Diverse Strategies for Modulating Insulin Resistance: Causal or Consequential Inference on Metabolic Parameters in Treatment-Naïve Subjects with Type 2 Diabetes
Abstract
1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Laboratory Measurements
2.3. Data Analyses
3. Results
3.1. Baseline Characteristics and Associations between Insulin Resistance and Diabetic Parameters in Newly Diagnosed, Treatment-Naïve Subjects with Type 2 Diabetes at Baseline (All Subjects)
3.2. Alterations in Diabetic Parameters following Very Low-Calorie (Tight) Japanese Diet, Pioglitazone, or Canagliflozin Monotherapy in Treatment-Naïve Subjects with T2DM
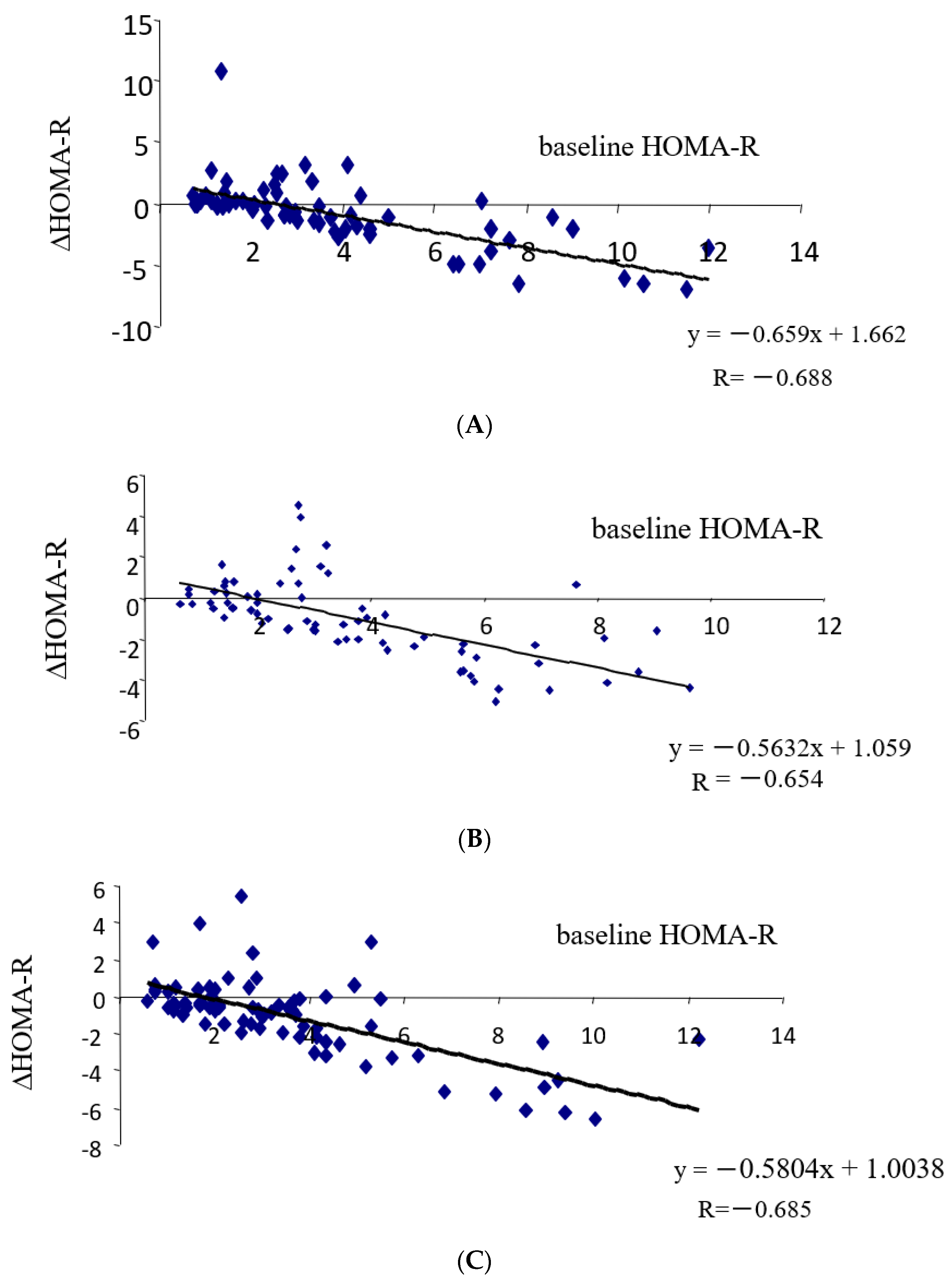
3.3. Correlation between Changes in Insulin Resistance and Diabetic Parameters with Very Low Calorie (Tight) Japanese Diet, Pioglitazone or Canagliflozin
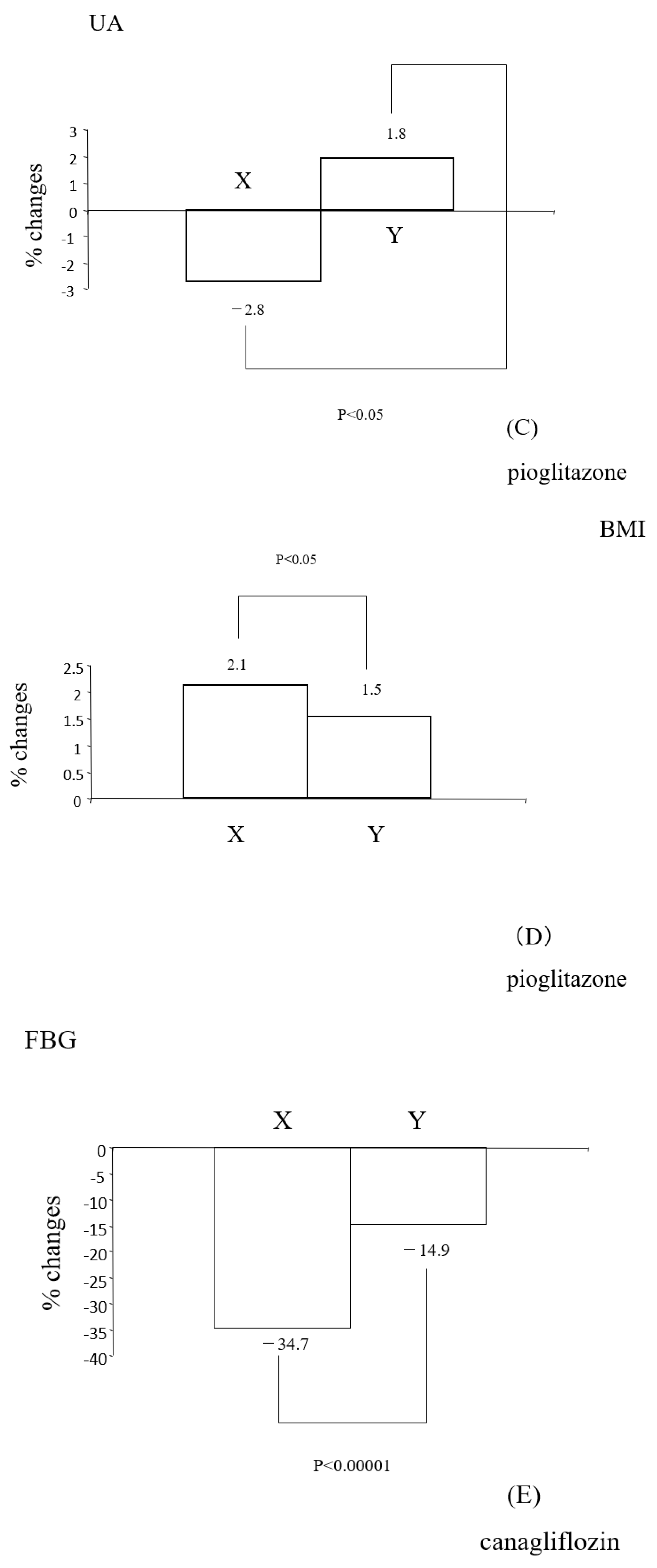
3.4. Differential Regulations of Diabetic Parameters in Two Groups with Distinct Changes in Insulin Resistance
4. Discussion
4.1. Characteristics of Diabetic Parameters in Newly Diagnosed, Drug-Naïve Japanese Patients with T2DM
4.2. Link between Changes in Insulin Resistance and Diabetic Parameters
4.2.1. FBG
- (1)
- With a tight Japanese diet, initial reductions in post-meal glucose (reduced input) occur, followed by the amelioration of glucotoxicity (reduction in insulin resistance and the enhancement of beta-cell function). In the long term, reduced caloric intake leads to weight loss, subsequently decreasing insulin resistance and FBG.
- (2)
- With pioglitazone, an initial reduction in insulin resistance occurs, followed by decreases in blood glucose levels. In the long term, enhanced insulin sensitivity may lead to weight gain and ameliorate beta-cell dysfunction.
- (3)
- With SGLT-2 inhibitors, as anticipated from their mode of action, initial reductions in both fasting and post-meal glucose (increased output) occur. Subsequently, glucotoxicity is alleviated (reduction in insulin resistance and the enhancement of beta-cell function). In the long term, weight reduction follows, leading to decreases in insulin resistance and blood glucose levels.
4.2.2. HbA1c
4.2.3. Beta-Cell Function
4.2.4. Weight
- (a)
- (b)
- With pioglitazone, on the contrary, more significant weight increases were noted in individuals with reduced insulin resistance (Figure 2D). Reductions in insulin resistance correlated with increased weight (Table 5B). The precise mechanism behind weight gain with pioglitazone remains unclear, but it has been hypothesized that the activation of PPARγ leads to an increase in the number and size of fat cells, resulting in increased fat storage [20]. In addition, the improvement in insulin sensitivity with this drug (referred to as group X in this paper) is accompanied by an increase in weight due to heightened lipogenesis [8]. These could contribute to an overall gain in body fat, leading to increased body weight.
- (c)
4.2.5. Lipids
- (a)
- (b)
- With pioglitazone, the significant down-regulation of TG and up-regulation of HDL-C were observed (Table 4B), consistent with other reports [22]. Changes in insulin resistance correlated with changes in T-C, TG, and non-HDL-C (Table 5B) but not with HDL-C (Table 5B). Significant reductions in TG and TG/HDL-C were observed in individuals with reduced insulin resistance (group 6BX). Collectively, pioglitazone appears to have favorable effects on certain lipid parameters, and the reductions in these lipids seem to be linked to reductions in insulin resistance.
- (c)
- Effects on lipids with SGLT-2 inhibitors are controversial [23]. In this study, with canagliflozin, the insignificant down-regulation of TG and significant up-regulation of HDL-C were observed (Table 4C). Changes in insulin resistance had no correlation with changes in HDL-C but showed a tendency to correlate with changes in TG (Table 5C). TG significantly decreased only in individuals with reduced insulin resistance (group 6CX). Thus, it appears that the modulation of insulin resistance with this SGLT-2 inhibitor is somewhat associated with TG but is not clearly associated with other lipid parameters.
4.2.6. UA
4.3. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | type 2 diabetes |
| SGLT-2 | sodium-glucose co-transporter |
| BMI | body mass index |
| FBG | fasting blood glucose |
| HOMA-R | homeostasis model assessment-R |
| HOMA-B | homeostasis model assessment-B |
| T-C | total cholesterol |
| TG | triglyceride |
| HDL-C | high density lipoprotein cholesterol |
| UA | uric acid |
References
- DeFronzo, R.A.; Ferrannini, E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Insulin resistance: Definition and consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl. S2), S135–S148. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diab Rep. 2019, 19, 151. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J.; Paquot, N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: A review of the clinical evidence. Diabetes Metab. 2014, 40, S4–S11. [Google Scholar] [CrossRef]
- Kutoh, E. Differential regulations of lipid profiles between Japanese responders and nonresponders treated with pioglitazone. Postgrad. Med. 2011, 123, 45–52. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Inzucchi, S.; Abdul-Ghani, M.; Nissen, S.E. Pioglitazone: The forgotten, cost-effective cardioprotective drug for type 2 diabetes. Diab Vasc. Dis. Res. 2019, 16, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Kutoh, E.; Kuto, A.N.; Ozawa, E.; Kurihara, R.; Akiyama, M. Regulation of Adipose Tissue Insulin Resistance and Diabetic Parameters in Drug Naïve Subjects with Type 2 Diabetes Treated with Canagliflozin Monotherapy. Drug Res. 2023, 73, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A. Measuring and estimating insulin resistance in clinical and research settings. Obesity 2022, 30, 1549–1563. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Sakamoto, N.; Koh, N. Very low calorie diet therapy of patients with diabetes mellitus. Nihon Rinsho 1990, 48, 894–898. (In Japanese) [Google Scholar] [PubMed]
- Sakata, T. A very-low-calorie conventional Japanese diet: Its implications for prevention of obesity. Obes. Res. 1995, 3 (Suppl. S2), 233s–239s. [Google Scholar] [CrossRef] [PubMed]
- Kutoh, E.; Ukai, Y. Alogliptin as an initial therapy in patients with newly diagnosed, drug naïve type 2 diabetes: A randomized, control trial. Endocrine 2012, 41, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Amrhein, V.; Korner-Nievergelt, F.; Roth, T. The earth is flat (p > 0.05): Significance thresholds and the crisis of unreplicable research. PeerJ 2017, 5, e3544. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Tohidi, M.; Derakhshan, A.; Hasheminia, M.; Azizi, F.; Hadaegh, F. Cut-off points of homeostasis model assessment of insulin resistance, beta-cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran Lipid and Glucose Study. Acta Diabetol. 2015, 52, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Importance of identifying the overweight patient who will benefit the most by losing weight. Ann. Intern. Med. 2003, 138, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M.; Okemah, J.; O′Neil, P.M. Body Weight Considerations in the Management of Type 2 Diabetes. Adv. Ther. 2019, 36, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Yokoh, H.; Kobayashi, K.; Sato, Y.; Takemoto, M.; Uchida, D.; Kanatsuka, A.; Kuribayashi, N.; Terano, T.; Hashimoto, N.; Sakurai, K.; et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin compared with alpha-glucosidase inhibitor in Japanese patients with type 2 diabetes inadequately controlled on metformin or pioglitazone alone (Study for an Ultimate Combination Therapy to Control Diabetes with Sitagliptin-1): A multicenter, randomized, open-label, non-inferiority trial. J. Diabetes Investig. 2015, 6, 182–191. [Google Scholar]
- Kutoh, E.; Kuto, A.N.; Wada, A.; Hayashi, J.; Kurihara, R. Sitagliptin as an Initial Therapy and Differential Regulations of Metabolic Parameters Depending on its Glycemic Response in Subjects with Type 2 Diabetes. Drug Res. 2021, 71, 157–165. [Google Scholar] [CrossRef]
- Kubota, N.; Terauchi, Y.; Miki, H.; Tamemoto, H.; Yamauchi, T.; Komeda, K.; Satoh, S.; Nakano, R.; Ishii, C.; Sugiyama, T.; et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell. 1999, 4, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Pathophysiology of Diabetic Dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, S.; Miskin, B.; Glazer, N.B.; Prince, M.J.; Robertson, K.E.; Pioglitazone 026 Study Group. The impact of pioglitazone on glycemic control and atherogenic dyslipidemia in patients with type 2 diabetes mellitus. Coron. Artery Dis. 2001, 12, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Premji, R.; Nylen, E.S.; Naser, N.; Gandhi, S.; Burman, K.D.; Sen, S. Lipid Profile Changes Associated with SGLT-2 Inhibitors and GLP-1 Agonists in Diabetes and Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2022, 20, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Tassone, E.J.; Cimellaro, A.; Perticone, M.; Hribal, M.L.; Sciacqua, A.; Andreozzi, F.; Sesti, G.; Perticone, F. Uric Acid Impairs Insulin Signaling by Promoting Enpp1 Binding to Insulin Receptor in Human Umbilical Vein Endothelial Cells. Front. Endocrinol. 2018, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, Y.; Huang, T.; Zhang, Y.; Li, Z.; Luo, C.; Luo, Y.; Yuan, H.; Hisatome, I.; Yamamoto, T.; et al. High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochem. Biophys. Res. Commun. 2014, 447, 707–714. [Google Scholar] [CrossRef]
- Wasada, T.; Katsumori, K.; Saeki, A.; Iwatani, M. Hyperuricemia and insulin resistance. Nihon Rinsho 1996, 54, 3293–3296. [Google Scholar]


| Baseline | |
|---|---|
| F/M | 49/163 |
| age | 52.3 ± 12.6 |
| FBG (mg/dL) | 202.6 ± 56.3 |
| HbA1c (%) | 9.75 ± 1.96 |
| insulin (μL/mL) | 7.44 ± 5.05 |
| HOMA-R | 3.64 ± 2.52 |
| HOMA-B | 23.23 ± 20.44 |
| BMI | 25.89 ± 4.93 |
| T-C (mg/dL) | 215.3 ± 42.2 |
| TG (mg/dL) | 181.7 ± 160.4 |
| HDL-C (mg/dL) | 52.6 ± 12.8 |
| non-HDL-C (mg/dL) | 148.0 ± 61.8 |
| TG/HDL-C | 3.86 ± 4.16 |
| UA (mg/dL) | 4.96 ± 1.33 |
| Baseline HOMA-R vs. Baseline | R | p-Values |
|---|---|---|
| age | −0.145 | <0.04 |
| FBG (mg/dL) | 0.295 | <0.00001 |
| HbA1c (%) | 0.082 | n.s. |
| insulin (μL/mL) | 0.886 | <0.00001 |
| HOMA-B | 0.535 | <0.00001 |
| BMI | 0.466 | <0.00001 |
| T-C (mg/dL) | −0.019 | n.s. |
| TG (mg/dL) | 0.127 | 0.064 |
| HDL-C (mg/dL) | −0.131 | 0.056 |
| nonHDL-C (mg/dL) | 0.019 | n.s. |
| TG/HDL-C | 0.12 | 0.0817 |
| UA (mg/dL) | 0.279 | 0.00001 |
| A | B | p-Values | |
|---|---|---|---|
| N | 107 | 105 | n.s. |
| age | 54.1 ± 11.4 | 50.6 ± 13.5 | <0.05 |
| FBG (mg/dL) | 191.8 ± 54.4 | 213.6 ± 56.4 | <0.005 |
| HbA1c (%) | 9.72 ± 2.10 | 9.78 ± 1.82 | n.s. |
| insulin (μL/mL) | 4.13 ± 2.35 | 10.81 ± 4.83 | <0.00001 |
| HOMA-R | 1.87 ± 1.04 | 5.46 ± 2.29 | <0.00001 |
| HOMA-B | 14.68 ± 11.89 | 31.94 ± 23.48 | <0.00001 |
| BMI | 24.03 ± 4.03 | 27.79 ± 5.07 | <0.00001 |
| T-C (mg/dL) | 219.4 ± 45.5 | 211.2 ± 38.3 | n.s. |
| TG (mg/dL) | 163.7 ± 176.0 | 200.0 ± 141.3 | 0.099 |
| HDL-C (mg/dL) | 54.5 ± 14.4 | 50.7 ± 10.7 | <0.04 |
| nonHDL-C (mg/dL) | 137.8 ± 74.7 | 160.5 ± 37.8 | 0.051 |
| TG/HDL-C | 3.32 ± 4.16 | 4.40 ± 4.09 | 0.058 |
| UA (mg/dL) | 4.69 ± 1.25 | 5.25 ± 1.36 | <0.003 |
| (A) | ||||
| Baseline | 3 Months | p-Values | % Changes | |
| F/M | 15/50 | |||
| age | 50.8 ± 12.9 | |||
| FBG (mg/dL) | 189.4 ± 49.3 | 167.6 ± 51.9 | <0.0004 | −11.5 |
| HbA1c (%) | 9.08 ± 1.32 | 7.96 ± 1.52 | <0.00001 | −12.3 |
| insulin (μL/mL) | 8.04 ± 5.25 | 7.20 ± 5.02 | n.s. | −10.4 |
| HOMA-R | 3.80 ± 2.28 | 2.95 ± 2.18 | <0.01 | −22.3 |
| HOMA-B | 26.23 ± 19.94 | 30.60 ± 24.92 | <0.05 | 16.6 |
| BMI | 26.20 ± 4.97 | 25.29 ± 4.77 | <0.00001 | −3.4 |
| T-C (mg/dL) | 208.0 ± 31.7 | 201.6 ± 33.7 | p < 0.05 | −3 |
| TG (mg/dL) | 157.2 ± 100.9 | 142.2 ± 85.6 | n.s. | −9.5 |
| HDL-C (mg/dL) | 53.2 ± 11.7 | 53.2 ± 11.5 | n.s. | 0 |
| nonHDL-C (mg/dL) | 116.9 ± 71.7 | 110.8 ± 70.2 | <0.03 | −5.2 |
| TG/HDL-C | 3.18 ± 2.28 | 2.87 ± 2.05 | n.s. | −9.7 |
| UA (mg/dL) | 4.84 ± 1.39 | 5.13 ± 1.47 | <0.002 | 5.9 |
| (B) | ||||
| Baseline | 3 Months | p-Values | % Changes | |
| F/M | 17/53 | |||
| age | 53.0 ± 11.7 | |||
| HOMA-R | 3.62 ± 2.27 | 2.64 ± 1.78 | <0.00001 | −27 |
| FBG (mg/dL) | 214.4 ± 53.1 | 170.2 ± 63.3 | <0.00001 | −20.6 |
| HbA1c (%) | 9.85 ± 1.60 | 8.37 ± 1.69 | <0.00001 | −15 |
| insulin (μL/mL) | 6.93 ± 4.36 | 6.61 ± 4.70 | n.s. | −4.6 |
| HOMA-B | 19.00 ± 15.42 | 32.31 ± 38.79 | <0.003 | 70 |
| BMI | 25.20 ± 5.23 | 25.64 ± 5.30 | <0.00001 | 1.7 |
| T-C (mg/dL) | 210.3 ± 37.8 | 213.0 ± 36.2 | n.s. | 1.2 |
| TG (mg/dL) | 177.9 ± 122.6 | 145.4 ± 88.7 | <0.0007 | −18.2 |
| HDL-C (mg/dL) | 49.9 ± 11.3 | 56.5 ± 16.0 | <0.00001 | 13.2 |
| nonHDL-C (mg/dL) | 160.4 ± 38.7 | 156.44 ± 38.8 | n.s. | −2.4 |
| TG/HDL-C | 3.56 ± 10.7 | 2.57 ± 5.52 | <0.0002 | −27.8 |
| UA (mg/dL) | 4.67 ± 1.31 | 4.64 ± 1.21 | n.s. | −0.6 |
| (C) | ||||
| Baseline | 3 Months | p-Values | % Changes | |
| F/M | 18/59 | |||
| age | 53.5 ± 12.5 | |||
| HOMA-R | 3.53 ± 2.72 | 2.48 ± 2.05 | <0.00001 | −29.7 |
| FBG (mg/dL) | 203.1 ± 64.8 | 150.6 ± 47.9 | <0.00001 | −25.8 |
| HbA1c (%) | 10.24 ± 2.61 | 8.34 ± 1.97 | <0.00001 | −18.5 |
| insulin (μL/mL) | 7.38 ± 5.92 | 6.85 ± 6.28 | n.s. | −7.1 |
| HOMA-B | 24.53 ± 26.76 | 33.46 ± 43.00 | <0.00001 | 36.4 |
| BMI | 26.26 ± 5.64 | 25.82 ± 5.66 | <0.00001 | −1.6 |
| T-C (mg/dL) | 226.1 ± 49.7 | 224.2 ± 46.1 | n.s. | −0.8 |
| TG (mg/dL) | 205.3 ± 213.0 | 193.1 ± 233.7 | 0.087 | −5.9 |
| HDL-C (mg/dL) | 54.0 ± 14.4 | 56.2 ± 14.8 | <0.03 | 4 |
| nonHDL-C (mg/dL) | 167.7 ± 58.7 | 163.7 ± 54.7 | n.s. | −2.3 |
| TG/HDL-C | 4.44 ± 5.77 | 4.19 ± 7.24 | n.s. | −5.6 |
| UA (mg/dL) | 5.33 ± 1.25 | 5.31 ± 1.26 | n.s. | −0.3 |
| (A) | ||
| ΔHOMA-R vs. | R | p-Values |
| baseline HOMA-R | −0.688 | <0.00001 |
| ΔFBG | 0.599 | <0.00001 |
| ΔHbA1c | 0.256 | <0.04 |
| Δinsulin | 0.932 | <0.00001 |
| ΔHOMA-B | 0.452 | <0.0002 |
| ΔBMI | 0.102 | n.s. |
| ΔT-C | −0.078 | n.s. |
| ΔTG | 0.137 | n.s. |
| ΔHDL-C | 0.022 | n.s. |
| ΔnonHDL-C | −0.091 | n.s. |
| ΔTG/HDL-C | 0.154 | n.s. |
| ΔUA | −0.217 | 0.082 |
| (B) | ||
| ΔHOMA-R vs. | R | p-Values |
| baseline HOMA-R | −0.654 | <0.00001 |
| ΔFBG | 0.51 | <0.00001 |
| ΔHbA1c | 0.266 | <0.03 |
| Δinsulin | 0.771 | <0.00001 |
| ΔHOMA-B | 0.298 | <0.02 |
| ΔBMI | −0.342 | <0.04 |
| ΔT-C | 0.283 | <0.02 |
| ΔTG | 0.299 | <0.02 |
| ΔHDL-C | 0.087 | n.s. |
| ΔnonHDL-C | 0.26 | <0.03 |
| ΔTG/HDL-C | 0.23 | 0.055 |
| ΔUA | 0.077 | n.s. |
| (C) | ||
| ΔHOMA-R vs. | R | p-Values |
| baseline HOMA-R | −0.685 | <0.00001 |
| ΔFBG | 0.322 | <0.007 |
| ΔHbA1c | 0.118 | n.s. |
| Δinsulin | 0.849 | <0.00001 |
| ΔHOMA-B | 0.365 | <0.004 |
| ΔBMI | −0.178 | n.s. |
| ΔT-C | 0.041 | n.s. |
| ΔTG | 0.215 | 0.072 |
| ΔHDL-C | −0.041 | n.s. |
| ΔnonHDL-C | 0.06 | n.s. |
| ΔTG/HDL-C | 0.293 | <0.02 |
| ΔUA | −0.196 | 0.093 |
| (AX) | ||||
| Baseline | 3 Months | p-Values | % Changes | |
| N | 33 | |||
| age | 51.0 ± 13.4 | |||
| HOMA-R | 5.47 ± 2.89 | 2.83 ± 1.86 | <0.00001 | −48.2 |
| FBG (mg/dL) | 205.6 ± 47.8 | 155.9 ± 39.4 | <0.00001 | −24.1 |
| HbA1c (%) | 9.31 ± 1.37 | 7.79 ± 1.43 | <0.00001 | −16.3 |
| insulin (μL/mL) | 10.92 ± 5.27 | 7.23 ± 4.00 | <0.00001 | −33.7 |
| HOMA-B | 31.70 ± 21.67 | 20.59 ± 16.48 | <0.00001 | −35 |
| BMI | 27.65 ± 4.79 | 26.59 ± 4.88 | <0.00001 | −3.8 |
| T-C (mg/dL) | 208.4 ± 31.4 | 200.7 ± 37.5 | 0.094 | −3.6 |
| TG (mg/dL) | 171.0 ± 81.5 | 153.4 ± 81.3 | n.s. | −10.2 |
| HDL-C (mg/dL) | 52.0 ± 9.4 | 52.7 ± 11.4 | n.s. | 1.3 |
| nonHDL-C (mg/dL) | 156.3 ± 29.1 | 148.0 ± 33.8 | <0.05 | −5.3 |
| TG/HDL-C | 3.49 ± 1.95 | 3.08 ± 1.90 | n.s. | −11.7 |
| UA (mg/dL) | 4.93 ± 1.45 | 5.40 ± 1.59 | <0.0002 | 9.5 |
| (AY) | ||||
| Baseline | 3 Months | p-Values | % Changes | |
| N | 32 | |||
| age | 50.6 ± 12.7 | |||
| HOMA-R | 2.08 ± 1.35 | 3.08 ± 2.50 | <0.01 | 48 |
| FBG (mg/dL) | 172.6 ± 45.6 | 179.7 ± 60.4 | n.s. | 4.1 |
| HbA1c (%) | 8.85 ± 1.25 | 8.15 ± 1.60 | <0.00001 | −7.9 |
| insulin (μL/mL) | 5.07 ± 3.22 | 7.16 ± 5.96 | <0.02 | 41.2 |
| HOMA-B | 20.59 ± 16.48 | 28.39 ± 26.80 | <0.04 | 37.8 |
| BMI | 24.71 ± 4.77 | 23.96 ± 4.33 | <0.007 | −3 |
| T-C (mg/dL) | 207.6 ± 32.6 | 202.6 ± 29.9 | n.s. | −2.4 |
| TG (mg/dL) | 143.0 ± 117.3 | 130.6 ± 89.6 | n.s. | −8.6 |
| HDL-C (mg/dL) | 54.4 ± 13.7 | 53.8 ± 11.7 | n.s. | −1.1 |
| nonHDL-C (mg/dL) | 153.1 ± 31.1 | 148.8 ± 28.8 | n.s. | −2.8 |
| TG/HDL-C | 2.85 ± 2.58 | 2.66 ± 2.21 | n.s. | −6.6 |
| UA (mg/dL) | 4.75 ± 1.35 | 4.86 ± 1.31 | n.s. | 2.3 |
| (BX) | ||||
| Baseline | 3 Months | p-Values | % Changes | |
| N | 35 | |||
| age | 52.2 ± 11.9 | |||
| HOMA-R | 5.03 ± 2.15 | 2.52 ± 1.58 | <0.00001 | −49.9 |
| FBG (mg/dL) | 223.1 ± 58.1 | 150.7 ± 50.0 | <0.00001 | −32.4 |
| HbA1c (%) | 9.78 ± 1.59 | 7.93 ± 1.68 | <0.00001 | −18.9 |
| insulin (μL/mL) | 9.55 ± 4.39 | 6.88 ± 3.70 | <0.00001 | −27.9 |
| HOMA-B | 26.07 ± 18.21 | 35.02 ± 23.19 | <0.007 | 34.3 |
| BMI | 26.21 ± 5.44 | 26.77 ± 5.58 | <0.00001 | 2.1 |
| T-C (mg/dL) | 216.0 ± 42.7 | 211.0 ± 40.7 | n.s. | −2.3 |
| TG (mg/dL) | 207.8 ± 147.4 | 151.4 ± 101.4 | <0.0004 | −27.1 |
| HDL-C (mg/dL) | 48.7 ± 10.5 | 54.2 ± 13.4 | <0.0005 | 11.2 |
| nonHDL-C (mg/dL) | 167.2 ± 40.6 | 156.8 ± 40.9 | 0.052 | −6.2 |
| TG/HDL-C | 4.73 ± 4.01 | 3.10 ± 2.53 | <0.001 | −34.4 |
| UA (mg/dL) | 5.12 ± 1.42 | 4.98 ± 1.20 | n.s. | −2.7 |
| (BY) | ||||
| Baseline | 3 Months | p-Values | % Changes | |
| N | 35 | |||
| age | 53.8 ± 11.6 | |||
| HOMA-R | 2.22 ± 1.33 | 2.76 ± 1.97 | <0.02 | 24.3 |
| FBG (mg/dL) | 205.7 ± 46.8 | 189.6 ± 69.6 | 0.082 | −7.8 |
| HbA1c (%) | 9.91 ± 1.64 | 8.81 ± 1.61 | <0.00001 | −11 |
| insulin (μL/mL) | 4.32 ± 2.30 | 6.35 ± 5.56 | <0.01 | 46.9 |
| HOMA-B | 11.93 ± 6.92 | 29.59 ± 50.01 | <0.04 | 148 |
| BMI | 23.89 ± 4.09 | 24.25 ± 4.19 | <0.00001 | 1.5 |
| T-C (mg/dL) | 204.7 ± 31.7 | 215.0 ± 31.7 | 0.078 | 5 |
| TG (mg/dL) | 144.3 ± 81.1 | 133.5 ± 70.9 | n.s. | −7.4 |
| HDL-C (mg/dL) | 51.7 ± 12.2 | 59.2 ± 18.2 | <0.0005 | 14.5 |
| nonHDL-C (mg/dL) | 153.6 ± 35.9 | 156.0 ± 37.2 | n.s. | 1.5 |
| TG/HDL-C | 2.93 ± 1.83 | 2.60 ± 1.76 | n.s. | −11.2 |
| UA (mg/dL) | 4.22 ± 1.03 | 4.30 ± 1.13 | n.s. | 1.8 |
| (CX) | ||||
| Baseline | 3 Months | p-Values | % Changes | |
| N | 39 | |||
| age | 52.46 ± 13.05 | |||
| HOMA-R | 4.79 ± 2.74 | 2.32 ± 1.75 | <0.00001 | −51.5 |
| FBG (mg/dL) | 220.0 ± 61.6 | 143.6 ± 31.5 | <0.00001 | −34.7 |
| HbA1c (%) | 10.75 ± 2.74 | 8.59 ± 2.18 | <0.00001 | −20 |
| insulin (μL/mL) | 9.40 ± 6.23 | 6.64 ± 5.26 | <0.00001 | −29.3 |
| HOMA-B | 27.95 ± 28.09 | 33.30 ± 28.94 | n.s. | 19.1 |
| BMI | 26.88 ± 4.67 | 26.50 ± 5.01 | <0.05 | −1.4 |
| T-C (mg/dL) | 223.7 ± 48.4 | 220.0 ± 44.7 | n.s. | −1.6 |
| TG (mg/dL) | 201.5 ± 133.5 | 175.0 ± 123.0 | <0.04 | −13.1 |
| HDL-C (mg/dL) | 53.0 ± 15.7 | 55.1 ± 15.8 | n.s. | 3.9 |
| nonHDL-C (mg/dL) | 170.7 ± 50.7 | 165.0 ± 46.0 | n.s. | −3.3 |
| TG/HDL-C | 4.47 ± 4.73 | 3.70 ± 3.84 | <0.02 | −17.2 |
| UA (mg/dL) | 5.13 ± 1.14 | 5.33 ± 1.17 | n.s. | 3.8 |
| (CY) | ||||
| Baseline | 3 Months | p-Values | % Changes | |
| N | 38 | |||
| age | 53.71 ± 13.32 | |||
| HOMA-R | 2.25 ± 1.30 | 2.65 ± 1.97 | <0.05 | 17.7 |
| FBG (mg/dL) | 185.7 ± 59.7 | 157.9 ± 57.5 | <0.0002 | −14.9 |
| HbA1c (%) | 9.71 ± 2.14 | 8.09 ± 1.76 | <0.0001 | −16.6 |
| insulin (μL/mL) | 5.31 ± 3.52 | 7.07 ± 5.37 | <0.01 | 33.1 |
| HOMA-B | 21.02 ± 19.03 | 33.63 ± 32.56 | <0.007 | 59.9 |
| BMI | 25.61 ± 4.53 | 25.13 ± 4.27 | <0.0004 | −1.8 |
| T-C (mg/dL) | 228.4 ± 54.4 | 228.6 ± 49.6 | n.s. | 0 |
| TG (mg/dL) | 213.5 ± 283.5 | 211.6 ± 319.7 | n.s. | −0.8 |
| HDL-C (mg/dL) | 55.5 ± 13.4 | 57.3 ± 13.8 | n.s. | 3.2 |
| nonHDL-C (mg/dL) | 172.8 ± 55.4 | 171.2 ± 51.2 | n.s. | −0.9 |
| TG/HDL-C | 4.45 ± 6.72 | 4.69 ± 9.60 | n.s. | 5.3 |
| UA (mg/dL) | 5.54 ± 1.30 | 5.29 ± 1.37 | 0.079 | −4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutoh, E.; Kuto, A.N.; Okada, R.; Akiyama, M.; Kurihara, R. Diverse Strategies for Modulating Insulin Resistance: Causal or Consequential Inference on Metabolic Parameters in Treatment-Naïve Subjects with Type 2 Diabetes. Medicina 2024, 60, 991. https://doi.org/10.3390/medicina60060991
Kutoh E, Kuto AN, Okada R, Akiyama M, Kurihara R. Diverse Strategies for Modulating Insulin Resistance: Causal or Consequential Inference on Metabolic Parameters in Treatment-Naïve Subjects with Type 2 Diabetes. Medicina. 2024; 60(6):991. https://doi.org/10.3390/medicina60060991
Chicago/Turabian StyleKutoh, Eiji, Alexandra N. Kuto, Rumiko Okada, Midori Akiyama, and Rumi Kurihara. 2024. "Diverse Strategies for Modulating Insulin Resistance: Causal or Consequential Inference on Metabolic Parameters in Treatment-Naïve Subjects with Type 2 Diabetes" Medicina 60, no. 6: 991. https://doi.org/10.3390/medicina60060991
APA StyleKutoh, E., Kuto, A. N., Okada, R., Akiyama, M., & Kurihara, R. (2024). Diverse Strategies for Modulating Insulin Resistance: Causal or Consequential Inference on Metabolic Parameters in Treatment-Naïve Subjects with Type 2 Diabetes. Medicina, 60(6), 991. https://doi.org/10.3390/medicina60060991






