Ultrasound Imaging of Thoracolumbar Fascia: A Systematic Review
Abstract
:1. Introduction
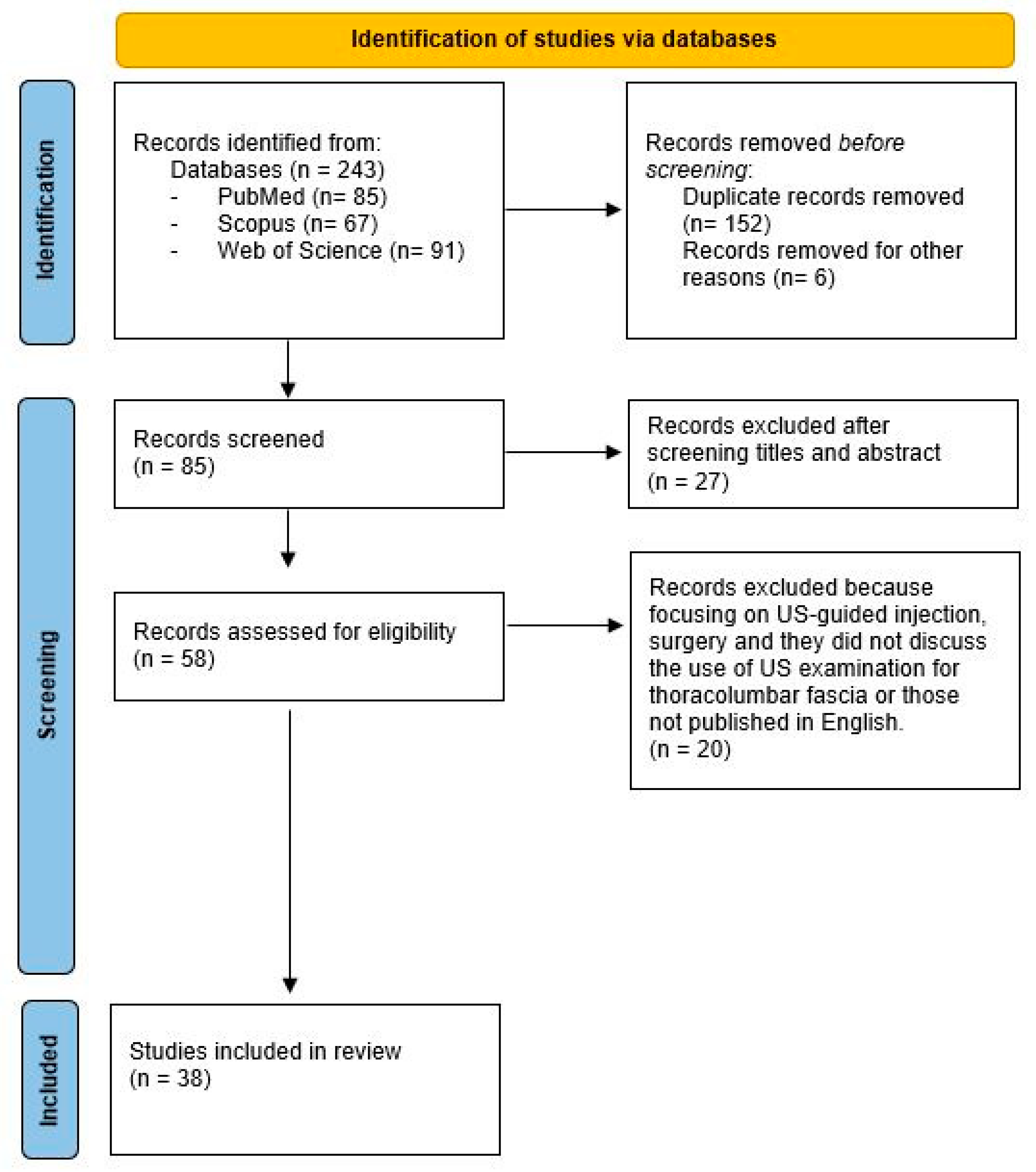
2. Materials and Methods
2.1. Data Extraction
- General characteristic of the paper: first author, year of publication, study design.
- Study population characteristics. Number of patients or healthy volunteers, age, gender, and TLF status (normal vs abnormal).
- Measurements methods: type of probe, type of US imaging, positions of patients or healthy volunteer.
- Reliability.
- Outcomes: evaluated parameters.
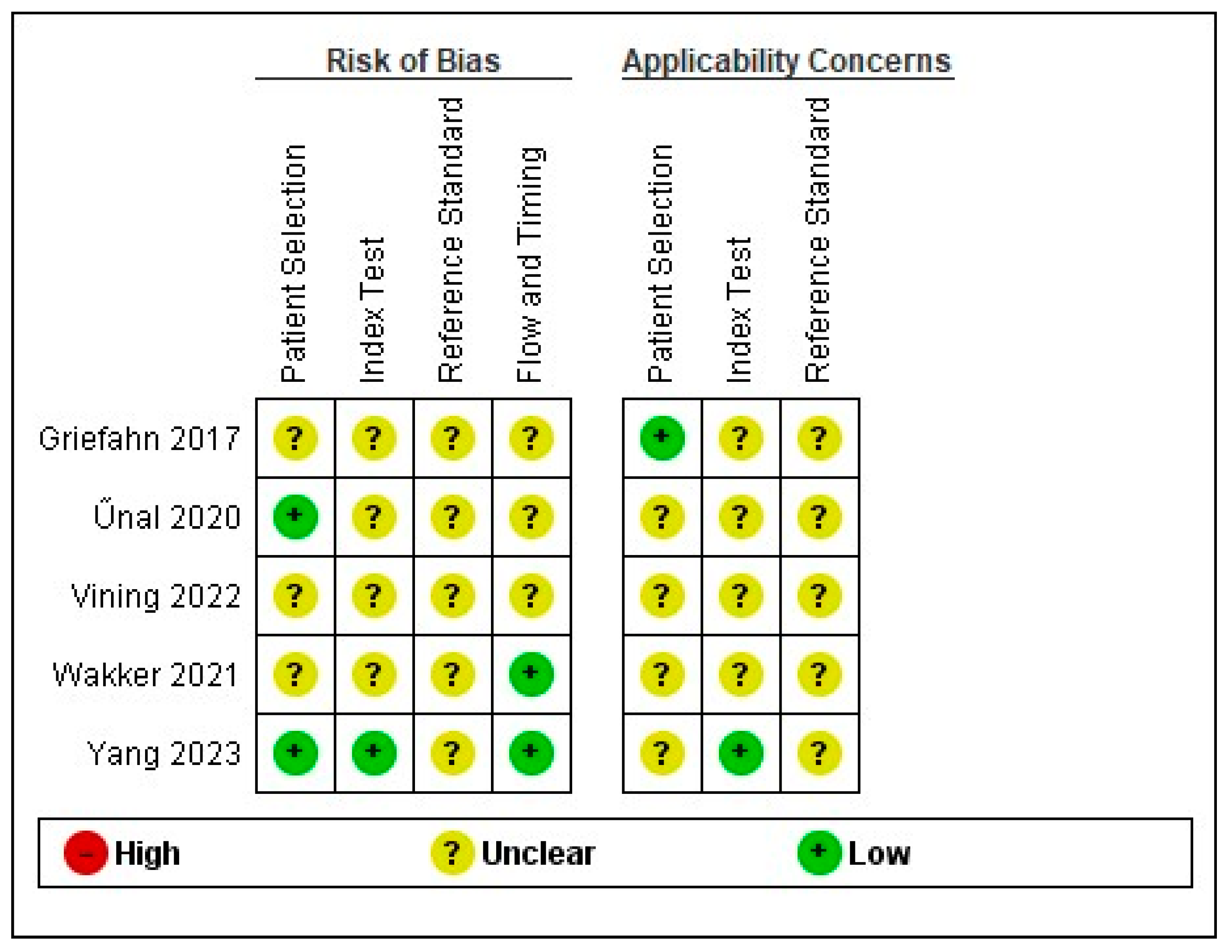
2.2. Risk of Bias
3. Results
3.1. General Characteristics of Studies
3.2. Type of Population
3.3. Assessed Fasciae and Other Musculoskeletal Structures
| Authors and Year | Type of Paper | Number of Participants | Population | Sex | Age (Years Old) | Type of Anatomical Structure | Type of Probe (Frequency) | Type of US Imaging | Position | Parameters | Reliability | Aim |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yerli, S (2024) [17] | A cross-sectional study | 60 | Painful scoliosis, non-painful scoliosis and HV. | 44 F and 16 M | 16.3 ± 4y. | TLF | Linear probe | B-mode | Same protocol of [22] | Thickness | ICC: 0.84 | To examine the thickening of the TLF was observed in subjects with scoliosis, whereby, in the presence of CLBP, it was further intensified. |
| Kellis E. (2023) [18] | Original article | 14 | HV | M | 23.7 ± 7.31 y. | TLF, STF and SMF |
| B-mode SWE | Measured at rest (passive condition) and during submaximal isometric knee flexion efforts (active condition) with the hip at neutral position and the knee flexed at 0°, 45°, and 90°. |
| - | To examine the effect of passive and active knee flexion efforts on the stiffness of the TLF, STF and SMF. |
| Gumruk Aslan S. (2023) [19] | A cross-sectional study | 50 |
| - | - | TLF LMM | - | B-mode | - | Thickness TLF and lumbar multifidus muscle | - | To quantitatively assess the thickness of TLF and LMM in younger-middle aged individuals, both those experiencing CLBP and those without LBP. |
| Brandl A. (2023) [20] | A case–control study | 48 |
| 8 M and 8 F in each group | 18–60 y. | TLF | Philips Lumify linear transducer 4–12 MHz | Cine B-mode | 60-degree TL flexion controlled using a digital goniometer. Trunk extension over 8 s to the neutral position. Ultrasound TLFD measurement was performed in the starting and ending positions. | TLF deformation (TLFD) between the latissimus dorsi muscle junction and an artificial reference | ICC = 0.97 | To investigate TLF deformation in athletes and non-athletes with and without acute low back pain. |
| Vining R. (2023) [21] | Clinical trial | 40 | Self-reporting LBP ≥ 1 year. | 14 F 26 M | 21–65 y. (mean 40 y.) | TLF | Terason 12L5 device, set at 10 MHz) | B-mode and CINE | Probe oriented parallel, 2–3 cm laterally to the L2–3 spinous process interspace at a point where target tissue layers were most visible. Prone-relaxed position on a table moving the lower extremities downward 15°, for five cycles at 0.5 Hz. To assess paraspinal muscle contraction effects, participants raised the head slightly from the table. | TLF shear strain | - | To assess TLF mobility and CLBP: Phase 1 of a pilot and feasibility study assessing repeated measures and the influence of paraspinal muscle contraction. |
| Pirri C. (2023) [22] | A cross-sectional study | 92 |
| 47 F 45 M | CNLBP: 28.96 ± 10.54 y. HV: 27.09 ± 12.38 y. | TLF | Edge II, Sonosite, FUJIFILM, 6–15 MHz | B-mode | Relaxed prone position and the US transducer was placed parallel to the spine, approximately 2–3 cm lateral to the L3 spinous process | TLF thickness in the longitudinal and transverse axes | Intra-rater reliability: Long axis (CNLBP: ICC(3,k): 0.91; HV ICC(3,k): 0.92). Transverse axis:(CNLBP: ICC(3,k): 0.88; HV: ICC3, k: 0.88). | To measure and compare by ultrasound imaging the thickness of the TLF at the bilateral L3 level of the lumbar spine in the longitudinal and transverse axes in chronic non-specific LBP and in healthy subjects, demonstrating an increase in non-specific LBP patients. |
| Devantery K. (2023) [23] | A before-and-after experimental study | 49 | LBP between 12th rib and gluteal fold for more than six months;
| 25 M 24 F | >18 y.; | TLF ESM | Aixplorer Ultimate, SuperSonic Imagine, Aix-en-Provence, France; SL 10–2 MHz | B-mode; SWE | The probe was placed 2 cm lateral to L2–L3 interspinous space, on the right and left sides | Stiffness Thickness | - | To evaluate the immediate effect of a standardized versus a simulated MFT on the stiffness of the TLF and ESM using shear-wave elastography. |
| Perez M. A. (2023) [24] | A pilot study | 54 | NSLBP (n = 23) HV (n = 31) | NS-LBP: 10 M 13 F; HV: 12 M 19 F | 18–60 y. | TLF Diaphragm | Vinno E35 (VINNO Technology, Suzhou, China) 7–18 MHz, with 38 mm footprint; Convex probe 1–5 MHz was used with 52 mm footprint. | B-mode (TLF) M-mode (diaphragm) | L4 vertebral level longitudinally over the anterior subcostal region in a supine position (bed slope of 45°) | Thickness of the TLF Diaphragmatic excursion | - | To perform a comparison based on the measurement of ultrasonographic parameters of the diaphragm, the lumbar multifidus muscles, and the TLF in subjects with and without NS-LBP. |
| Yang C. (2023) [25] | Randomized Controlled Trial | 66 |
| M | 22 ± 4.1 y. | TLF | A Mindray M7 scanner with a 4 cm, 10 MHz linear probe | B-mode | The probe centred at 2 cm lateral to the middle of the L2–L3 interspinous ligament | TLF thickness and echo intensity, perceived stiffness, lumbar flexibility, and skin temperature | Intra-observer: ICC = 0.95; inter-observer: ICC = 0.91. | To investigate the effects of PT on TLF morphology and other related outcomes. |
| Larivière C. (2023) [26] | Experimental laboratory study | 70 | CLBP | - | - | LM | - | B-mode | - | LuM echogenicity at three vertebral levels (L3/L4, L4/L5 and L5/S1); TLF posterior layer thickness PMCT thickness of the fasciae between STT and EO, between EO and IO, between IO and TrA, and between TrA and IA. | - | To explore whether these RUSI parameters (LuM echogenicity and fascia thicknesses), here after called dependent variables (DV) were linked to independent variables (IV) such as (1) other RUSI parameters (trunk muscle thickness and activation) and (2) physical and psychological measures. RUSI measures, as well as a clinical examination comprising physical tests and psychological questionnaires. |
| Tamartash H. (2023) [27] | A cross-sectional study | 131 | 68 LBP 63 HV | LBP:33 M 35 F HV: 32 M 31F | 40.2 ± 5.3 y. 41.7 ± 4.9 y. | thoracolumbar fascia (TLF) | SONON Ultrasound Imaging System with a 5–14 MHz linear probe. | B-mode and SWE | Prone position and placed their upper limbs in a relaxed position next to the body. The probe left side of L2–L3 vertebrae. These images were recorded “with stress” and “without stress” to achieve the elastic modulus of the TLF. | Strain of TLF | - | To evaluate the changes in the elastic behavior of LF in patients with CNLBP based on ultrasound imaging |
| Bartsch K. (2023) [28] | Experimental study | 1 | Multi-layered phantom model | - | - | TLF | Philips Lumify with L12–L4 linear transducer | B-mode | In two states: with stress and without stress. For the stress state scenario, compressive stress is imposed by the ultrasound transducer. | Tissue stiffness and Stress | ICC (2,2) = 0.75–0.98 | To compare different stiffness measurement tools reliability on a multi-layered phantom tissue model (MPTM). |
| Brandl A. (2022) [29] | Case-control study | 10 with LBP; 10 HV | Acute LBP were matched to HV | LBP: 4 M 6 F HV: 4 M 6 F | 43.6 ± 15.9 (LBP group) 39.0 ± 15.0 (control group) | TLF | Mindray DP2200, linear transducer 75L38HB, 5–10 MHz, sampling rate 7.5 MHz | B -mode and dynamic US | The transducer was then moved laterally along the line from the L1 spinous process in the sagittal section until the junction of the LD muscle with the TLF was visible. | Deformation of TLF defined by the distance between the intersection of the artificial reference and the underside of the PLF and the muscle–fascia junction of the LD and the TLF. | - | To reveal time-dependent relationships between biomechanical and neuromotor factors. |
| Vining R. (2022) [30] | clinical trial | 20 | CLBP following spinal manipulation and over an 8-week course of multimodal chiropractic care. | 11 F 9 M | 21–65 y. (mean 40 y.) | TLF | Terason T3000 ultrasound system with a transducer set at 10 MHz and programmed to record a cine-loop for 20 s in B-mode at a 25 Hz frame rate. | B-mode and cine | Ultrasound imaging occurred 2–3 cm lateral to L2–3 while participants relaxed prone on an automated table moving the lower extremities downward 15 degrees, for five cycles at 0.5 Hz. | TLF shear strain and TLF mobility | - | To assess TLF shear strain in persons with chronic low back pain following spinal manipulation and over an 8-week course of multimodal chiropractic care. |
| Turan Z. (2022) [31] | Cross-sectional study | 30 | HV | 15 M; 15 F | 28.8 ± 8.1 y. | Transversus abdominis and internal oblique muscles | Esaote MyLab Class C ultrasound device equipped with 55 mm convex transducer (CA 541, B-mode, frequency 5 MHz). | B-mode | Evaluated using ultrasound during four positions (rest, abdominal hollowing, bridge, and bridge with arm extension). | Thickness of transversus abdominis and internal oblique muscles | - | To evaluate the changes in the ultrasonographic thickness of transversus abdominis and internal oblique muscles during bridge with arm extension compared to bridge and abdominal hollowing. |
| Ushida K. (2022) [32] | 17 | CLBP | - | - | Paravertebral muscles and perimuscular connective tissues | - | B-mode | Measurements located lateral to the midpoint between L2-3 and L4-5 spines. | Thickness and echogenicity of the paravertebral muscles and PMCT. | - | To investigate the relationship between paravertebral muscles and PMCT of the TLF region and the four types of pain in patients suffering from CLBP. | |
| Venkatesan P. (2022) [33] | Case-control study | 144; Experimental group: yoga; Control Group: exercise based on DNS | Lumbar muscle in CLBP for longer than 3 months | 18–45 y. | - | TLF | - | B-mode | TLF thickness | - | - | To compare the effects of yoga and dynamic neuromuscular stabilization exercise on CSA, fat infiltration of LMM with magnetic resonance imaging, and TLF thickness using musculoskeletal ultrasound imaging in CLBP. |
| Larivière C. (2021) [34] | Cross sectional study | 64 | 34 LBP 30 HV | 15 M 15 F | 18–65 y. | Dorsal trunk and ventrolateral abdominal wall soft tissues | A 5–2 MHz curvilinear array transducer for lumbar spine, while a 12–5 MHz 50-mm linear array transducer for PMCT of the abdominal wall. | B-mode | USI measures were collected at rest on an exam table, in supine and prone positions), standardized task to assess muscle activation. | Muscle thickness, PMCT thickness; STTABD over the lateral abdominal wall. | ICC(3,1) ¼ 0.92 and 0.96 for left and right sides, respectively. | To test the medium-term (8 weeks) test-retest reliability of the corresponding RUSI measures. |
| Pirri C. (2021) [35] | Case reports | 1 | Sedentary work at computer | F | 35 years | TLF | Linear 4–16 MHz and convex 1–7 MHz | B-mode | - | - | - | To identify the reason of LBP. |
| Larivière C. (2021) [36] | Cross sectional study | 64 | 30 HV and 34 LBP | HV: 15 M 15 F LBP: 15 M 18 F | 18–65 y. | Dorsal trunk and ventrolateral abdominal wall soft tissues | 5–2 MHz curvilinear array transducer (6.5 cm footprint), while the PMCT of abdominal wall using a 12–5 MHz 50-mm linear array transducer. | B-mode | The lumbar spine structures were imaged in the parasagittal plane | (1) Lumbar multifidus (LM) echogenicity at three vertebral levels (L3/L4, L4/L5 and L5/S1); (2) PLF thickness of the thoracolumbar fascia; (3) Thickness of the fasciae surrounding EO, IO and TrA. | - | To identify the main potential determinants of US measures of LM muscle fatty infiltrations, TLF thickness and thicknesses of PMCT surrounding the abdominal wall muscles. |
| Chen B. (2021) [37] | Cohort study | 20 | HV | M | 18.4 ± 0.7 y. | TLF | Ultrasound transducer | SWE | Seven postures. (1) Rest, (2) sitting, (3) sitting-forward 30°, (4) sitting forward 60°, (5) standing, (6) standing-forward 30°, and (7) standing-forward 60°. | Stiffness | - | To use SWE to study the relationship between shear modulus and different body postures of TLF. Acquire physiologically meaningful information from the stiffness-posture graph to better quantify passive flexion responses. |
| Wakker J. (2021) [38] | Prospective clinical trial | 267 | HV | 166 F; 101 M | 36.1 ± 15.5 y. | TLF | Siemens Acuson S3000 TM 4.-9 MHz linear transducer. | B-mode a colour-coded elastogram, was positioned in the TLF | Lying prone with the arms adjacent to the body. | Stiffness | Intra-rater reliability ICC was between 0.857 and 0.979. The ICC for the inter-rater reliability was 0.931. | Determining the normal values for acoustic radiation force impulse (ARFI) SWE of TLF and define possible factors of influence. |
| Cheung W.K. (2020) [39] | Review | - | LBP and HV | - | - | TrA; MF + TLF | 5- to 7-MHz linear, 5-MHz curved and 2 to 5 MHz curvilinear arrays | B-mode DOPPLER SWE | TrA thickness at the end of expiration; TLF: linear probe longitudinally 2 cm lateral to the midline at the level of the L2–3 interspace. MF: linear probe at 4 cm lateral to L3 over the longissimus muscle group. | Thickness, Doppler and Stiffness. | The ultrasound measurements had moderate and good between-day inter-rater reliability. | To highlight the current understanding of how medical ultrasound has been used for diagnosis and study of low back pain and discusses potential new applications. |
| Larivière C. (2020) [40] | Cross sectional study | 64 | 30 HV 34 LBP | HV: 15 M 15 F/LBP: 15 M 18 F | 18–65 y. | Dorsal trunk and ventrolateral abdominal wall soft tissues | A 5–2 MHz curvilinear array transducer with 6.5 cm footprint for lumbar spine; a 12–5 MHz 50-mm linear array transducer for PMCT of the abdominal wall. | B-mode | Images were collected on an exam table, in supine (ventrolateral abdominal wall) and prone (dorsal soft tissues), just before and during an isometric standardized task to induce muscle activation. | Thickness | - | To compare three quantitative measures of these tissues, using US imaging. |
| Chen B. (2020) [41] | 20 | Healthy | M | 18.4 ± 0.7 y. | TLF | Aixplorer ultrasound device with a 40 mm linear array 10–2 MHz. | B-mode SWE | At the L3–L4. Horizontally 2 cm from the right side of the L2–3 and the L3–4 midline. All subjects performed in postures: sitting, sitting-forward 60° | Elasticity | Intra-operator (ICC = 0.860–0.938) and inter-operator (ICC = 0.904–0.944) | To examine the intra and inter-operator reliability of SWE device in quantifying the shear modulus of TLF and the device’s abilities to examine the shear modulus of the TLF during upper body forward. | |
| Ünal M. (2020) [42] | Randomized Controlled Trial | 40 | CLBP | - | 25–65 y. | TLF | Siemens Acuson X 700 and a Linear 10.7 MHz probe | B-mode | On the right and left sides of the dorsum with the patient in the prone position | Morphological structure of TLF. | - | The aim of this study was to comparatively investigate the effects of MIT against PNE on pain and function in patients with CLBP. |
| Blain M. (2019) [43] | 15 | HV, right-handed | 6 F; 9 M | 24 ± 4 y. | Paraspinal muscular compartment (PMC) | - | B-mode SWE | The transducer was oriented longitudinally centred at L3–L4 level, at 2 cm from the midline bilaterally. Performed in 5 postures including various trunk and arm positions. | Stiffness | ICC showed good to excellent intra-rater reliability. | The aims of this study were (1) to test the reliability of SWE in MFM and ESM in prone and sited position; (2) to investigate the role of the tensioning of the pTLF, via stretching of LD, on LPM stiffness. | |
| Vita M. (2019) [44] | Prospective study | 29 | 17 users, and 12 nonusers of hormonal contraceptives | F | 18–29 y. (mean, 22.5 years) | TLF, FL and PF | SuperSonicAixplorer ultrasound machine with a linear array transducer SL 15–4 MHz. | B-mode SWE | Relaxed prone position with hands placed beside their thighs. The examined side respected the dorsal myofascial line. | Thickness Stiffness | - | To examine the influence of hormonal changes during the menstrual cycle on deep fasciae. |
| De Coninck K. (2018) [45] | Cross-sectional study | 30 | 21 medical doctors, 7 physiotherapists and 2 radiologists. | 13.03 ± 9.6 y. of experience | TLF | 18 MHz linear array transducer (Esaote LA435) | B-mode | Intervertebral level 2–3, as fascial planes are the most parallel to the skin at this level. | Architectural disorganisation of TLF | - | To determine the inter-rater reliability for the rating of morphological characteristics of thoracolumbar fascia in ultrasound images, on Likert-type scale, by a range of clinicians. | |
| Fullerton B. D (2018) [46] | Cases report | 2 |
|
|
| TLF | Linear-array high frequency transducer | B-mode | Prone position | Thickness of the TLF | - | To identify the alteration in TLF. |
| Panagos A. (2018) [47] | Case report | 1 | Chronic LBP | M | 65 y. | TLF | - | B-mode | Right paraspinal muscles at the L5-S1 vertebral body level. | Thickness. | - | To identify the reason of LBP. |
| Todorov PT. (2018) [3] | Review | - | Patients with low back pain (LBP) | - | - | Lumbar and pelvic ligaments, muscles and entheses, TLF and the sacroiliac joints | 10 MHz linear transducer | B-mode | Linear transducer in the longitudinal plane on a point 2 cm lateral to the midpoint at L2–L3 level. | Thickness and echogenicity | - | To review the literature on the diagnostic value of US in different conditions that could cause LBP. |
| Langevin H.M (2018) [48] | Animal study | 20 | Swine | - | 4–6 wks | TLF | Terason 3000 scanner with a 4.0-mm, 10-MHz linear array transducer | B-mode + cine | L3–4 level in transverse axis, and the edge of the probe aligned with the lateral border of the vertebral body. Cine recording acquired during passive flexion of the trunk. | Thickness, Shear Strain and tissue displacement within the connective tissue layers of the TLF. | - | To determine whether the abnormalities in fascia mobility caused by an unilateral TLF injury and movement restriction can be reversed by removing the movement restriction, with or without the implementation of daily stretching for one month. |
| Wong KK. (2017) [49] | Cross-sectional study. | 10 | Healthy | M | 22.8 ± 2.0 y. | PLF | Terason t3000 system, with a 5–12 MHz and 38 mm linear-array transducer | B-mode | Prone position with the shoulder internally rotated, palm up, elbow extended, and head in neutral position performed a press-down to MVC in the prone position. | Deformation of the PLF. | Moderate to good reliability of all parameters ICC (3,3) ranging from 0.95 to 0.98. | To quantify the immediate effects of MR on fascial properties of the PLF in healthy men. |
| Griefahn A. (2017) [50] | Randomized and controlled trial | 38 | Healthy athletic active | 25 M 13 F | Mean 23.34 y. | TLF | A MyLab One Esaote, ultrasound machine with a 13–6 MHz linear probe | Cine B-mode | Sit position on the treatment table with their feet having contact to the ground and exercise the TL flexion, probe located 2 cm lateral and to the right of the spine, at L2–L3. | To calculate how displaceable the various layers of the TLF are. | ICC ranging from 0.79 to 0.9 and 0.76 to 0.79 | To determine whether there is a significant difference in the mobility of the TLF among three treatment groups. |
| Tu S.J. (2016) [51] | Observational Study | 12 | HV | 8 M 4 F | 22.9 ± 3.59 y. | TL tissue | Voluson i, GE with a frequency 4–12 MHz linear probe. | B-mode + cine | Transducer 3 cm lateral to the middle of the L2 and L3 spinous processes; patients perform speed-guided lumbar flexion-extension tasks in two states (without taping and with KT). | Lumbar tissue movements | - | To assess the impact of KT on the movements of the TLF tissue. |
| Bishop J H (2016) [52] | Experimental design | 20 | Castrated male domestic swine | - | 4–6 weeks old | TLF | Terason 3000 scanner with a 4.0 mm, 10 MHz linear array transducer | B-mode + ultrasound cine-recordings | Bilaterally at L2–3, L3–4 and L4–5 levels with the ultrasound probe oriented transversely, and the edge of the probe aligned with the lateral border of the vertebral body | Tissue displacement: during passive flexion of the trunk + tissue thickness | - | They used a porcine model to test the hypothesis that similar ultrasound findings can be produced experimentally in a porcine model by combining a local injury of fascia with movement restriction using a “hobble” device linking one foot to a chest harness for 8 weeks. |
| Darrieutort-Laffite C- (2014) [53] | Review | - | - | - | - | TLF and the various components of the lumbar spine | Transducer frequencies ranged from 2 to 9 MHz; linear 3–11 MHz probe, with the trapezoid mode when needed to expand the field of view. | B-mode | The transducer is placed on the midline, along the spinous processes, in the longitudinal direction. Seated or bent forward or in the prone position with a cushion under the abdomen. | - | - | To discuss a systematic approach to the ultrasonographic assessment of the lumbar spine. |
| Langevin H. M (2011) [54] | Original article | 121 | 50 no—LBP 71 LBP ≥ 12 months duration | 24 M 26 F 38 M 33 F | 44.6 ± 1.8 41.8 ± 2.3 | TLF | Terason 3000 ultrasound machine; 10 MHz (12L5) linear array transducer | B-mode + cine | Bilaterally of the back during passive trunk flexion using a motorized articulated table with the hinge point of the table at L4–5 and probe located longitudinally 2 cm lateral to the midline at the level of the L2–3 interspace | Displacement within TLF; PMCT thickness and echogenicity | - | To quantify shear plane motion within the TLF using ultrasound elasticity imaging in human subjects with and without CLBP. |
| Type of Studies | N |
|---|---|
| Cross-sectional study | 11 |
| Original article | 6 |
| Experimental laboratory study | 4 |
| Case-control study | 3 |
| Case report | 3 |
| Randomized clinical trial | 3 |
| Clinical trial | 2 |
| Review | 3 |
| Pilot study | 1 |
| Before and after experimental study | 1 |
| Prospective study | 1 |
3.4. US Equipment Charcteristics and Type of Probe
3.5. Positioning of Patient and Protocol
3.6. Parameters Evaluated with the Measurements
3.7. Reliability
3.8. Aims of Studies
3.9. Risk of Bias Assessment and Applicability Concern
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pirri, C.; Pirri, N.; Stecco, C.; Macchi, V.; Porzionato, A.; De Caro, R.; Özçakar, L. ‘Ultrasound Examination’ of the Musculoskeletal System: Bibliometric/Visualized Analyses on the Terminology (Change). Tomography 2023, 9, 352–361. [Google Scholar] [CrossRef]
- Özçakar, L.; Ricci, V.; Mezian, K.; Pirri, C. A New and Dedicated Video Gallery: EURO-MUSCULUS/USPRM Protocols for Dynamic Ultrasound Examination of the Joints. Am. J. Phys. Med. Rehabil. 2022, 101, 201–202. [Google Scholar] [CrossRef]
- Todorov, P.T.; Nestorova, R.; Batalov, A. Diagnostic value of musculoskeletal ultrasound in patients with low back pain—A review of the literature. Med. Ultrason. 2018, 1, 80–87. [Google Scholar] [CrossRef]
- Crisco, J.J.; Panjabi, M.M.; Yamamoto, I.; Oxland, T.R. Euler stability of the human ligamentous lumbar spine. Part II: Experiment. Clin. Biomech 1992, 7, 27–32. [Google Scholar] [CrossRef]
- Bergmark, A. Stability of the lumbar spine. A study in mechanical engineering. Acta Orthop. Scand. Suppl. 1989, 230, 1–54. [Google Scholar] [CrossRef]
- Cholewicki, J.; Panjabi, M.M.; Khachatryan, A. Stabilizing function of trunk flexor-extensor muscles around a neutral spine posture. Spine 1997, 22, 2207–2212. [Google Scholar] [CrossRef]
- Willard, F.H. The muscular, ligamentous, and neural structure of the lumbosacrum and its relationship to low back pain. In Movement, Stability & Lumbopelvic Pain, 2nd ed.; Vleeming, A., Mooney, V., Stoeckart, R., Eds.; Churchill Livingstone Elsevier: Edinburgh, Scotland, 2007; pp. 5–45. [Google Scholar]
- Clemente, C.D. Gray’s Anatomy of the Human Body; Lea & Febiger: Philadelphia, PA, USA, 1985. [Google Scholar]
- Willard, F.H.; Vleeming, A.; Schuenke, M.D.; Danneels, L.; Schleip, R. The thoracolumbar fascia: Anatomy, function and clinical considerations. J. Anat. 2012, 221, 507–536. [Google Scholar] [CrossRef]
- Schuenke, M.D.; Vleeming, A.; Van Hoof, T.; Willard, F.H. A description of the lumbar interfascial triangle and its relation with the lateral raphe: Anatomical constituents of load transfer through the lateral margin of the thoracolumbar fascia. J. Anat. 2012, 221, 568–576. [Google Scholar] [CrossRef]
- Stecco, C. Functional Atlas of the Human Fascial System, 1st ed.; Churchill Livingstone Elsevier: Edinburgh, Scotland, 2015. [Google Scholar]
- Vleeming, A.; Pool-Goudzwaard, A.L.; Stoeckart, R.; van Wingerden, J.P.; Snijders, C.J. The posterior layer of the thoracolumbar fascia. Its function in load transfer from spine to legs. Spine 1995, 20, 753–758. [Google Scholar] [CrossRef]
- Carvalhais, V.O.; Do, C.; Ocarino, J.d.M.; Araujo, V.L.; Souza, T.R.; PLP, S.; Fonseca, S.T. Myofascial force transmission between the latissimus dorsi and gluteus maximus muscles: An in vivo experiment. J. Biomech. 2013, 46, 1003–1007. [Google Scholar] [CrossRef]
- Pavan, P.G.; Stecco, A.; Stern, R.; Stecco, C. Painful connections: Densification versus fibrosis of fascia. Curr. Pain Headache Rep. 2014, 18, 441. [Google Scholar] [CrossRef] [PubMed]
- Corey, S.M.; Vizzard, M.A.; Bouffard, N.A.; Badger, G.J.; Langevin, H.M. Stretching of the back improves gait, mechanical sensitivity and connective tissue inflammation in a rodent model. PLoS ONE 2012, 7, e29831. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Yerli, S.; Yinanç, S.B.; Yağcı, G.; Erbahçeci, F.; Özçakar, L. Thoracolumbar fascia and chronic low back pain in idiopathic lumbar scoliosis: An ultrasonographic study. Eur. Spine J. 2024, 33, 2469–2475. [Google Scholar] [CrossRef]
- Kellis, E.; Kekelekis, A.; Drakonaki, E.E. Is thoracolumbar fascia shear-wave modulus affected by active and passive knee flexion? J. Anat. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gumruk Aslan, S.; Koylu Uyar, S.; Gurcay, E. Potential role of thoracolumbar fascia in younger middle-aged patients with chronic low back pain. Int. J. Neurosci 2023, 1–7, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Wilke, J.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Thoracolumbar fascia deformation during deadlifting and trunk extension in individuals with and without back pain. Front. Med. 2023, 10, 1177146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vining, R.; Onifer, S.M.; Twist, E.; Ziegler, A.M.; Corber, L.; Long, C.R. Thoracolumbar fascia mobility and chronic low back pain: Phase 1 of a pilot and feasibility study assessing repeated measures and the influence of paraspinal muscle contraction. J. Bodyw. Mov. Ther. 2023, 34, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Pirri, N.; Guidolin, D.; Macchi, V.; Porzionato, A.; De Caro, R.; Stecco, C. Ultrasound Imaging of Thoracolumbar Fascia Thickness: Chronic Non-Specific Lower Back Pain versus Healthy Subjects; A Sign of a “Frozen Back”? Diagnostics 2023, 13, 1436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devantéry, K.; Morin, M.; Grimard, J.; Gaudreault, N. Effects of a Myofascial Technique on the Stiffness and Thickness of the Thoracolumbar Fascia and Lumbar Erector Spinae Muscles in Adults with Chronic Low Back Pain: A Randomized before-and-after Experimental Study. Bioengineering 2023, 10, 332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perez, A.M.; Fernández-Carnero, S.; Sicilia-Gomez-de-Parada, C.; Cuenca-Zaldívar, N.; Naranjo-Cinto, F.; Pecos-Martín, D.; Gallego-Izquierdo, T.; Nuñez-Nagy, S. Diaphragmatic Activation Correlated with Lumbar Multifidus Muscles and Thoracolumbar Fascia by B-Mode and M-Mode Ultrasonography in Subjects with and without Non-Specific Low Back Pain: A Pilot Study. Medicina 2023, 59, 315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, C.; Huang, X.; Li, Y.; Sucharit, W.; Sirasaporn, P.; Eungpinichpong, W. Acute Effects of Percussive Massage Therapy on Thoracolumbar Fascia Thickness and Ultrasound Echo Intensity in Healthy Male Individuals: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 1073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larivière, C.; Preuss, R.; Gagnon, D.H.; Mecheri, H.; Driscoll, M.; Henry, S.M. The relationship between clinical examination measures and ultrasound measures of fascia thickness surrounding trunk muscles or lumbar multifidus fatty infiltrations: An exploratory study. J. Anat. 2023, 242, 666–682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tamartash, H.; Bahrpeyma, F.; Mokhtari Dizaji, M. Ultrasound evidence of altered lumbar fascia in patients with low back pain. Clin. Anat. 2023, 36, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, K.; Brandl, A.; Weber, P.; Wilke, J.; Bensamoun, S.F.; Bauermeister, W.; Klingler, W.; Schleip, R. Assessing reliability and validity of different stiffness measurement tools on a multi-layered phantom tissue model. Sci. Rep. 2023, 13, 815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brandl, A.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Associations between Deformation of the Thoracolumbar Fascia and Activation of the Erector Spinae and Multifidus Muscle in Patients with Acute Low Back Pain and Healthy Controls: A Matched Pair Case-Control Study. Life 2022, 12, 1735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vining, R.; Onifer, S.M.; Twist, E.; Ziegler, A.M.; Corber, L.; Long, C.R. Thoracolumbar fascia mobility and chronic low back pain: Phase 2 of a pilot and feasibility study including multimodal chiropractic care. Chiropr. Man Therap. 2022, 30, 46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turan, Z.; Özyemişçi-Taşkıran, Ö. The effect of activation of thoracolumbar fascia on the thickness of abdominal muscles: An ultrasonographic study. Turk J. Phys. Med. Rehabil. 2022, 68, 169–174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ushida, K.; Akeda, K.; Momosaki, R.; Yokochi, A.; Shimada, T.; Ito, T.; Maruyama, K. Intermittent pain in patients with chronic low back pain is associated with abnormalities in muscles and fascia. Int. J. Rehabil. Res. 2022, 45, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P.; K, S.; Kishen, T.J.; Janardhan, S.; Kumar, C.S. Comparison of yoga and dynamic neuromuscular stabilization exercise in chronic low back pain on magnetic resonance imaging of lumbar multifidus- protocol for a randomized controlled trial. Contemp. Clin. Trials Commun. 2022, 28, 100937. [Google Scholar] [CrossRef]
- Larivière, C.; Gagnon, D.H.; Preuss, R. Structural remodeling of the lumbar multifidus, thoracolumbar fascia and lateral abdominal wall perimuscular connective tissues: Medium-term test-retest reliability of ultrasound measures. J. Bodyw. Mov. Ther. 2021, 27, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Pirri, C.; Ricci, V.; Stecco, C.; Özçakar, L. Clinical and Ultrasound Examination of the Thoracolumbar Fascia: The Hands and the Probe Together. Am. J. Phys. Med. Rehabil. 2021, 100, e157–e158. [Google Scholar] [CrossRef] [PubMed]
- Larivière, C.; Henry, S.M.; Preuss, R. Structural remodeling of the lumbar multifidus, thoracolumbar fascia and lateral abdominal wall perimuscular connective tissues: A search for its potential determinants. J. Anat. 2021, 238, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, C.; Lin, M.; Deng, W.; Zhang, Z. Effects of body postures on the shear modulus of thoracolumbar fascia: A shear wave elastography study. Med. Biol. Eng. Comput. 2021, 59, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Wakker, J.; Kratzer, W.; Schmidberger, J.; Graeter, T.; Elasto Study Group. Elasticity standard values of the thoracolumbar fascia assessed with acoustic radiation force impulse elastography on healthy volunteers: A cross section study. J. Bodyw. Mov. Ther. 2021, 26, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.K.; Cheung, J.P.Y.; Lee, W.N. Role of Ultrasound in Low Back Pain: A Review. Ultrasound Med. Biol. 2020, 46, 1344–1358. [Google Scholar] [CrossRef]
- Larivière, C.; Preuss, R.; Gagnon, D.H.; Mecheri, H.; Henry, S.M. Structural remodelling of the lumbar multifidus, thoracolumbar fascia and lateral abdominal wall perimuscular connective tissues: A cross-sectional and comparative ultrasound study. J. Bodyw. Mov. Ther. 2020, 24, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhao, H.; Liao, L.; Zhang, Z.; Liu, C. Reliability of shear-wave elastography in assessing thoracolumbar fascia elasticity in healthy male. Sci. Rep. 2020, 10, 19952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ünal, M.; Evci, K.E.; Kocatürk, M.; Algun, Z.C. Investigating the effects of myofascial induction therapy techniques on pain, function and quality of life in patients with chronic low back pain. J. Bodyw. Mov. Ther. 2020, 24, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Blain, M.; Bedretdinova, D.; Bellin, M.F.; Rocher, L.; Gagey, O.; Soubeyrand, M.; Creze, M. Influence of thoracolumbar fascia stretching on lumbar back muscle stiffness: A supersonic shear wave elastography approach. Clin. Anat. 2019, 32, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Vita, M.; Sedlackova, Z.; Herman, M.; Furst, T.; Smekal, D.; Cech, Z. Influence of female hormones on fascia elasticity: An elastography study. Clin. Anat. 2019, 32, 941–947. [Google Scholar] [CrossRef] [PubMed]
- De Coninck, K.; Hambly, K.; Dickinson, J.W.; Passfield, L. Measuring the morphological characteristics of thoracolumbar fascia in ultrasound images: An inter-rater reliability study. BMC Musculoskelet. Disord. 2018, 19, 180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fullerton, B.D. Prolotherapy for the Thoracolumbar Myofascial System. Phys. Med. Rehabil. Clin. N. Am. 2018, 29, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Panagos, A. Resolution of a Greater Than 50-year History of Severe, Chronic Low Back Pain Following an Ultrasound-guided Platelet-rich Plasma Infiltration of the Thoracolumbar Fascia. Cureus 2018, 10, e3457. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Langevin, H.M.; Bishop, J.; Maple, R.; Badger, G.J.; Fox, J.R. Effect of Stretching on Thoracolumbar Fascia Injury and Movement Restriction in a Porcine Model. Am. J. Phys. Med. Rehabil. 2018, 97, 187–191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, K.K.; Chai, H.M.; Chen, Y.J.; Wang, C.L.; Shau, Y.W.; Wang, S.F. Mechanical deformation of posterior thoracolumbar fascia after myofascial release in healthy men: A study of dynamic ultrasound imaging. Musculoskelet. Sci. Pract. 2017, 27, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Griefahn, A.; Oehlmann, J.; Zalpour, C.; von Piekartz, H. Do exercises with the Foam Roller have a short-term impact on the thoracolumbar fascia? —A randomized controlled trial. J. Bodyw. Mov. Ther. 2017, 21, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.J.; Woledge, R.C.; Morrissey, D. Does ‘Kinesio tape’ alter thoracolumbar fascia movement during lumbar flexion? An observational laboratory study. J. Bodyw. Mov. Ther. 2016, 20, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.H.; Fox, J.R.; Maple, R.; Loretan, C.; Badger, G.J.; Henry, S.M.; Vizzard, M.A.; Langevin, H.M. Ultrasound Evaluation of the Combined Effects of Thoracolumbar Fascia Injury and Movement Restriction in a Porcine Model. PLoS ONE 2016, 11, e0147393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Darrieutort-Laffite, C.; Hamel, O.; Glémarec, J.; Maugars, Y.; Le Goff, B. Ultrasonography of the lumbar spine: Sonoanatomy and practical applications. Jt. Bone Spine 2014, 81, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; Greenan-Naumann, A.C.; Bouffard, N.A.; Konofagou, E.E.; Lee, W.N.; Triano, J.J.; Henry, S.M. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet. Disord. 2011, 12, 203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Population | Patients or healthy volunteers who underwent Ultrasound Imaging of Thoracolumbar fascia |
| Intervention | Ultrasound Imaging |
| Comparison | Ultrasound Imaging of other types of fasciae |
| Outcome | Parameters of thickness, echogenicity, stiffness, displacement |
| References | Selection | Comparability (Matched Analysis) | Assessment of Outcome | Outcomes | Adequacy of Follow-Up of Cohorts | NOS Score | |||
|---|---|---|---|---|---|---|---|---|---|
| Consecutive or Obviously Representative Series of Cases | Representativeness of Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest Was Not Present at the Start of Study | Follow up Long Enough for the Outcome | |||||
| Yerli [17] | * | * | * | - | ** | * | - | - | 6 |
| Kellis [18] | - | * | * | - | - | * | - | - | 3 |
| Gumruk [19] | * | * | * | * | ** | * | - | - | 6 |
| Vining [21] | * | * | * | * | - | * | - | - | 5 |
| Pirri [22] | * | * | * | * | ** | * | - | - | 7 |
| Devantery [23] | - | - | * | * | - | * | - | - | 3 |
| Perez [24] | * | * | * | - | * | * | - | - | 5 |
| Larivière [26] | - | * | - | - | - | * | - | - | 2 |
| Tamartash [27] | * | * | * | * | * | * | - | - | 6 |
| Bartsch [28] | - | - | - | - | - | * | - | - | 1 |
| Turan [31] | - | - | * | - | - | * | - | - | 2 |
| Ushida [32] | - | - | * | - | - | * | - | - | 2 |
| Larivière [34] | - | * | - | - | * | * | - | - | 3 |
| Larivière [36] | - | * | - | - | * | * | - | - | 3 |
| Chen [37] | - | - | * | - | * | * | - | - | 3 |
| Larivière [40] | - | * | * | - | * | * | - | - | 4 |
| Chen [41] | - | - | - | - | * | * | - | - | 2 |
| Blain [43] | - | - | - | - | * | * | - | - | 2 |
| Vita [44] | - | - | * | - | * | * | - | - | 3 |
| De Coninck [45] | - | * | * | - | * | * | - | - | 4 |
| Langevin [48] | - | - | * | - | * | * | - | - | 3 |
| Wong [49] | - | - | - | - | * | * | - | - | 2 |
| Tu [51] | - | - | - | - | * | * | - | - | 2 |
| Bishop [52] | - | - | * | - | - | * | - | - | 2 |
| Langevin [54] | - | * | * | - | * | * | - | - | 4 |
| References | Selection | Comparability of Cohorts | Ascertainment of Exposure | Outcomes | Non-Response Rate | NOS Score | |||
|---|---|---|---|---|---|---|---|---|---|
| Adequate Case Definition | Representativeness of Cases | Selection of Controls | Definition of Controls | Same Method of Ascertainment | |||||
| Brandl [20] | * | * | * | * | * | * | * | - | 7 |
| Brandl [29] | * | - | * | - | - | * | - | - | 3 |
| Venkatesan [33] | * | - | * | * | * | * | - | - | 5 |
| References | Were Patient’s Demographic Characteristics Clearly Described? | Was the Patient’s History Clearly Described and Presented as a Timeline? | Was the Current Clinical Condition of the Patient on Presentation Clearly Described? | Were Diagnostic Tests or Assessment Methods and the Results Clearly Described? | Was the Intervention(s) or Treatment Procedure(s) Clearly Described? | Was the Post-Intervention Clinical Condition Clearly Described? | Were Adverse Events (Harms) or Unanticipated Events Identified and Described? | Does the Case Report Provide Takeaway Lessons? |
|---|---|---|---|---|---|---|---|---|
| Pirri [35] | Y | Y | Y | Y | Y | Y | - | Y |
| Fullerton [46] | Y | N | Y | Y | - | - | - | - |
| Panagos [47] | Y | N | Y | Y | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirri, C.; Pirri, N.; Macchi, V.; Porzionato, A.; De Caro, R.; Stecco, C. Ultrasound Imaging of Thoracolumbar Fascia: A Systematic Review. Medicina 2024, 60, 1090. https://doi.org/10.3390/medicina60071090
Pirri C, Pirri N, Macchi V, Porzionato A, De Caro R, Stecco C. Ultrasound Imaging of Thoracolumbar Fascia: A Systematic Review. Medicina. 2024; 60(7):1090. https://doi.org/10.3390/medicina60071090
Chicago/Turabian StylePirri, Carmelo, Nina Pirri, Veronica Macchi, Andrea Porzionato, Raffaele De Caro, and Carla Stecco. 2024. "Ultrasound Imaging of Thoracolumbar Fascia: A Systematic Review" Medicina 60, no. 7: 1090. https://doi.org/10.3390/medicina60071090
APA StylePirri, C., Pirri, N., Macchi, V., Porzionato, A., De Caro, R., & Stecco, C. (2024). Ultrasound Imaging of Thoracolumbar Fascia: A Systematic Review. Medicina, 60(7), 1090. https://doi.org/10.3390/medicina60071090











