Antioxidant Status in Patients after Breast Mastopexy and Augmentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Methods
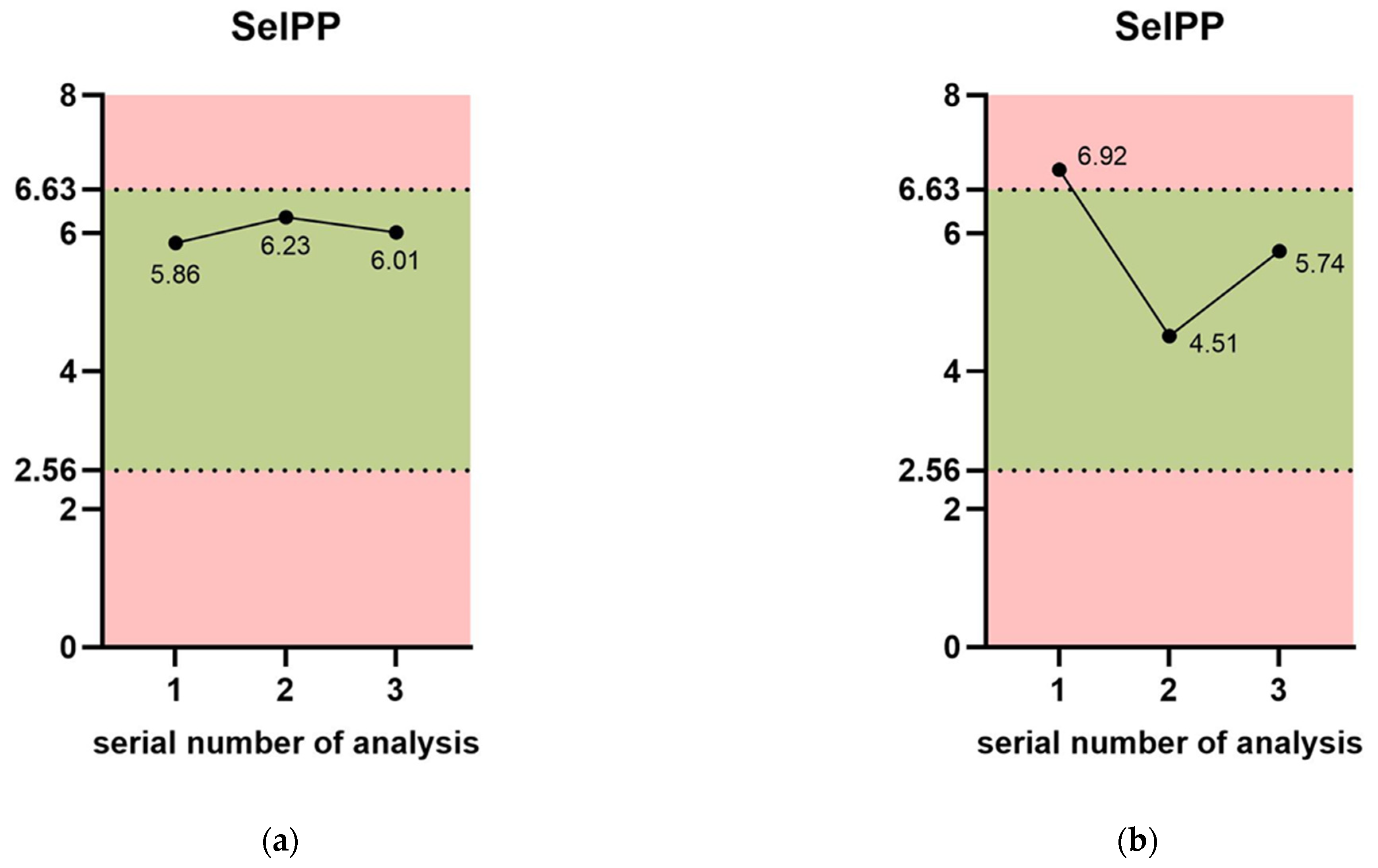
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colaris, M.J.L.; Ruhl, T.; Beier, J.P. Effects of Silicone Breast Implants on Human Cell Types In Vitro: A Closer Look on Host and Implant. Aesthetic Plast. Surg. 2022, 46, 2208–2217. [Google Scholar] [CrossRef]
- Suh, L.J.; Khan, I.; Kelley-Patteson, C.; Mohan, G.; Hassanein, A.H.; Sinha, M. Breast Implant-Associated Immunological Disorders. J. Immunol. Res. 2022, 2022, 8536149. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.E.; Seitz, A.P.; Boudreau, R.M.; Skinner, M.J.B.; Beydoun, A.B.; Kaval, N.; Caldwell, C.C.; Gulbins, E.M.; Edwards, M.J.; Gobble, R.M. Doxycycline-Coated Silicone Breast Implants Reduce Acute Surgical-Site Infection and Inflammation. Plast. Reconstr. Surg. 2020, 146, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Santos-Vizcaino, E.; Salvador, A.; Vairo, C.; Igartua, M.; Hernandez, R.M.; Correa, L.; Villullas, S.; Gainza, G. Overcoming the inflammatory stage of non-healing wounds: In vitro mechanism of action of negatively charged microspheres (NCMs). Nanomaterials 2020, 10, 1108. [Google Scholar] [CrossRef]
- do Monte, F.A.; Ahuja, N.; Awad, K.R.; Pan, Z.; Young, S.; Kim, H.K.; Aswath, P.; Brotto, M.; Varanasi, V.G. Silicon Oxynitrophosphide Nanoscale Coating Enhances Antioxidant Marker-Induced Angiogenesis During in vivo Cranial Bone-Defect Healing. J. Bone Miner. Res. Plus 2021, 5, e10425. [Google Scholar] [CrossRef]
- Awad, K.; Ahuja, N.; Fiedler, M.; Peper, S.; Wang, Z.; Aswath, P.; Brotto, M.; Varanasi, V. Ionic silicon protects oxidative damage and promotes skeletal muscle cell regeneration. Int. J. Mol. Sci. 2021, 22, 497. [Google Scholar] [CrossRef]
- Lee, K.-I.; Su, C.-C.; Fang, K.-M.; Wu, C.-C.; Wu, C.-T.; Chen, Y.-W. Ultrafine silicon dioxide nanoparticles cause lung epithelial cells apoptosis via oxidative stress-activated PI3K/Akt-mediated mitochondria- and endoplasmic reticulum stress-dependent signaling pathways. Sci. Rep. 2020, 10, 9928. [Google Scholar] [CrossRef]
- Monte, F.; Cebe, T.; Ripperger, D.; Ighani, F.; Kojouharov, H.V.; Chen, B.M.; Kim, H.K.W.; Aswath, P.B.; Varanasi, V.G. Ionic silicon improves endothelial cells’ survival under toxic oxidative stress by overexpressing angiogenic markers and antioxidant enzymes. J. Tissue Eng. Regen. Med. 2018, 12, 2203–2220. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, N.F.S.; Polizelli, M.U.; Muralha, F.P.; de Morais, C.N.L.; Junior, O.M.S.; Maia, M.; Melo, G.B.; Farah, M.E. Ocular inflammation after agitation of siliconized and silicone oil-free syringes: A randomized, double-blind, controlled clinical trial. Int. J. Retin. Vitr. 2022, 8, 41. [Google Scholar] [CrossRef]
- Chauhan, K.; Sabarwal, S.; Soni, D.; Karkhur, S. Silicone oil-associated orbital cellulitis with lipogranulomatous inflammation in the setting of HIV: A management challenge and clinicopathological correlation. BMJ Case Rep. 2021, 14, e239118. [Google Scholar] [CrossRef]
- Thorarinsdottir, H.; Kander, T.; Johansson, D.; Nilsson, B.; Klarin, B.; Sanchez, J. Blood compatibility of widely used central venous catheters; an experimental study. Sci. Rep. 2022, 12, 8600. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, E.; Chatterjee, K. Human Disorders Affecting the Selenocysteine Incorporation Pathway Cause Systemic Selenoprotein Deficiency. Antioxid. Redox Signal. 2020, 33, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Méplan, C.; Hughes, D.J. The role of selenium in health and disease: Emerging and recurring trends. Nutrients 2020, 12, 1049. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free. Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Avissar, N.; Ornt, D.B.; Yagil, Y.; Horowitz, S.; Watkins, R.H.; Kerl, E.A.; Takahashi, K.; Palmer, I.S.; Cohen, H.J. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am. J. Physiol.-Cell Physiol. 1994, 266, C367–C375. [Google Scholar] [CrossRef] [PubMed]
- Schmutzler, C.; Mentrup, B.; Schomburg, L.; Hoang-Vu, C.; Herzog, V.; Köhrle, J. Selenoproteins of the thyroid gland: Expression, localization and possible function of glutathione peroxidase 3. Biol. Chem. 2007, 388, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Faruq, O.; Chien, P.N.; Dönmez, N.; Nam, S.-Y.; Heo, C.-Y. Functionalization of silicone surface with drugs and polymers for regulation of capsular contracture. Polymers 2021, 13, 2731. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-Y.; Ji, H.B.; Shin, B.H.; Chien, P.N.; Donmez, N.; Zhang, X.R.; Huh, B.K.; Kim, M.J.; Bin Choy, Y.; Heo, C.Y. Silicone breast implant coated with triamcinolone inhibited breast-implant-induced fibrosis in a porcine model. Materials 2021, 14, 3917. [Google Scholar] [CrossRef]
- Jwa, S.-J.; Won, J.-M.; Kim, D.-H.; Kim, K.-B.; Lee, J.-B.; Heo, M.; Shim, K.-S.; Jo, H.-S.; Lee, W.-J.; Roh, T.-S.; et al. Breast Tissue Restoration after the Partial Mastectomy Using Polycaprolactone Scaffold. Polymers 2022, 14, 3817. [Google Scholar] [CrossRef]
- Yoo, B.Y.; Kim, B.H.; Lee, J.S.; Shin, B.H.; Kwon, H.; Koh, W.-G.; Heo, C.Y. Dual surface modification of PDMS-based silicone implants to suppress capsular contracture. Acta Biomater. 2018, 76, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Holben, D.H.; Smith, A.M. The diverse role of selenium within selenoproteins: A review. J. Am. Diet. Assoc. 1999, 99, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, R.; Gundimeda, U.; Zhou, S.; Bui, H.; Holmgren, A. Redox regulation of protein kinase C by selenometabolites and selenoprotein thioredoxin reductase limits cancer prevention by selenium. Free. Radic. Biol. Med. 2018, 127, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Veisa, V.; Kalere, I.; Zake, T.; Strele, I.; Makrecka-Kuka, M.; Upmale-Engela, S.; Skesters, A.; Rezeberga, D.; Lejnieks, A.; Pudule, I.; et al. Assessment of iodine and selenium nutritional status in women of reproductive age in Latvia. Medicina 2021, 57, 1211. [Google Scholar] [CrossRef] [PubMed]
- Šķesters, A.; Lece, A.; Kustovs, D.; Zolovs, M. Selenium Status and Oxidative Stress in SARS-CoV-2 Patients. Medicina 2023, 59, 527. [Google Scholar] [CrossRef] [PubMed]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, selenoproteins and viral infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef]
- Alfthan, G. A micromethod for the determination of selenium in tissues and biological fluids by single-test-tube fluorimetry. Anal. Chim. Acta 1984, 165, 187–194. [Google Scholar] [CrossRef]
- Arteel, G.E.; Briviba, K.; Sies, H. Protection against peroxynitrite. FEBS Lett. 1999, 445, 226–230. [Google Scholar] [CrossRef]
- Vijayalakshmi, P.; Geetha, C.; Mohanan, P. Assessment of oxidative stress and chromosomal aberration inducing potential of three medical grade silicone polymer materials. J. Biomater. Appl. 2013, 27, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.J.; Bosch-Morell, F.; Romero, M.J.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, G.; Zhao, E.R.; Li, J.; Hableel, G.; Lemaster, J.E.; Bai, Y.; Sen, G.L.; Jokerst, J.V. Cellular toxicity of silicon carbide nanomaterials as a function of morphology. Biomaterials 2018, 179, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Tonello, S.; Carniato, F.; Rizzi, M.; Migliario, M.; Rocchetti, V.; Marchese, L.; Renò, F. Charged polyhedral oligomeric silsesquioxanes trigger in vitro METosis via both oxidative stress and autophagy. Life Sci. 2017, 190, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Skesters, A.; Kustovs, D.; Lece, A.; Moreino, E.; Petrosina, E.; Rainsford, K.D. Selenium, selenoprotein P, and oxidative stress levels in SARS-CoV-2 patients during illness and recovery. Inflammopharmacology 2022, 30, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Mangiapane, E.; Pessione, A.; Pessione, E. Selenium and Selenoproteins: An Overview on Different Biological Systems. Curr. Protein Pept. Sci. 2014, 15, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- Marampon, F.; Gravina, G.L.; Festuccia, C.; Popov, V.M.; Colapietro, E.A.; Sanità, P.; Musio, D.; De Felice, F.; Lenzi, A.; Jannini, E.A.; et al. Vitamin D protects endothelial cells from irradiation-induced senescence and apoptosis by modulating MAPK/SirT1 axis. J. Endocrinol. Investig. 2016, 39, 411–422. [Google Scholar] [CrossRef]
- Peng, X.; Vaishnav, A.; Murillo, G.; Alimirah, F.; Torres, K.E.; Mehta, R.G. Protection against cellular stress by 25-hydroxyvitamin D3 in breast epithelial cells. J. Cell. Biochem. 2010, 110, 1324–1333. [Google Scholar] [CrossRef]
- Weitsman, G.; Ravid, A.; Liberman, U.; Koren, R. Vitamin D Enhances Caspase-Dependent and Independent TNF-Induced Breast Cancer Cell Death: The Role of Reactive Oxygen Species. Ann. N. Y. Acad. Sci. 2003, 1010, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Tohari, A.M.; Zhou, X.; Shu, X. Protection against oxidative stress by vitamin D in cone cells. Cell Biochem. Funct. 2016, 34, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Ismail, A. Vitamin D treatment protects against and reverses oxidative stress induced muscle proteolysis. J. Steroid Biochem. Mol. Biol. 2015, 152, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Hamden, K.; Carreau, S.; Jamoussi, K.; Miladi, S.; Lajmi, S.; Aloulou, D.; Ayadi, F.; Elfeki, A. 1α,25 dihydroxyvitamin D3: Therapeutic and preventive effects against oxidative stress, hepatic, pancreatic and renal injury in alloxan-induced diabetes in rats. J. Nutr. Sci. Vitaminol. 2009, 55, 215–222. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurševičs, K.; Jurševičs, E.; Krasiļņikova, J.; Šķesters, A.; Lece, A.; Skadiņš, I. Antioxidant Status in Patients after Breast Mastopexy and Augmentation. Medicina 2024, 60, 1046. https://doi.org/10.3390/medicina60071046
Jurševičs K, Jurševičs E, Krasiļņikova J, Šķesters A, Lece A, Skadiņš I. Antioxidant Status in Patients after Breast Mastopexy and Augmentation. Medicina. 2024; 60(7):1046. https://doi.org/10.3390/medicina60071046
Chicago/Turabian StyleJurševičs, Kirils, Eduards Jurševičs, Jeļena Krasiļņikova, Andrejs Šķesters, Anna Lece, and Ingus Skadiņš. 2024. "Antioxidant Status in Patients after Breast Mastopexy and Augmentation" Medicina 60, no. 7: 1046. https://doi.org/10.3390/medicina60071046
APA StyleJurševičs, K., Jurševičs, E., Krasiļņikova, J., Šķesters, A., Lece, A., & Skadiņš, I. (2024). Antioxidant Status in Patients after Breast Mastopexy and Augmentation. Medicina, 60(7), 1046. https://doi.org/10.3390/medicina60071046




