The Efficacy of a Multidisciplinary Approach and Diagnostic–Therapeutic Algorithm for Vertebral Metastases with Spinal Cord Compression
Abstract
1. Introduction
2. Materials and Methods
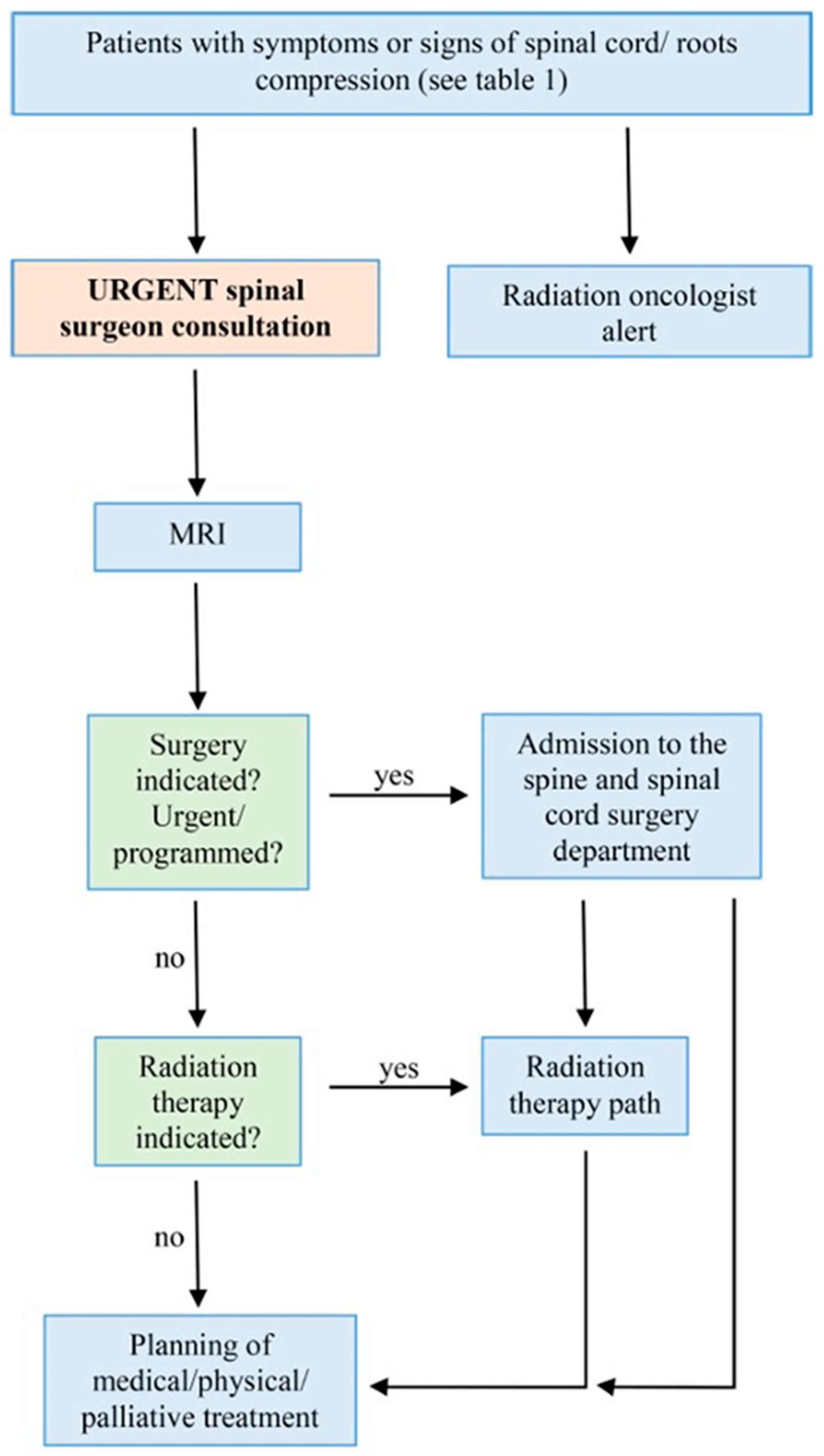
2.1. Initial Recognition and Alert Symptoms
- Nocturnal neck or back pain: persistent pain that worsens at night;
- Axial mechanical pain: pain in the spine that is exacerbated by movement and alleviated by rest;
- Sudden onset of axial pain: acute, severe pain in the spine;
- Radicular pain: pain radiating to the arms or legs, which may be accompanied by numbness, tingling, or dysesthesia;
- Walking or balance difficulties: new or worsening problems with walking or maintaining balance;
- Weakness in arms/hands: muscle weakness affecting one or more muscles in the arms or hands.
- Bladder or bowel control disorders: issues such as urinary retention or incontinence.
2.2. Role of the Spine Surgeon and the Radiation Oncologist
- Assessing neurological deficits: evaluating the patient for any motor or sensory deficits to determine the extent of spinal cord/root compression;
- Collaboration with the neuroradiologist: working closely with the neuroradiologist to arrange for timely magnetic resonance imaging (MRI).
2.3. Role of the Oncologist, the Physiatrist, and the Palliative Care Physician
3. Results
3.1. Increase in Total Oncological Patients Evaluated
3.2. Increase in Patients with Suspected Spinal Cord Compression
3.3. Increase in Surgical Procedures
3.4. Changes in Number of Intrahospital Consultations and Number of Outpatients with Spine Metastasis
4. Discussion
5. Limitations
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, D.A.; Fornasier, V.L.; MacNab, I. Spinal Metastases: The Obvious, the Occult, and the Impostors. Spine 1990, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, J.J.; Choi, D.; Versteeg, A.; Albert, T.; Arts, M.; Balabaud, L.; Bunger, C.; Buchowski, J.M.; Chung, C.K.; Coppes, M.H.; et al. Characteristics of Patients Who Survived 2 Years after Surgery for Spinal Metastases: Can We Avoid Inappropriate Patient Selection? J. Clin. Oncol. 2016, 34, 3054–3061. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.H.; Klimo, P., Jr.; Vrionis, F.D. Metastatic Spinal Cord Compression. J. Natl. Compr. Cancer Netw. 2005, 3, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Siege, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Levack, P.; Graham, J.; Colliem, D.; Grant, R.; Kidd, J.; Kunkler, I.; Gibsond, A.; Hurman, D.; McMillan, N.; Rampling, R.; et al. Scottish Cord Compression Study Group: Don’t Wait for a Sensory Level—Listen to the Symptoms: A Prospective Audit of the Delays in Diagnosis of Malignant Cord Compression. Clin. Oncol. 2002, 14, 472–480. [Google Scholar] [CrossRef]
- Loblaw, D.A.; Laperriere, N.J. Emergency Treatment of Malignant Extradural Spinal Cord Compression: An Evidence-Based Guideline. J. Clin. Oncol. 1998, 16, 1613–1624. [Google Scholar] [CrossRef]
- Boussios, S.; Cooke, D.; Hayward, C.; Kanellos, F.S.; Tsiouris, A.K.; Chatziantoniou, A.A.; Zakynthinakis-Kyriakou, N.; Karathanasi, A. Metastatic Spinal Cord Compression: Unraveling the Diagnostic and Therapeutic Challenges. Anticancer Res. 2018, 38, 4987–4997. [Google Scholar] [CrossRef]
- Prasad, D.; Schiff, D. Malignant Spinal-Cord Compression. Lancet Oncol. 2005, 6, 15–24. [Google Scholar] [CrossRef]
- Rispoli, R.; Reverberi, C.; Targato, G.; D’Agostini, S.; Fasola, G.; Trovò, M.; Calci, M.; Fanin, R.; Cappelletto, B. Multidisciplinary Approach to Patients with Metastatic Spinal Cord Compression: A Diagnostic Therapeutic Algorithm to Improve the Neurological Outcome. Front. Oncol. 2022, 12, 902928. [Google Scholar] [CrossRef]
- Spratt, D.E.; Beeler, W.H.; de Moraes, F.Y.; Rhines, L.D.; Gemmete, J.J.; Chaudhary, N.; Shultz, D.B.; Smith, S.R.; Berlin, A.; Dahele, M.; et al. An integrated multidisciplinary algorithm for the management of spinal metastases: An International Spine Oncology Consortium report. Lancet Oncol. 2017, 18, e720–e730. [Google Scholar] [CrossRef]
- Lemaire, A.; George, B.; Maindet, C.; Burnod, A.; Allano, G.; Minello, C. Opening Up Disruptive Ways of Management in Cancer Pain: The Concept of Multimorphic Pain. Support. Care Cancer 2019, 27, 3159–3170. [Google Scholar] [CrossRef] [PubMed]
- Paton, G.R.; Frangou, E.; Fourney, D.R. Contemporary Treatment Strategy for Spinal Metastasis: The “LMNOP” System. Can. J. Neurol. Sci. 2011, 38, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Fourney, D.R.; Frangou, E.M.; Ryken, T.C.; Di Paola, C.P.; Shaffrey, C.I.; Berven, S.H.; Bilsky, M.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; et al. Spinal Instability Neoplastic Score: An Analysis of Reliability and Validity from the Spine Oncology Study Group. J. Clin. Oncol. 2011, 29, 3072–3077. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.H.; Burch, S.; Buckley, J.; Schmidt, M.H.; Fehlings, M.G.; Vrionis, F.D.; Fisher, C.G. Instability and Impending Instability of the Thoracolumbar Spine in Patients with Spinal Metastases: A Systematic Review. Int. J. Oncol. 2011, 38, 5–12. [Google Scholar] [PubMed]
- Widmann, G.; Henninger, B.; Kremser, C.; Jaschke, W. MRI Sequences in Head & Neck Radiology—State of the Art. Rofo 2017, 189, 413–422. [Google Scholar] [PubMed]
- Raya, J.G.; Dietrich, O.; Reiser, M.F.; Baur-Melnyk, A. Methods and Applications of Diffusion Imaging of Vertebral Bone Marrow. J. Magn. Reson. Imaging 2006, 24, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Buhmann-Kirchhoff, S.; Becker, C.; Duerr, H.R.; Reiser, M.; Baur-Melnyk, A. Detection of Osseous Metastases of the Spine: Comparison of High Resolution Multi-Detector-CT with MRI. Eur. J. Radiol. 2009, 69, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Allan, L.; Baker, L.; Dewar, J.; Eljamel, S.; Grant, R.M.; Houston, J.G.; McLeay, T.; Munro, A.J.; Levack, P. Suspected Malignant Cord Compression-Improving Time to Diagnosis via a ‘Hotline’: A Prospective Audit. Br. J. Cancer 2009, 100, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.; Sharkey, R.; Kua, T.; Schofield, L.; Richardson, D.; Panchmatia, N.; Papanastasopoulos, P.; Williams, M.; Falconer, A.; Power, D.; et al. Malignant Spinal Cord Compression: NICE Guidance, Improvements and Challenges. QJM 2014, 107, 277–282. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Metastatic Spinal Cord Compression in Adults: Risk Assessment, Diagnosis and Management Clinical Guideline. National Collaborating Centre for Cancer 2008. Available online: https://www.nice.org.uk/guidance/cg75/evidence/full-guideline-242052589 (accessed on 20 April 2024).
- Ntilikina, Y.; Collinet, A.; Tigan, L.V.; Fabacher, T.; J-Paul, S.; Charles, Y.P. Comparison of Open Versus Minimally Invasive Surgery in the Treatment of Thoracolumbar Metastases. Orthop. Traumatol. Surg. Res. 2022, 108, 103274. [Google Scholar] [CrossRef]
- Perna, A.; Smakaj, A.; Vitiello, R.; Velluto, C.; Proietti, L.; Tamburrelli, F.C.; Maccauro, G. Posterior Percutaneous Pedicle Screws Fixation Versus Open Surgical Instrumented Fusion for Thoraco-Lumbar Spinal Metastases Palliative Management: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 884928. [Google Scholar] [CrossRef] [PubMed]
- Cappelletto, B.; Del Fabro, P.; Meo, A. Decompression and Surgical Stabilization in the Palliative Treatment of Vertebral Metastases. La Chir. Degli Organi Di Mov. 1998, 83, 167–176. [Google Scholar]
- Hoskin, P.J.; Hopkins, K.; Misra, V.; Holt, T.; McMenemin, R.; Dubois, D.; McKinna, F.; Foran, B.; Madhavan, K.; MacGregor, C.; et al. SCORAD III: Randomized Noninferiority Phase III Trial of Single-Dose Radiotherapy (RT) Compared to Multifraction RT in Patients (Pts) with Metastatic Spinal Canal Compression (SCC). J. Clin. Oncol. 2019, 35, LBA10004. [Google Scholar] [CrossRef]
- Greco, C.; Pares, O.; Pimentel, N.; Moser, E.; Louro, V.; Morales, X.; Salas, B.; Fuks, Z. Spinal Metastases: From Conventional Fractionated Radiotherapy to Single-Dose SBRT. Rep. Pract. Oncol. Radiother. 2015, 20, 454–463. [Google Scholar] [CrossRef]
- Van Tol, F.R.; Choi, D.; Verkooijen, H.M.; Oner, F.C.; Verlaan, J.J. Delayed Presentation to a Spine Surgeon is the Strongest Predictor of Poor Postoperative Outcome in Patients Surgically Treated for Symptomatic Spinal Metastases. Spine J. 2019, 19, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, S.M.; Kinnaird, W.; Lin, R.; Hu, Y.; Payne, H.A. 394P Reversing the trend of Friday peak for metastatic spinal cord compression referrals. Ann. Oncol. 2020, 31, S408. [Google Scholar] [CrossRef]
- Adeleke, S.; Hakim, R.; Dean, L.; Zahid, H.; Lin, R.; Karova, M.; Galante, J.R.; Kinnaird, W.; Taylor, K.; Payne, H.A.; et al. Reversing the Friday peak in metastatic cord compression referrals: Not as simple as previously thought? J. Clin. Oncol. 2021, 39, e14050. [Google Scholar] [CrossRef]
- Hameed, E.K.; Al-Ameri, L.T. Artificial Intelligence: The Gateway to Better Medical Diagnosis. AL-Kindy Coll. Med. J. 2024, 20, 1–3. [Google Scholar] [CrossRef]
| Pre-Protocol Period (Years 2019 and 2021) | Post-Protocol Period (Years 2022 and 2023) | |
|---|---|---|
| Oncological patients evaluated | 419 | 488 |
| Patients with suspected MSCC | 28 | 45 |
| Patients that underwent surgical procedures | 17 | 44 |
| Emergency/elective delayed urgency surgery | 10/7 | 25/19 |
| Intrahospital consultations | 105 | 82 |
| Outpatient consultation | 59 | 124 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rispoli, R.; Giorgiutti, F.; Veltri, C.; Copetti, E.; Imbruce’, P.; Iacopino, G.; Cappelletto, B. The Efficacy of a Multidisciplinary Approach and Diagnostic–Therapeutic Algorithm for Vertebral Metastases with Spinal Cord Compression. Medicina 2024, 60, 1020. https://doi.org/10.3390/medicina60071020
Rispoli R, Giorgiutti F, Veltri C, Copetti E, Imbruce’ P, Iacopino G, Cappelletto B. The Efficacy of a Multidisciplinary Approach and Diagnostic–Therapeutic Algorithm for Vertebral Metastases with Spinal Cord Compression. Medicina. 2024; 60(7):1020. https://doi.org/10.3390/medicina60071020
Chicago/Turabian StyleRispoli, Rossella, Fabrizia Giorgiutti, Claudio Veltri, Edi Copetti, Pietro Imbruce’, Giorgia Iacopino, and Barbara Cappelletto. 2024. "The Efficacy of a Multidisciplinary Approach and Diagnostic–Therapeutic Algorithm for Vertebral Metastases with Spinal Cord Compression" Medicina 60, no. 7: 1020. https://doi.org/10.3390/medicina60071020
APA StyleRispoli, R., Giorgiutti, F., Veltri, C., Copetti, E., Imbruce’, P., Iacopino, G., & Cappelletto, B. (2024). The Efficacy of a Multidisciplinary Approach and Diagnostic–Therapeutic Algorithm for Vertebral Metastases with Spinal Cord Compression. Medicina, 60(7), 1020. https://doi.org/10.3390/medicina60071020






