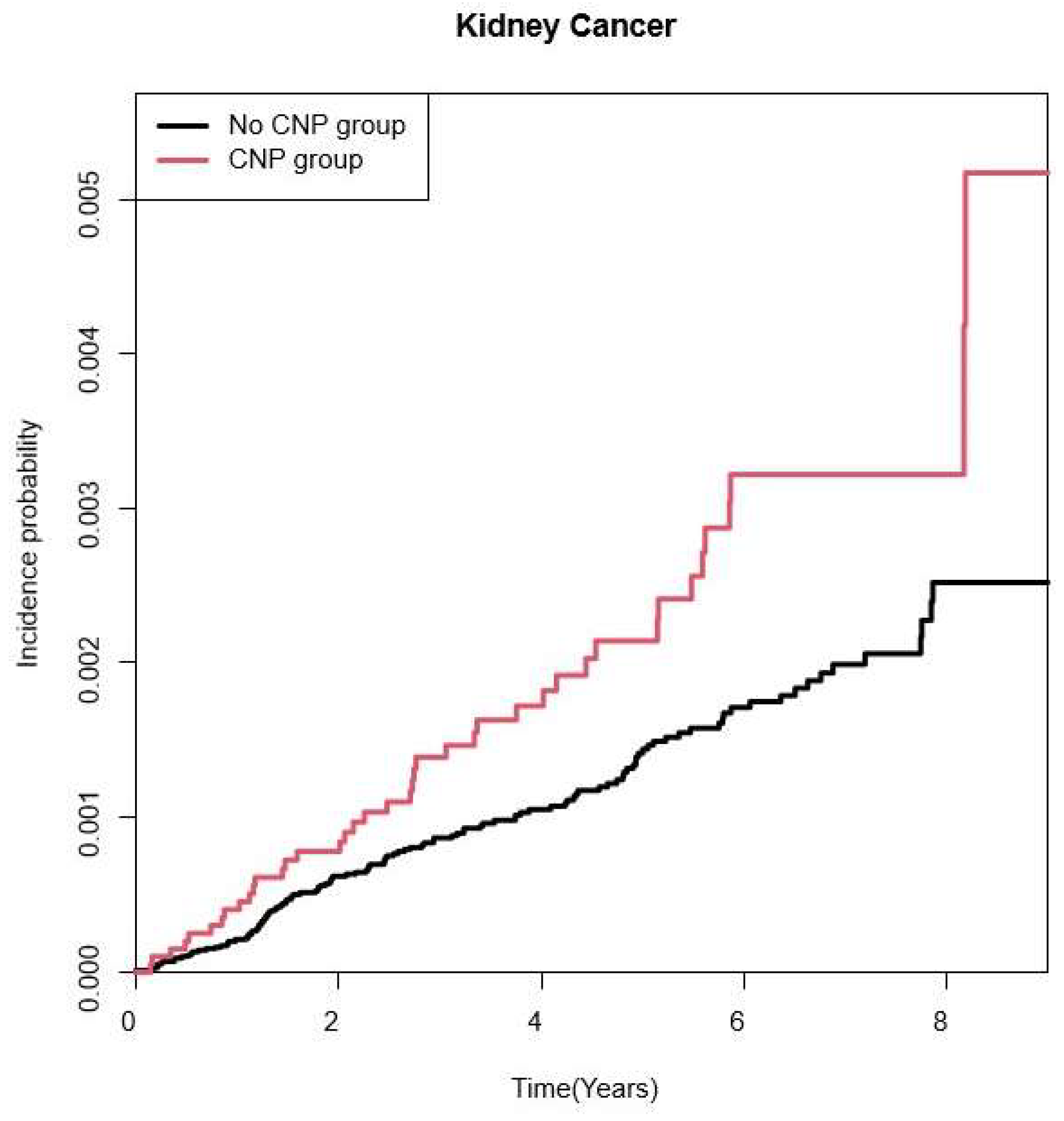
3.2. Risk for Kidney Cancer According to CNP
3.3. Subgroup Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, E.H.; Kim, S.J.; Lee, J.Y.; Cho, B.J. The incidence and etiology of sixth cranial nerve palsy in Koreans: A 10-year nationwide cohort study. Sci. Rep. 2019, 9, 18419. [Google Scholar] [CrossRef] [PubMed]
- Dosunmu, E.O.; Hatt, S.R.; Leske, D.A.; Hodge, D.O.; Holmes, J.M. Incidence and Etiology of Presumed Fourth Cranial Nerve Palsy: A Population-based Study. Am. J. Ophthalmol. 2018, 185, 110–114. [Google Scholar] [CrossRef]
- Fang, C.; Leavitt, J.A.; Hodge, D.O.; Holmes, J.M.; Mohney, B.G.; Chen, J.J. Incidence and Etiologies of Acquired Third Nerve Palsy Using a Population-Based Method. JAMA Ophthalmol. 2017, 135, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.H.; Kim, S.J.; Lee, J.Y.; Cho, B.J. The Incidence and Etiologies of Third Cranial Nerve Palsy in Koreans: A 10-year Nationwide Cohort Study. Ophthalmic Epidemiol. 2020, 27, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.H.; Kim, S.J.; Lee, J.Y.; Cho, B.J. The incidence and presumed aetiologies of fourth cranial nerve palsy in Korea: A 10-year nationwide cohort study. Eye 2021, 35, 3012–3019. [Google Scholar] [CrossRef]
- Bagheri, A.; Fallahi, M.R.; Abrishami, M.; Salour, H.; Aletaha, M. Clinical features and outcomes of treatment for fourth nerve palsy. J. Ophthalmic Vis. Res. 2010, 5, 27–31. [Google Scholar] [PubMed]
- Jacobson, D.M. Relative pupil-sparing third nerve palsy: Etiology and clinical variables predictive of a mass. Neurology 2001, 56, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Wilker, S.C.; Rucker, J.C.; Newman, N.J.; Biousse, V.; Tomsak, R.L. Pain in ischaemic ocular motor cranial nerve palsies. Br. J. Ophthalmol. 2009, 93, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Tamhankar, M.A.; Biousse, V.; Ying, G.S.; Prasad, S.; Subramanian, P.S.; Lee, M.S.; Eggenberger, E.; Moss, H.E.; Pineles, S.; Bennett, J.; et al. Isolated third, fourth, and sixth cranial nerve palsies from presumed microvascular versus other causes: A prospective study. Ophthalmology 2013, 120, 2264–2269. [Google Scholar] [CrossRef]
- Hoi, C.P.; Chen, Y.T.; Fuh, J.L.; Yang, C.P.; Wang, S.J. Increased Risk of Stroke in Patients with Isolated Third, Fourth, or Sixth Cranial Nerve Palsies: A Nationwide Cohort Study. Cerebrovasc. Dis. 2016, 41, 273–282. [Google Scholar] [CrossRef]
- Park, S.J.; Yang, H.K.; Byun, S.J.; Park, K.H.; Hwang, J.M. Ocular motor cranial nerve palsy and increased risk of stroke in the general population. PLoS ONE 2018, 13, e0205428. [Google Scholar] [CrossRef] [PubMed]
- Rim, T.H.; Han, J.; Choi, Y.S.; Lee, T.; Kim, S.S. Stroke risk among adult patients with third, fourth or sixth cranial nerve palsy: A Nationwide Cohort Study. Acta Ophthalmol. 2017, 95, e656–e661. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Q.; Hou, H.; Zhu, K.; Wang, Q.; Liu, H.; Zhang, Q.; Ji, L.; Li, D. The association between BMI and kidney cancer risk: An updated dose-response meta-analysis in accordance with PRISMA guideline. Medicine 2018, 97, e12860. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hong, J.Y.; Han, K.; Shen, J.J. Association between Glycemic Status and the Risk of Kidney Cancer in Men and Women: A Nationwide Cohort Study. Diabetes Care 2023, 46, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, L.; Xu, T.; Hao, Y.; Zhang, X.; Liu, Z.; Xiao, Y.; Wang, X.; Zeng, Q. Association of dyslipidemia with renal cell carcinoma: A 1ratio2 matched case-control study. PLoS ONE 2013, 8, e59796. [Google Scholar] [CrossRef]
- Park, U.C.; Kim, S.J.; Hwang, J.M.; Yu, Y.S. Clinical features and natural history of acquired third, fourth, and sixth cranial nerve palsy. Eye 2008, 22, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Richards, B.W.; Jones, F.R., Jr.; Younge, B.R. Causes and prognosis in 4278 cases of paralysis of the oculomotor, trochlear, and abducens cranial nerves. Am. J. Ophthalmol. 1992, 113, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Kadomoto, S.; Izumi, K.; Mizokami, A. Epidemiology and Prevention of Renal Cell Carcinoma. Cancers 2022, 14, 4059. [Google Scholar] [CrossRef]
- Haggstrom, C.; Rapp, K.; Stocks, T.; Manjer, J.; Bjorge, T.; Ulmer, H.; Engeland, A.; Almqvist, M.; Concin, H.; Selmer, R.; et al. Metabolic factors associated with risk of renal cell carcinoma. PLoS ONE 2013, 8, e57475. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 1 March 2024).
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef]
- Macleod, L.C.; Hotaling, J.M.; Wright, J.L.; Davenport, M.T.; Gore, J.L.; Harper, J.; White, E. Risk factors for renal cell carcinoma in the VITAL study. J. Urol. 2013, 190, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Farrar, W.L. Perturbations in hypoxia detection: A shared link between hereditary and sporadic tumor formation? Med. Hypotheses 2006, 66, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Gago-Dominguez, M.; Castelao, J.E. Lipid peroxidation and renal cell carcinoma: Further supportive evidence and new mechanistic insights. Free Radic. Biol. Med. 2006, 40, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Kim, D.H. Risk factors and prognosis of isolated ischemic third, fourth, or sixth cranial nerve palsies in the Korean population. J. Neuroophthalmol. 2015, 35, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; Gambina, F.; Maggio, F. Ophthalmoplegia in diabetes mellitus: A retrospective study. Acta Diabetol. 2009, 46, 23–26. [Google Scholar] [CrossRef]
- Jacobson, D.M.; McCanna, T.D.; Layde, P.M. Risk factors for ischemic ocular motor nerve palsies. Arch. Ophthalmol. 1994, 112, 961–966. [Google Scholar] [CrossRef]
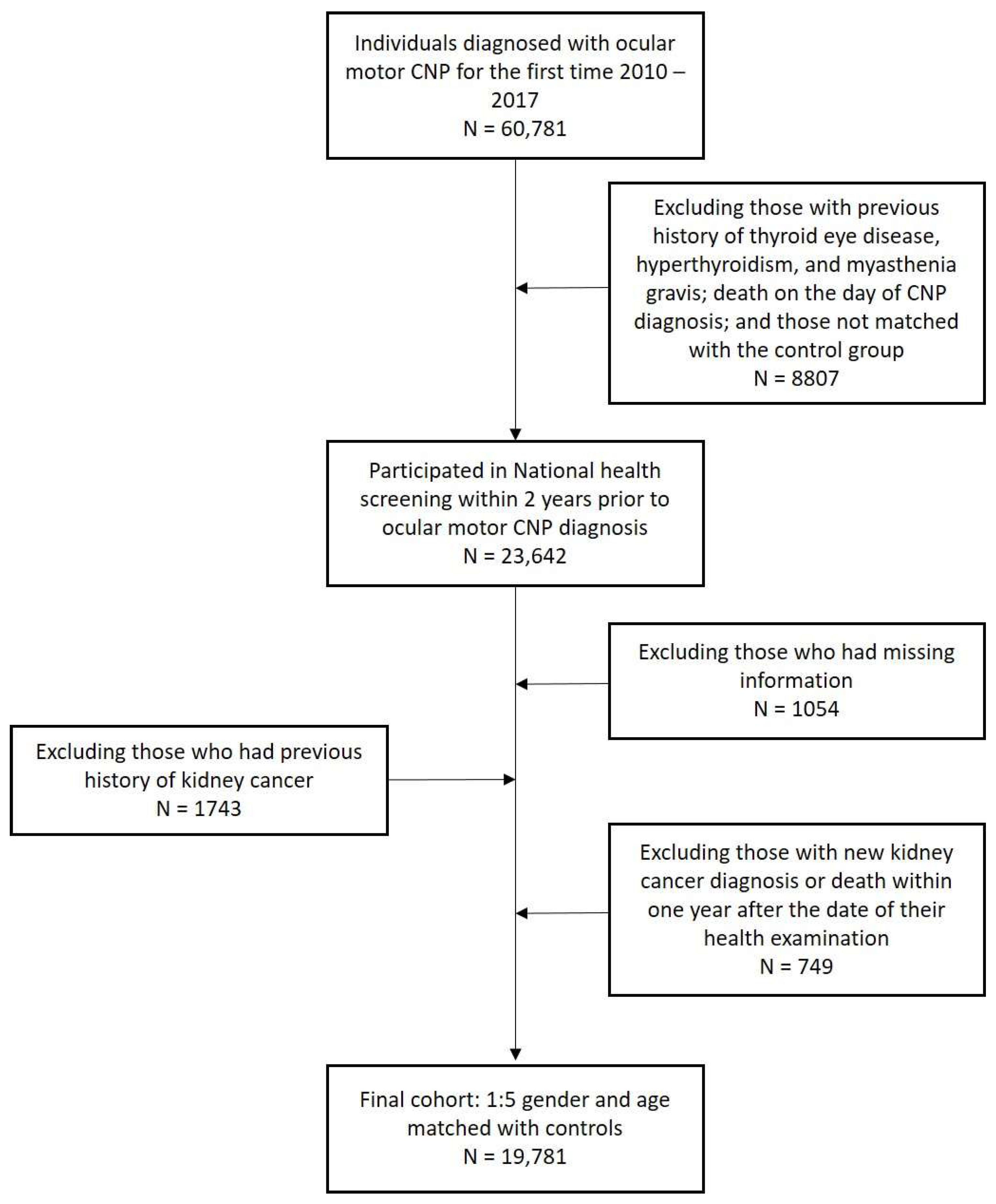
| Criteria | |
|---|---|
| Inclusion | Aged 20 years or more and underwent KNHIS health examinations |
| Diagnosed with ICD-10 code of CNP (H49.0, H49.1, H49.2) from 1 January 2010 to 31 December 2017 | |
| Exclusion | Dysthyroid exophthalmos |
| Thyrotoxicosis | |
| Myasthenia gravis | |
| Unmatchable to control group | |
| Died on the day of the diagnosis of CNP | |
| Did not undergo national health screening program within 2 years before diagnosis of CNP | |
| Missing health examination data | |
| Cancer history before cohort entry | |
| Newly diagnosed cancer within one year since diagnosis of CNP |
| Variables | Total | CNP | ||
|---|---|---|---|---|
| No | Yes | p-Value | ||
| 118,686 | 98,905 | 19,781 | ||
| Sex, male, n (%) | 74,778 (63) | 62,315 (63) | 12,463 (63) | 1 |
| Age, years | 59.82 ± 13.05 | 59.82 ± 13.05 | 59.82 ± 13.05 | 1 |
| 20–39 | 9282 (7.82) | 7735 (7.82) | 1547 (7.82) | 1 |
| 40–64 | 62,010 (52.25) | 51,675 (52.25) | 10,335(52.25) | |
| ≥65 | 47,394 (39.93) | 39,495 (39.93) | 7899 (39.93) | |
| Income status Q1, n (%) | 23,973 (20.2) | 20,126 (20.35) | 3847 (19.45) | 0.004 |
| Smoking status, smoker, n (%) | 26,318 (22.17) | 22,014 (22.26) | 4304 (21.76) | 0.1227 |
| Drinking amount, n (%) | ||||
| None | 67,224 (56.64) | 55,454 (56.07) | 11,770 (59.5) | <0.0001 |
| Mild | 42,361 (35.69) | 35,736 (36.13) | 6625 (33.49) | |
| Heavy | 9101 (7.67) | 7715 (7.8) | 1386 (7.01) | |
| Regular exercise, n (%) | 25,379 (21.38) | 21,104 (21.34) | 4275 (21.61) | 0.3909 |
| Obesity, n (%) | 44,288 (37.32) | 36,386 (36.79) | 7902 (39.95) | <0.0001 |
| DM, n (%) | 21,978 (18.52) | 16,029 (16.21) | 5949 (30.07) | <0.0001 |
| HTN, n (%) | 53,620 (45.18) | 43,508 (43.99) | 10,112 (51.12) | <0.0001 |
| Dyslipidemia, n (%) | 39,819 (33.55) | 31,729 (32.08) | 8090 (40.9) | <0.0001 |
| Chronic kidney disease | 8970 (7.56) | 7350 (7.43) | 1620 (8.19) | 0.0002 |
| Height, cm | 162.77 ± 9.1 | 162.75 ± 9.11 | 162.86 ± 9.05 | 0.1213 |
| Weight, kg | 64.15 ± 11.18 | 64.02 ± 11.11 | 64.81 ± 11.48 | <0.0001 |
| BMI, kg/m2 | 24.13 ± 3.15 | 24.09 ± 3.13 | 24.35 ± 3.24 | <0.0001 |
| Waist circumference (cm) | 82.83 ± 8.83 | 82.69 ± 8.78 | 83.56 ± 9.03 | <0.0001 |
| Fasting glucose (mg/dL) | 104.34 ± 29.94 | 102.75 ± 26.68 | 112.29 ± 41.74 | <0.0001 |
| Systolic BP (mmHg) | 125.77 ± 15.33 | 125.64 ± 15.26 | 126.43 ± 15.64 | <0.0001 |
| Diastolic BP (mmHg) | 77.29 ± 9.97 | 77.23 ± 9.91 | 77.6 ± 10.24 | <0.0001 |
| Total cholesterol (mg/dL) | 195.22 ± 38.36 | 195.52 ± 38.07 | 193.71 ± 39.77 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 87.78 ± 43.26 | 87.69 ± 41.6 | 88.2 ± 50.73 | 0.1326 |
| Renal cancer | 165 (0.14) | 124 (0.13) | 41 (0.21) | 0.0048 |
| F/U duration | ||||
| Mean ± SD | 4.42 ± 2.24 | 4.42 ± 2.24 | 4.38 ± 2.24 | 0.0345 |
| Median (Q1–Q3) | 4.19 (2.46–6.24) | 4.2 (2.47–6.24) | 4.16 (2.43–6.2) | 0.0706 |
| CNP | n | Renal Cancer | Duration | Incidence Rate (Per 1000 PY) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||||||
| Time lag of 1 year | No | 98,905 | 124 | 437,287.18 | 0.284 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Yes | 19,781 | 41 | 86,727.79 | 0.473 | 1.667 (1.171, 2.373) | 1.667 (1.171, 2.373) | 1.652 (1.16, 2.353) | 1.599 (1.116, 2.29) | |
| Time lag of3 years | No | 81,330 | 66 | 248,929.62 | 0.265 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Yes | 16,179 | 26 | 49,142.39 | 0.529 | 1.996 (1.268, 3.142) | 1.997 (1.268, 3.144) | 1.982 (1.258, 3.123) | 1.987 (1.252, 3.154) | |
| Subgroup | HR (95% CI) | p for Interaction | |
|---|---|---|---|
| Sex | Male | 1.418 (0.938, 2.143) | 0.2012 |
| Female | 2.437 (1.18, 5.034) | ||
| Age | 20–39 | . | . |
| 40–64 | 1.665 (0.997, 2.783) | ||
| 65– | 1.672 (1.014, 2.757) | ||
| Smoking | No | 1.642 (1.093, 2.468) | 0.7892 |
| Yes | 1.464 (0.696, 3.078) | ||
| Drinking amount | No | 2.108 (1.349, 3.294) | 0.1301 |
| Mild | 1.239 (0.64, 2.4) | ||
| Heavy | 0.359 (0.048, 2.707) | ||
| Regular exercise | No | 1.527 (1.015, 2.298) | 0.6297 |
| Yes | 1.876 (0.898, 3.919) | ||
| Income status | Other | 1.542 (1.036, 2.296) | 0.6679 |
| Low Q1 | 1.878 (0.833, 4.232) | ||
| Obesity | No | 1.767 (1.107, 2.822) | 0.5269 |
| Yes | 1.403 (0.81, 2.429) | ||
| DM | No | 1.487 (0.951, 2.324) | 0.5751 |
| Yes | 1.848 (0.997, 3.426) | ||
| HTN | No | 1.822 (1.037, 3.201) | 0.5648 |
| Yes | 1.473 (0.93, 2.333) | ||
| Dyslipidemia | No | 1.524 (0.959, 2.424) | 0.7442 |
| Yes | 1.72 (0.98, 3.018) | ||
| Chronic kidney disease | No | 1.456 (0.976, 2.171) | 0.2417 |
| Yes | 2.503 (1.102, 5.686) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Han, K.; Rhiu, S.; Jung, J.-h.; Park, K.-A.; Oh, S.Y. Cranial Nerve Palsy and Risk of Kidney Cancer: A Nationwide Population-Based Study. Medicina 2024, 60, 913. https://doi.org/10.3390/medicina60060913
Lee D, Han K, Rhiu S, Jung J-h, Park K-A, Oh SY. Cranial Nerve Palsy and Risk of Kidney Cancer: A Nationwide Population-Based Study. Medicina. 2024; 60(6):913. https://doi.org/10.3390/medicina60060913
Chicago/Turabian StyleLee, Dongyoung, Kyungdo Han, Soolienah Rhiu, Jin-hyung Jung, Kyung-Ah Park, and Sei Yeul Oh. 2024. "Cranial Nerve Palsy and Risk of Kidney Cancer: A Nationwide Population-Based Study" Medicina 60, no. 6: 913. https://doi.org/10.3390/medicina60060913





