Intradevice Repeatability and Interdevice Comparison of Two Specular Microscopy Devices in a Real-Life Setting: Tomey EM-4000 and Nidek CEM-530
Abstract
1. Introduction
2. Materials and Methods
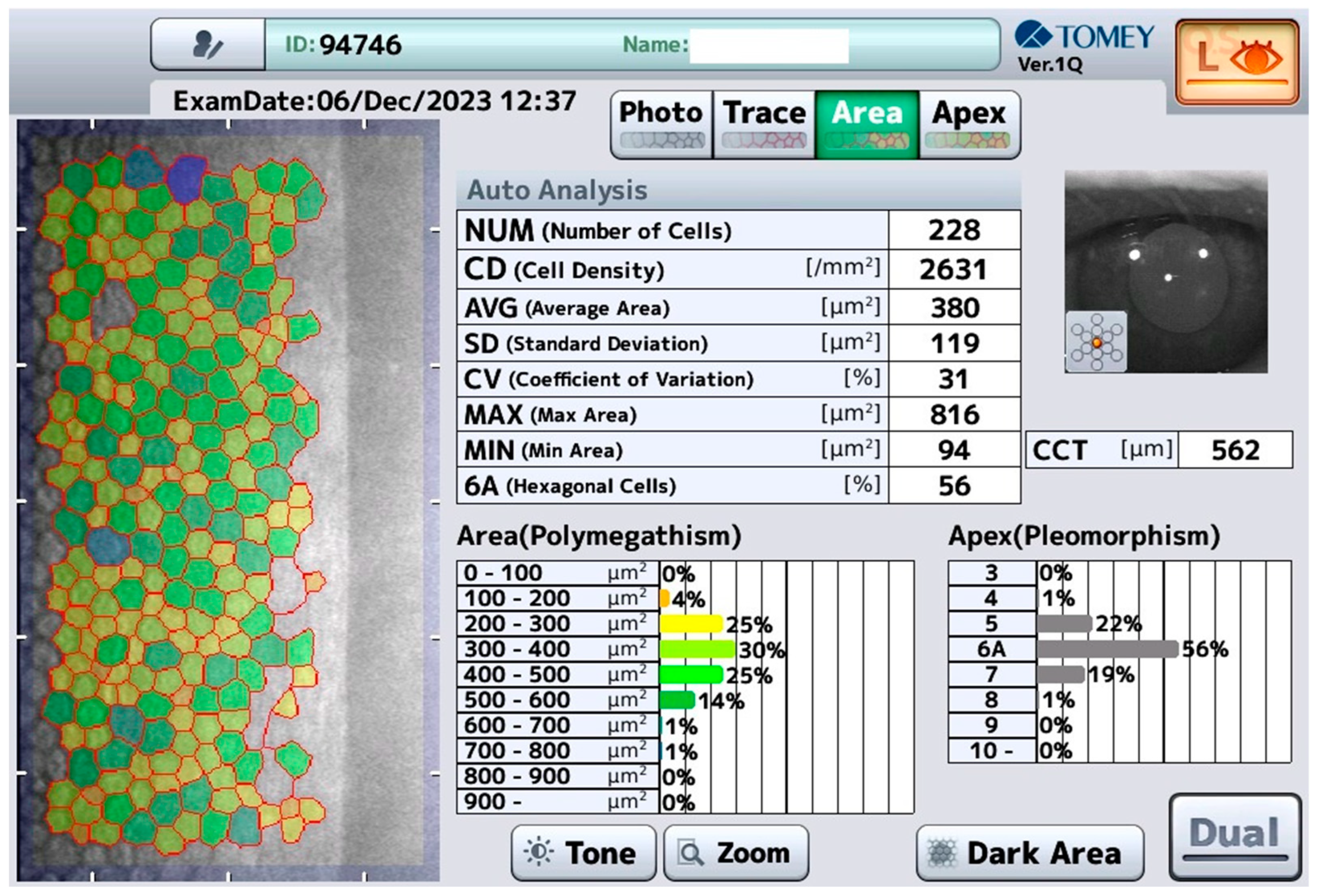
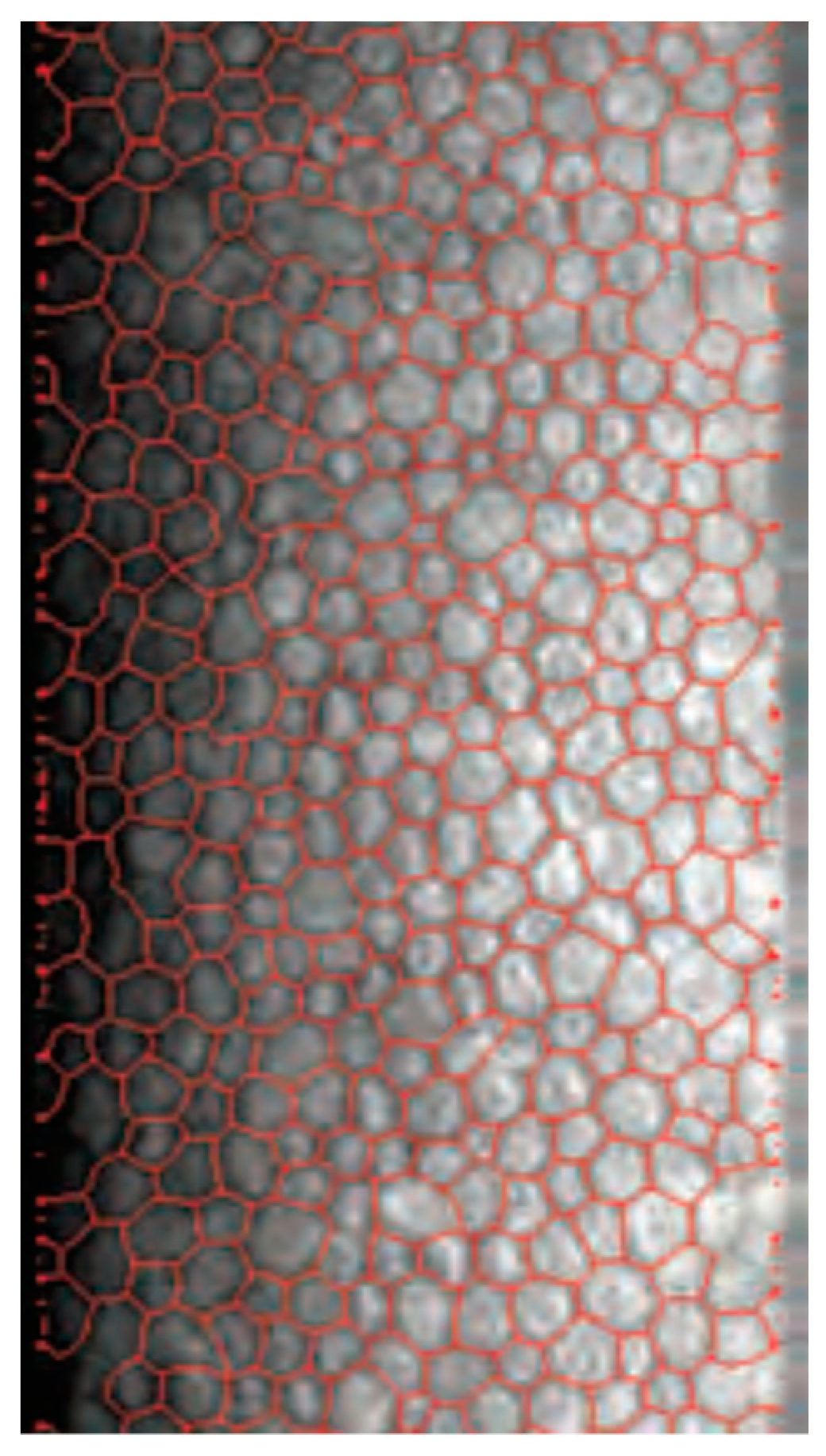
2.1. Tomey EM-4000 (Tomey, Nürnberg, Germany)
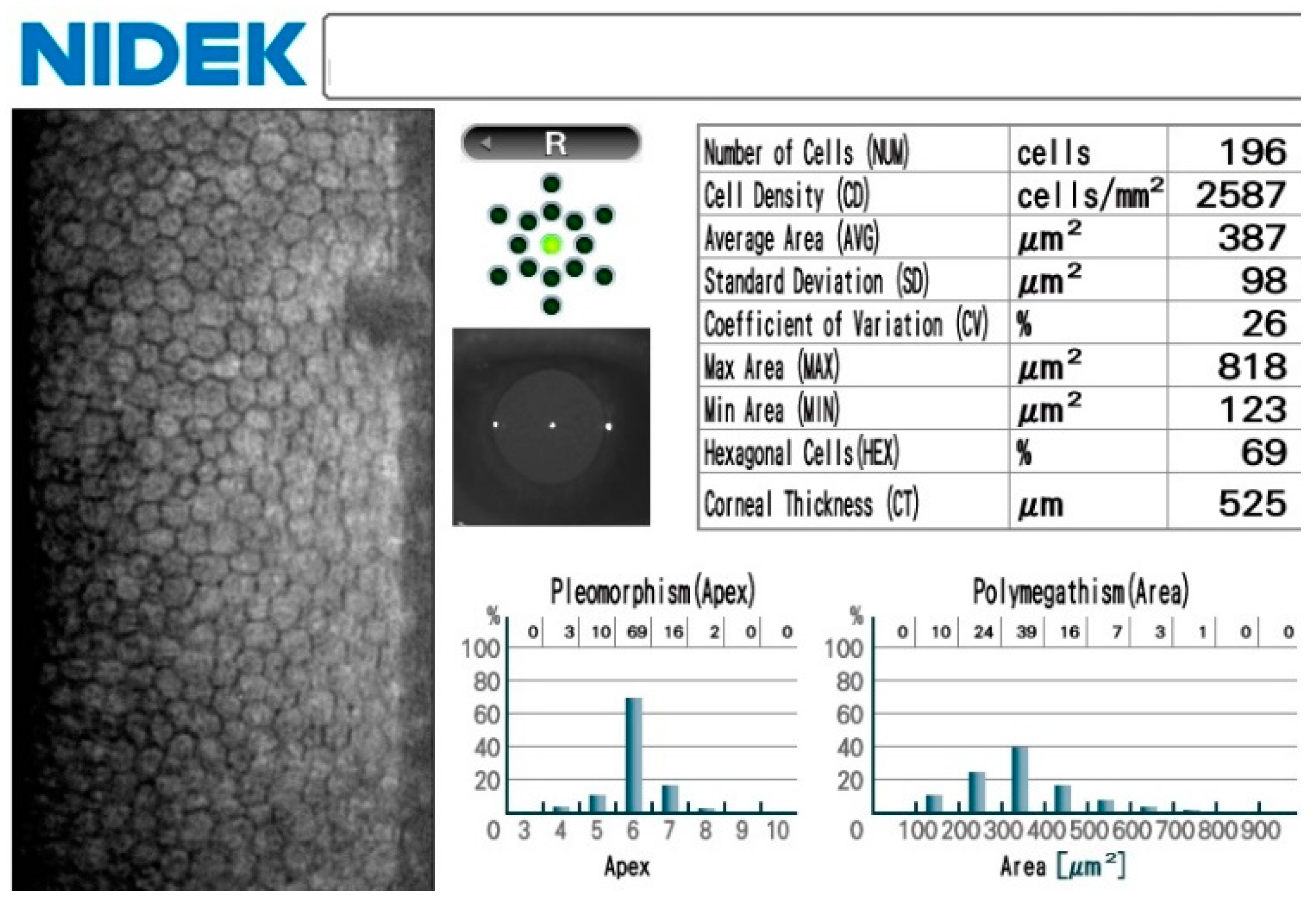
2.2. Nidek CEM-530 (Nidek, Tokyo, Japan)
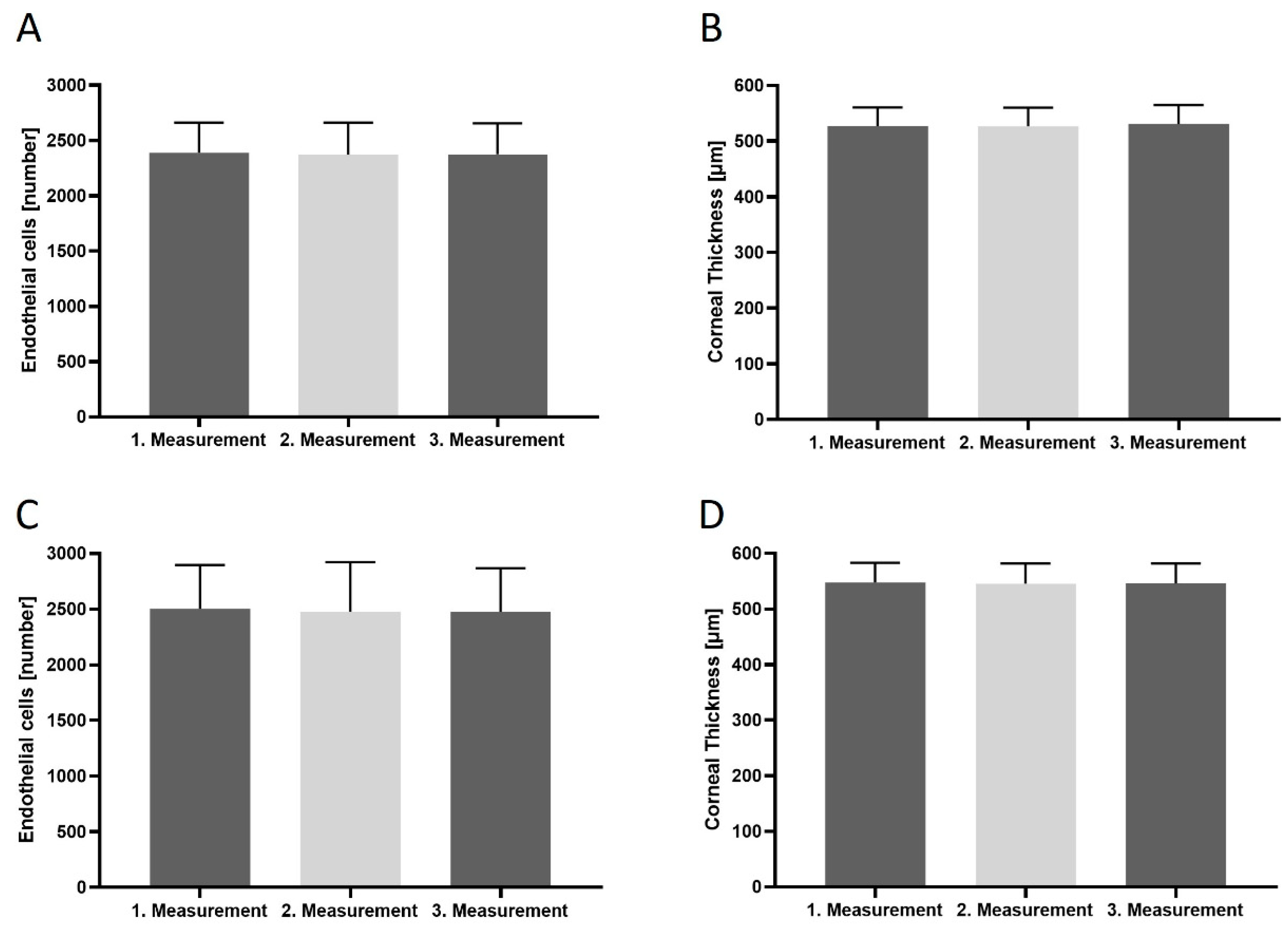
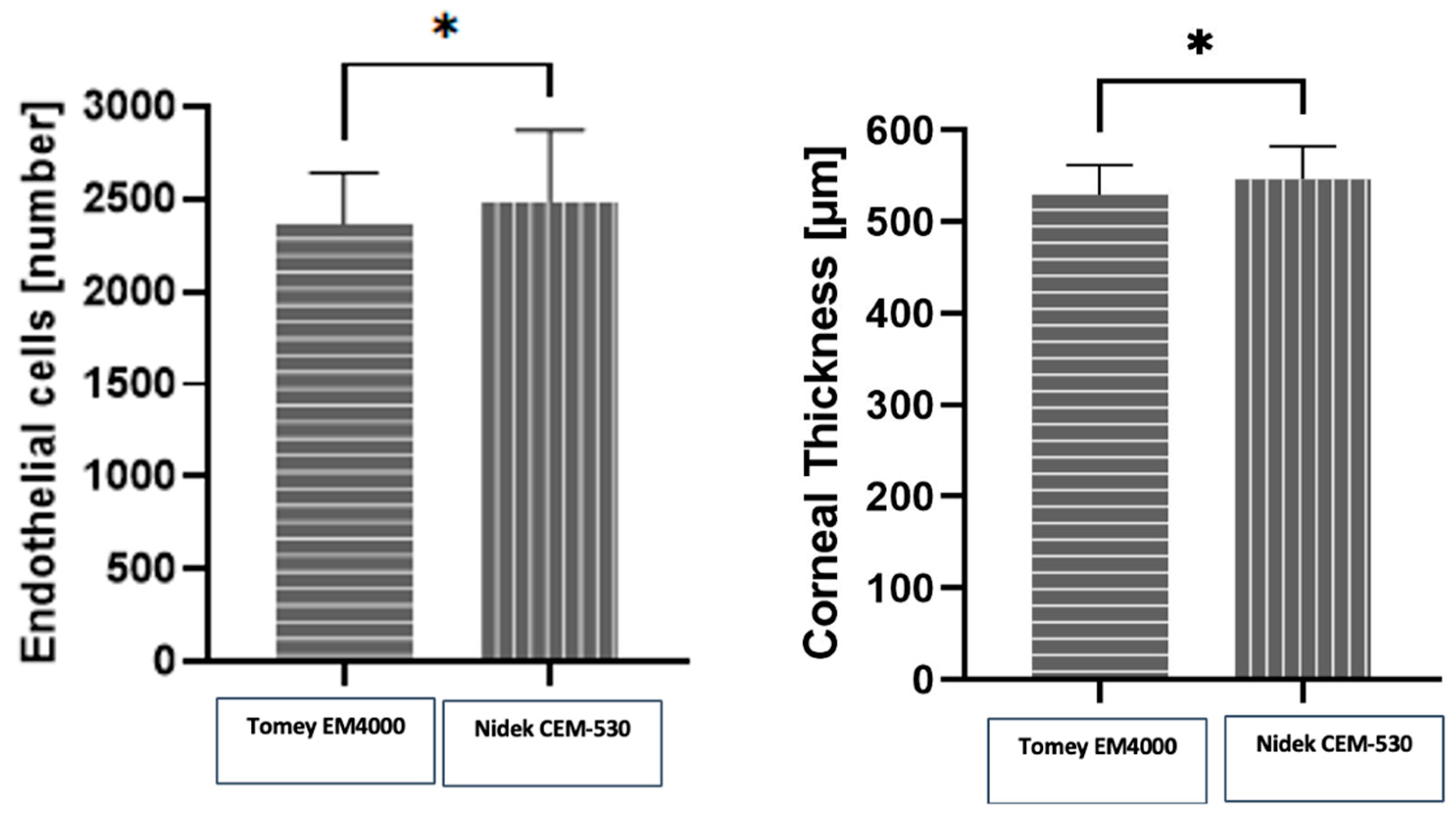
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar] [PubMed]
- Edelhauser, H.F. The balance between corneal transparency and edema: The Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Edelhauser, H.F. The resiliency of the corneal endothelium to refractive and intraocular surgery. Cornea 2000, 19, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Yee, R.W.; Matsuda, M.; Schultz, R.O.; Edelhauser, H.F. Changes in the normal corneal endothelial cellular pattern as a function of age. Curr. Eye Res. 1985, 4, 671–678. [Google Scholar] [CrossRef]
- Matsuda, M.; Yee, R.W.; Edelhauser, H.F. Comparison of the corneal endothelium in an American and a Japanese population. Arch. Ophthalmol. 1985, 103, 68–70. [Google Scholar] [CrossRef] [PubMed]
- McGlumphy, E.J.; Margo, J.A.; Haidara, M.; Brown, C.H.; Hoover, C.K.; Munir, W.M. Predictive Value of Corneal Donor Demographics on Endothelial Cell Density. Cornea 2018, 37, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S. Corneal endothelial cell dysfunction: Etiologies and management. Ther. Adv. Ophthalmol. 2018, 10, 2515841418815802. [Google Scholar] [CrossRef] [PubMed]
- McGowan, S.L.; Edelhauser, H.F.; Pfister, R.R.; Whikehart, D.R. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol. Vis. 2007, 13, 1984–2000. [Google Scholar]
- Ramirez-Garcia, M.A.; Khalifa, Y.M.; Buckley, M.R. Vulnerability of corneal endothelial cells to mechanical trauma from indentation forces assessed using contact mechanics and fluorescence microscopy. Exp. Eye Res. 2018, 175, 73–82. [Google Scholar] [CrossRef]
- McCarey, B.E.; Edelhauser, H.F.; Lynn, M.J. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea 2008, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nanda, G.G.; Alone, D.P. REVIEW: Current understanding of the pathogenesis of Fuchs’ endothelial corneal dystrophy. Mol. Vis. 2019, 25, 295–310. [Google Scholar] [PubMed]
- Chiou, A.G.; Kaufman, S.C.; Kaufman, H.E.; Beuerman, R.W. Clinical corneal confocal microscopy. Surv. Ophthalmol. 2006, 51, 482–500. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.M. Cellular membrane activity in the corneal endothelium of the intact eye. Experientia 1968, 24, 1094–1095. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.A.; Sandstrom, M.M.; Leibowitz, H.M. Clinical specular microscopy. I. Optical principles. Arch. Ophthalmol. 1979, 97, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Hirst, L.W.; Yamauchi, K.; Enger, C.; Vogelpohl, W.; Whittington, V. Quantitative analysis of wide-field specular microscopy. II. Precision of sampling from the central corneal endothelium. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1972–1979. [Google Scholar]
- Janson, B.J.; Alward, W.L.; Kwon, Y.H.; Bettis, D.I.; Fingert, J.H.; Provencher, L.M.; Goins, K.M.; Wagoner, M.D.; Greiner, M.A. Glaucoma-associated corneal endothelial cell damage: A review. Surv. Ophthalmol. 2018, 63, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Lass, J.H.; Benetz, B.A.; He, J.; Hamilton, C.; Von Tress, M.; Dickerson, J.; Lane, S. Corneal Endothelial Cell Loss and Morphometric Changes 5 Years after Phacoemulsification with or without CyPass Micro-Stent. Am. J. Ophthalmol. 2019, 208, 211–218. [Google Scholar] [CrossRef]
- Chaurasia, S.; Vanathi, M. Specular microscopy in clinical practice. Indian J. Ophthalmol. 2021, 69, 517–524. [Google Scholar] [CrossRef]
- Igo, R.P., Jr.; Kopplin, L.J.; Joseph, P.; Truitt, B.; Fondran, J.; Bardenstein, D.; Aldave, A.J.; Croasdale, C.R.; Price, M.O.; Rosenwasser, M.; et al. Differing roles for TCF4 and COL8A2 in central corneal thickness and fuchs endothelial corneal dystrophy. PLoS ONE 2012, 7, e46742. [Google Scholar] [CrossRef]
- Bolac, R.; Yildiz, E.; Balci, S. Anterior Corneal High-order Aberrations in Fuchs’ Endothelial Corneal Dystrophy Classified by Scheimpflug Tomography. Optom. Vis. Sci. 2023, 100, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Dudley, C.E.; Morell, A.J.; Duffey, M.E.; Patel, S.P. Effects of amantadine on corneal endothelium. Exp. Eye Res. 2019, 181, 208–212. [Google Scholar] [CrossRef]
- Mancera, N.; Wadia, H.P. Corneal Edema Associated with Systemic Dopaminergic Agents. Cornea 2019, 38, 1040–1042. [Google Scholar] [CrossRef]
- Madreperla, S.A.; Johnson, M.; O’Brien, T.P. Corneal endothelial dysfunction in digoxin toxicity. Am. J. Ophthalmol. 1992, 113, 211–212. [Google Scholar] [CrossRef]
- Höllhumer, R.; Moloney, G.; Jacob, K. Corneal edema with a systemic epidermal growth factor receptor inhibitor. Can. J. Ophthalmol. 2017, 52, e96–e97. [Google Scholar] [CrossRef] [PubMed]
- Powe, N.R.; Schein, O.D.; Gieser, S.C.; Tielsch, J.M.; Luthra, R.; Javitt, J.; Steinberg, E.P. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Arch. Ophthalmol. 1994, 112, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, B.; Kaur, K. Pseudophakic Bullous Keratopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Shi, Y.; Huang, J.; Conrick, P.; Hoover, C.; Lee, O.L. Discrepancies in Endothelial Cell Density Values of Human Donor Corneas Resulting from Comparison Between Specular Microscopes and Endothelial Analysis Methods. Cornea 2020, 39, 495–500. [Google Scholar] [CrossRef]
- van Rijn, G.A.; Wijnen, C.J.F.; van Dooren, B.T.; Cheng, Y.Y.; Beenakker, J.M.; Luyten, G.P. Improved Interchangeability with Different Corneal Specular Microscopes for Quantitative Endothelial Cell Analysis. Clin. Ophthalmol. 2020, 14, 61–70. [Google Scholar] [CrossRef]
- Karaca, I.; Yilmaz, S.G.; Palamar, M.; Ates, H. Comparison of central corneal thickness and endothelial cell measurements by Scheimpflug camera system and two noncontact specular microscopes. Int. Ophthalmol. 2018, 38, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Luft, N.; Hirnschall, N.; Schuschitz, S.; Draschl, P.; Findl, O. Comparison of 4 specular microscopes in healthy eyes and eyes with cornea guttata or corneal grafts. Cornea 2015, 34, 381–386. [Google Scholar] [CrossRef]
- Módis, L., Jr.; Szalai, E.; Németh, G.; Berta, A. Evaluation of a recently developed noncontact specular microscope in comparison with conventional pachymetry devices. Eur. J. Ophthalmol. 2010, 20, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Goldich, Y.; Marcovich, A.L.; Barkana, Y.; Hartstein, M.; Morad, Y.; Avni, I.; Zadok, D. Comparison of corneal endothelial cell density estimated with 2 noncontact specular microscopes. Eur. J. Ophthalmol. 2010, 20, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Módis, L., Jr.; Langenbucher, A.; Seitz, B. Corneal endothelial cell density and pachymetry measured by contact and noncontact specular microscopy. J. Cataract Refract. Surg. 2002, 28, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
| Demographic and Clinical Data | Mean ± SD (%) |
|---|---|
| Age (years; mean ± SD) | 61.05 ± 18.53 |
| Range | 22–85 |
| Gender | |
| Female | 24 (42.9%) |
| Male | 32 (57.1%) |
| Number of eyes included | 112 |
| Number of measurements | 668 |
| Excluded for poor quality | 28 |
| Tomey EM-4000 | 25 |
| Nidek CEM-530 | 3 |
| Measurements included in analysis | 640 |
| Results | |
| Tomey EM-4000 | |
| CD (cells/mm2) | 2390 ± 49.57 |
| Range | 799–3010 |
| CCT (μm) | 546 ± 5.104 |
| Range | 425–615 |
| Nidek CEM-530 | |
| CD (cells/mm2) | 2417 ± 80.09 |
| Range | 505–3461 |
| CCT (μm) | 546.3 ± 4.94 |
| Range | 431–621 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kecik, M.; Kropp, M.; Thumann, G.; Pajic, B.; Guber, J.; Guber, I. Intradevice Repeatability and Interdevice Comparison of Two Specular Microscopy Devices in a Real-Life Setting: Tomey EM-4000 and Nidek CEM-530. Medicina 2024, 60, 1110. https://doi.org/10.3390/medicina60071110
Kecik M, Kropp M, Thumann G, Pajic B, Guber J, Guber I. Intradevice Repeatability and Interdevice Comparison of Two Specular Microscopy Devices in a Real-Life Setting: Tomey EM-4000 and Nidek CEM-530. Medicina. 2024; 60(7):1110. https://doi.org/10.3390/medicina60071110
Chicago/Turabian StyleKecik, Mateusz, Martina Kropp, Gabriele Thumann, Bojan Pajic, Josef Guber, and Ivo Guber. 2024. "Intradevice Repeatability and Interdevice Comparison of Two Specular Microscopy Devices in a Real-Life Setting: Tomey EM-4000 and Nidek CEM-530" Medicina 60, no. 7: 1110. https://doi.org/10.3390/medicina60071110
APA StyleKecik, M., Kropp, M., Thumann, G., Pajic, B., Guber, J., & Guber, I. (2024). Intradevice Repeatability and Interdevice Comparison of Two Specular Microscopy Devices in a Real-Life Setting: Tomey EM-4000 and Nidek CEM-530. Medicina, 60(7), 1110. https://doi.org/10.3390/medicina60071110










