Smoking-Dependent Association of Serum Brain-Derived Neurotrophic Factor with Pulmonary Function Parameters in Chronic Obstructive Pulmonary Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Clinical Data Collection
2.3. Spirometric Analysis
2.4. Quantification of Serum BDNF Concentration
2.5. Statistical Methods
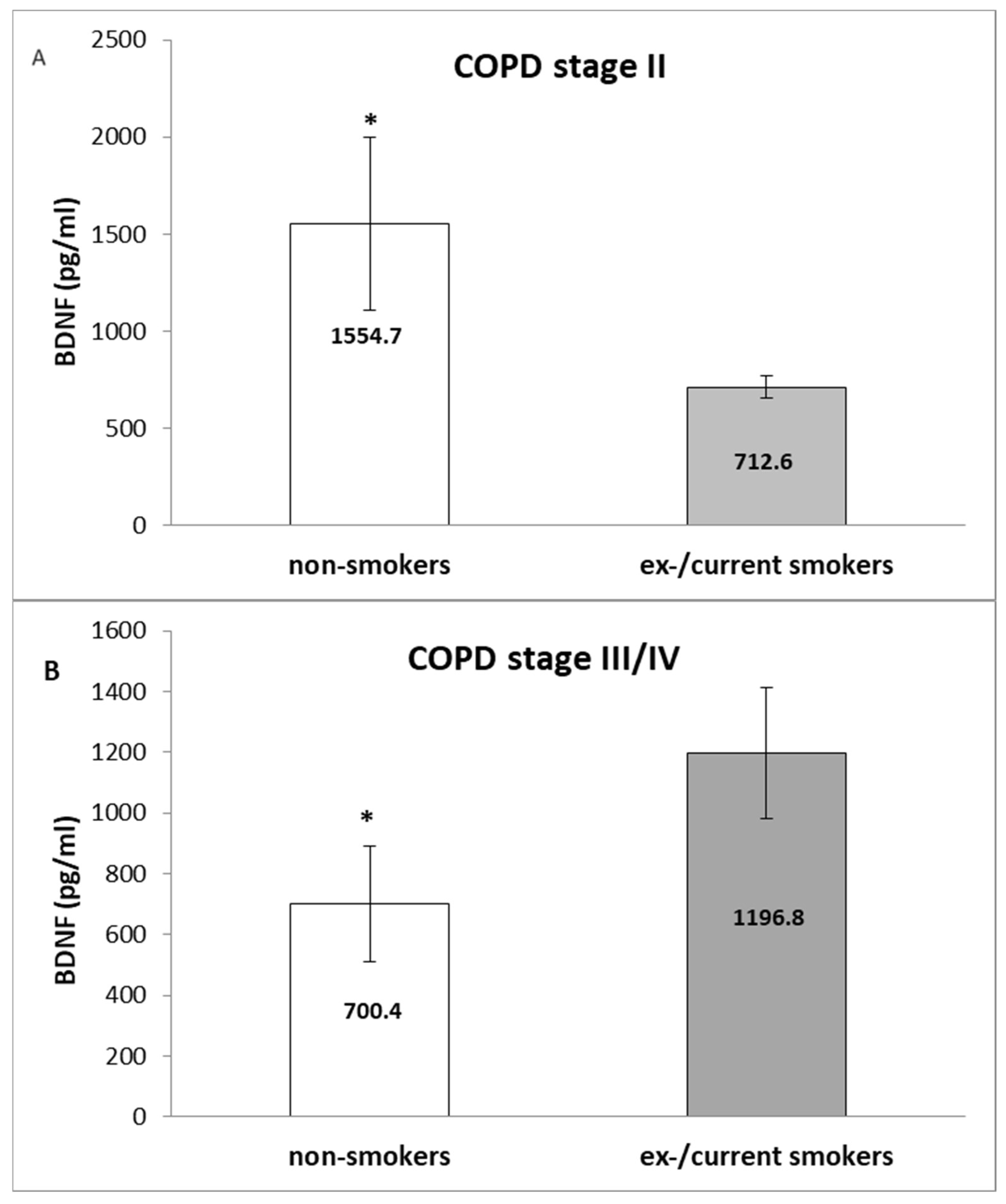
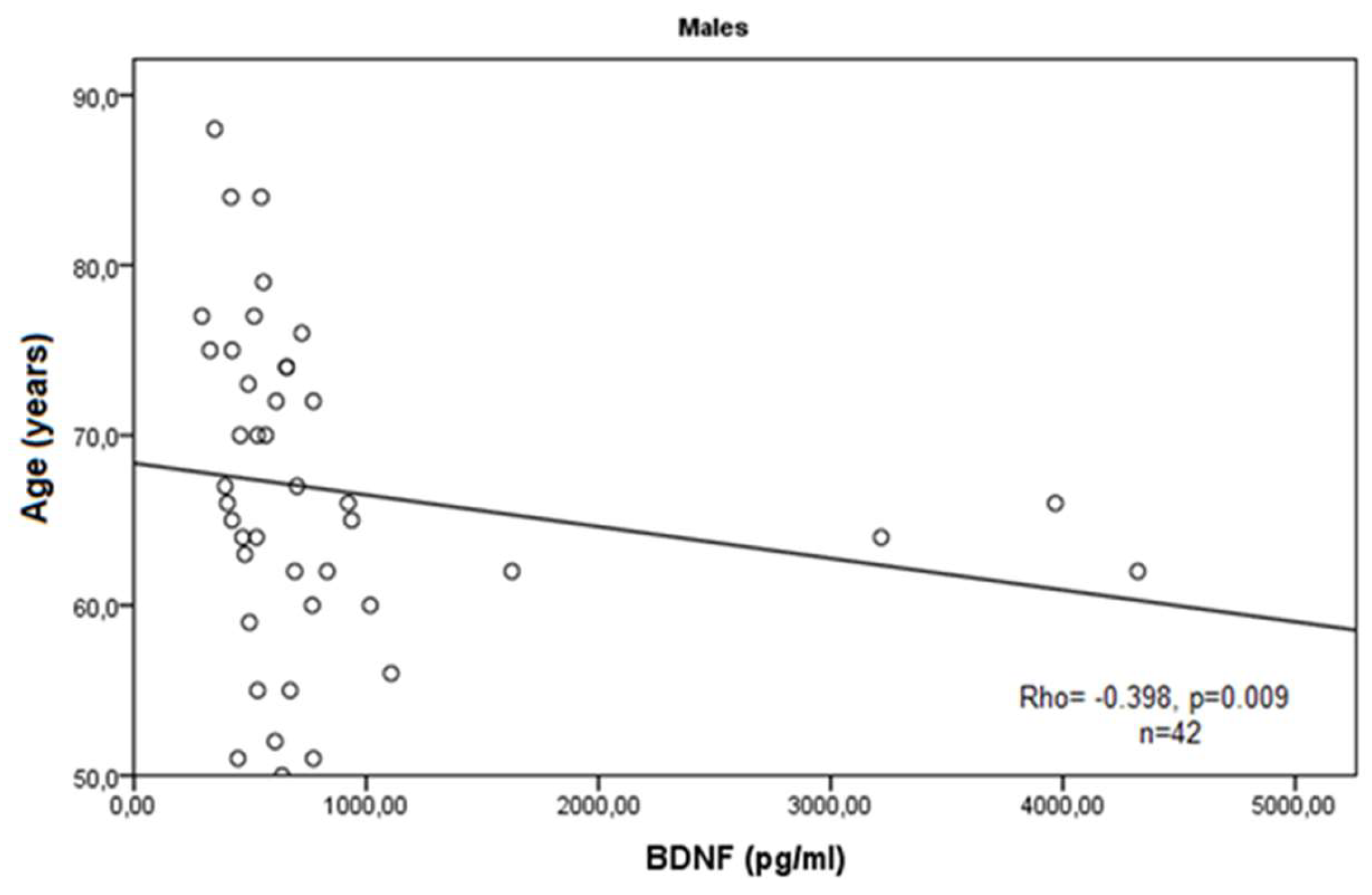
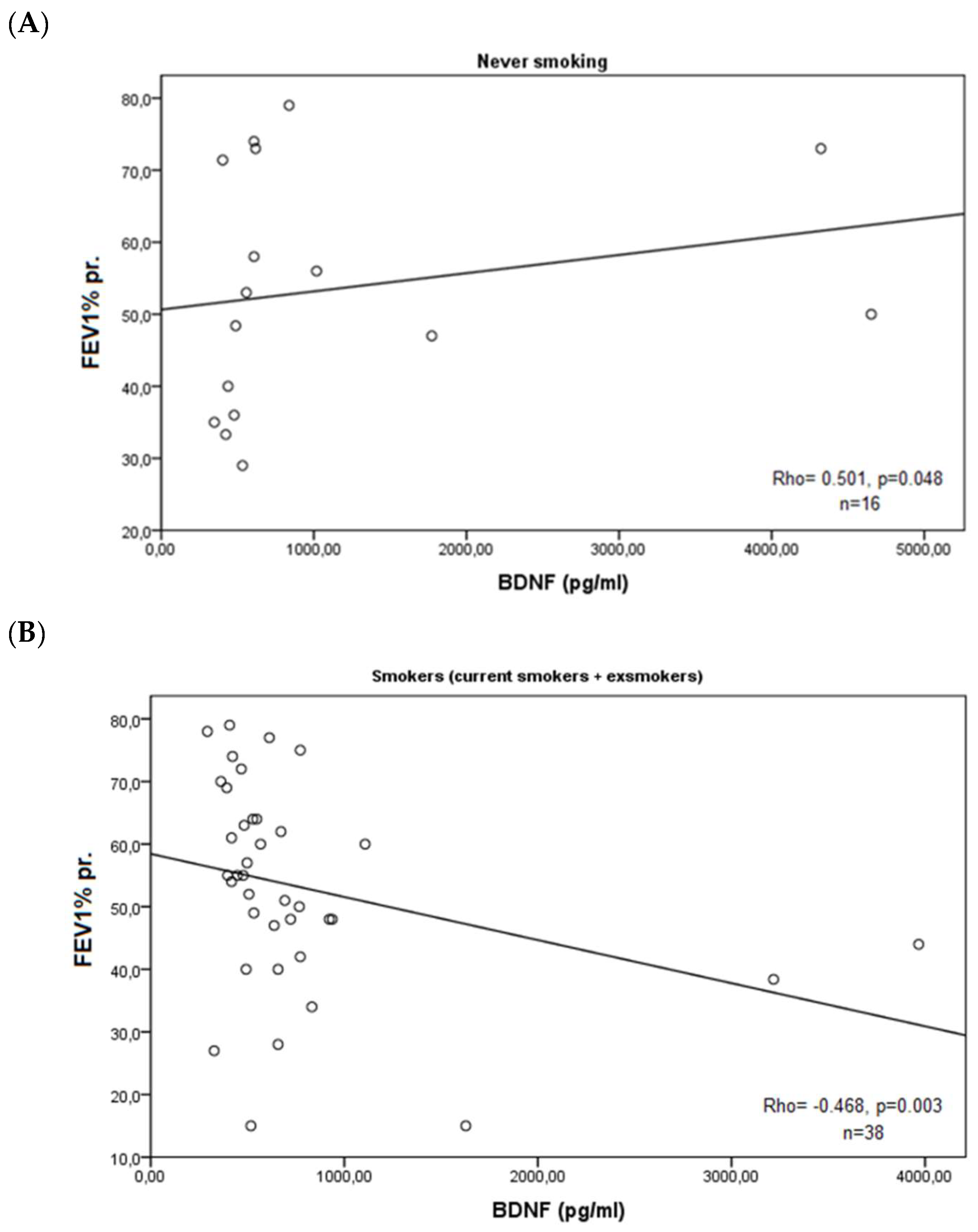
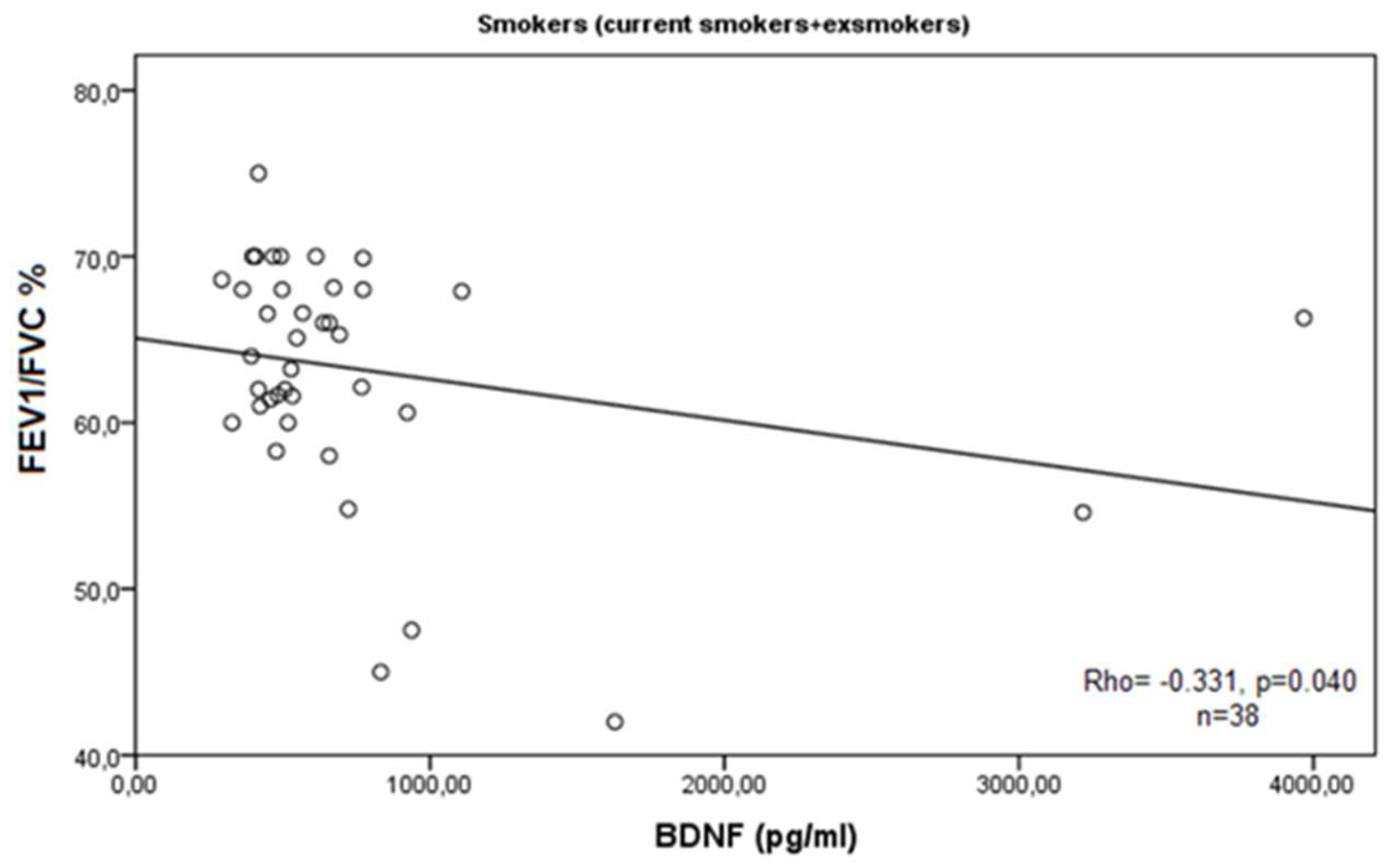
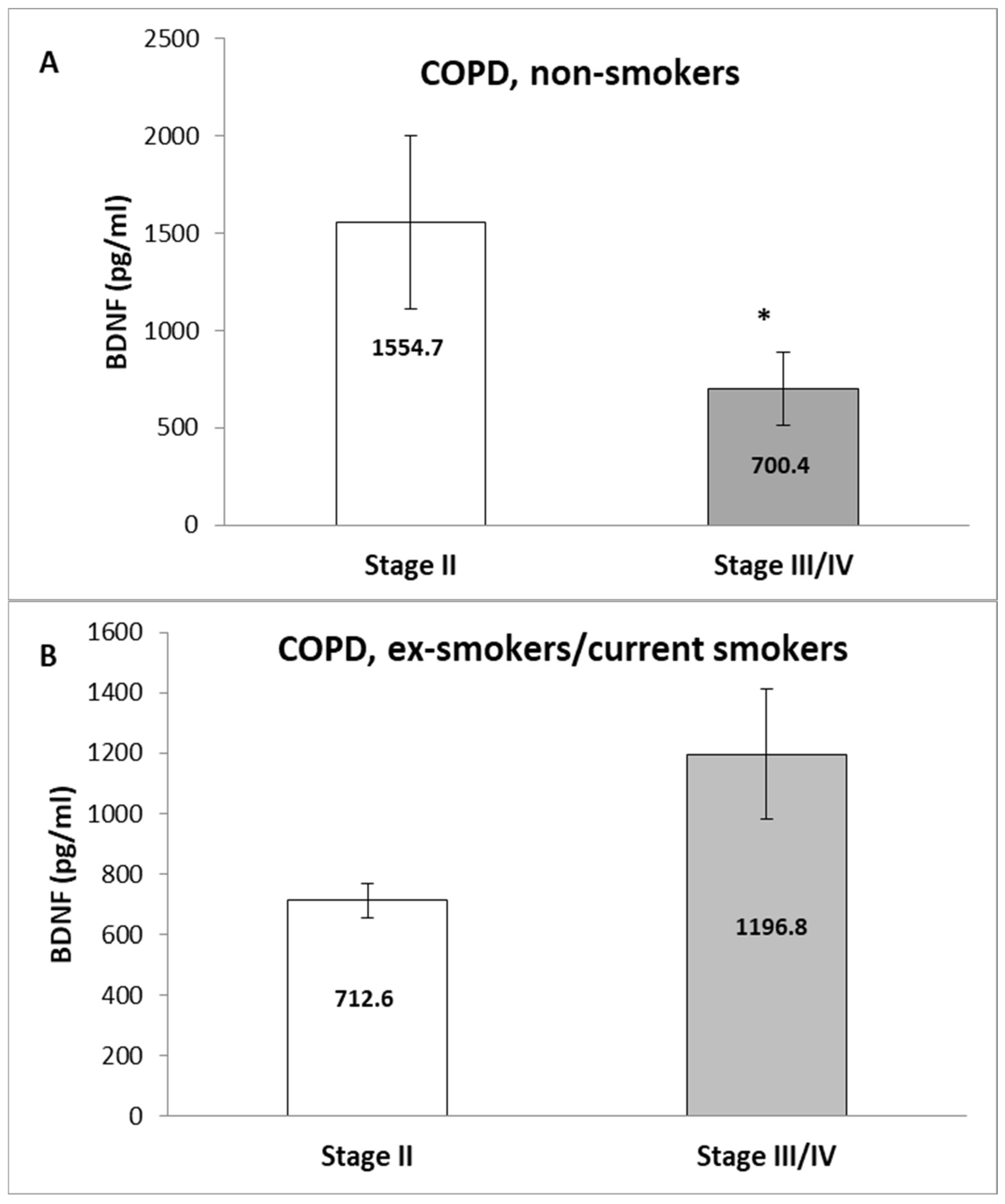
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bibel, M.; Hoppe, E.; Barde, Y.A. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J. 1999, 18, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Prakash, Y.; Thompson, M.A.; Meuchel, L.; Pabelick, C.M.; Mantilla, C.B.; Zaidi, S.; Martin, R.J. Neurotrophins in lung health and disease. Expert Rev. Respir. Med. 2010, 4, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Comprido, D.; Duarte, C.B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 2014, 76, 639–656. [Google Scholar] [CrossRef] [PubMed]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Mowla, S.J.; Farhadi, H.F.; Pareek, S.; Atwal, J.K.; Morris, S.J.; Seidah, N.G.; Murphy, R.A. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem. 2001, 276, 12660–12666. [Google Scholar] [CrossRef] [PubMed]
- Lessmann, V.; Brigadski, T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: An update. Neurosci. Res. 2009, 65, 11–22. [Google Scholar] [CrossRef]
- Teng, H.K.; Teng, K.K.; Lee, R.; Wright, S.; Tevar, S.; Almeida, R.D.; Kermani, P.; Torkin, R.; Chen, Z.Y.; Lee, F.S.; et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 2005, 25, 5455–5463. [Google Scholar] [CrossRef] [PubMed]
- Borodinova, A.A.; Salozhin, S.V. Differences in the Biological Functions of BDNF and proBDNF in the Central Nervous System. Neurosci. Behav. Physiol. 2017, 47, 251–265. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Chauhan, L.; Bhardwaj, A.; Sharma, A.; Fayaz, F.; Kumar, B.; Alhashmi, M.; AlHajri, N.; Alam, M.S.; Pottoo, F.H. Brain-Derived Neurotropic Factor in Neurodegenerative Disorders. Biomedicines 2022, 10, 1143. [Google Scholar] [CrossRef]
- Ziemssen, T.; Kümpfel, T.; Schneider, H.; Klinkert, W.E.; Neuhaus, O.; Hohlfeld, R. Secretion of brain-derived neu-rotrophic factor by glatiramer acetate-reactive T-helper cell lines: Implications for multiple sclerosis therapy. J. Neurol. Sci. 2005, 233, 109–112. [Google Scholar] [CrossRef]
- Nakahashi, T.; Fujimura, H.; Altar, C.A.; Li, J.; Kambayashi, J.; Tandon, N.N.; Sun, B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000, 470, 113–117. [Google Scholar] [CrossRef]
- Le Blanc, J.; Fleury, S.; Boukhatem, I.; Bélanger, J.C.; Welman, M.; Lordkipanidzé, M. Platelets Selectively Regulate the Release of BDNF, But Not That of Its Precursor Protein, proBDNF. Front. Immunol. 2020, 11, 575607. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, H.; Altar, C.A.; Chen, R.; Nakamura, T.; Nakahashi, T.; Kambayashi, J.; Sun, B.; Tandon, N.N. Brain-derived neu-rotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 2002, 87, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Lommatzsch, M.; Mannsfeldt, A.; Neuhaus-Steinmetz, U.; Fischer, A.; Schnoy, N.; Lewin, G.R.; Renz, H. Cellular sources of enhanced brain-derived neurotrophic factor production in a mouse model of allergic inflammation. Am. J. Respir. Cell Mol. Biol. 1999, 21, 537–546. [Google Scholar] [CrossRef]
- Watanabe, T.; Fajt, M.L.; Trudeau, J.B.; Voraphani, N.; Hu, H.; Zhou, X.; Holguin, F.; Wenzel, S.E. Brain-Derived Neurotrophic Factor Expression in Asthma. Association with Severity and Type 2 Inflammatory Processes. Am. J. Respir. Cell Mol. Biol. 2015, 53, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Stoll, P.; Wuertemberger, U.; Bratke, K.; Zingler, C.; Virchow, J.C.; Lommatzsch, M. Stage-dependent association of BDNF and TGF-β1 with lung function in stable COPD. Respir. Res. 2012, 13, 116. [Google Scholar] [CrossRef]
- Szczepankiewicz, A.; Bręborowicz, A.; Sobkowiak, P.; Popiel, A. Association of BDNF gene polymorphism with asthma in polish children. World Allergy Organ. J. 2010, 3, 235–238. [Google Scholar] [CrossRef]
- Zeilinger, S.; Pinto, L.A.; Nockher, W.A.; Depner, M.; Klopp, N.; Illig, T.; von Mutius, E.; Renz, H.; Kabesch, M. The effect of BDNF gene variants on asthma in German children. Allergy 2009, 64, 1790–1794. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, L.; Li, S.; Xu, B.; Xu, Y.; Li, H. Effect of BDNF on airway inflammation in a rat model of COPD. Exp. Ther. Med. 2021, 22, 1116. [Google Scholar] [CrossRef]
- de Araujo, C.L.P.; da Silva, I.R.V.; Reinaldo, G.P.; Peccin, P.K.; Pochmann, D.; Teixeira, P.J.Z.; Elsner, V.R.; Dal Lago, P. Pulmonary rehabilitation and BDNF levels in patients with chronic obstructive pulmonary disease: A pilot study. Respir. Physiol. Neurobiol. 2019, 259, 63–69. [Google Scholar] [CrossRef]
- Tacheva, T.; Zienolddiny, S.; Dimov, D.; Vlaykova, D.; Vlaykova, T. The leukocyte telomere length, single nucleotide polymorphisms near TERC gene and risk of COPD. PeerJ 2021, 9, e12190. [Google Scholar] [CrossRef] [PubMed]
- Zhelyazkova, Y.A.; Tacheva, T.T.; Dimov, D.M.; Vlaykova, D.G.; Bivolarska, A.V.; Vlaykova, T.I. Possible Role of Serum Leptin as Biomarker in COPD. Folia Med. 2019, 61, 512–521. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, 2017 Report. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14. [Google Scholar] [CrossRef]
- Mannino, D.M.; Buist, A.S. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet 2007, 370, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Plata, V.; Toso, J.; Lee, K.; Park, D.; Bilello, J.; Mullerova, H.; De Souza, M.M.; Vessey, R.; Celli, B. Profiling serum bi-omarkers in patients with COPD: Associations with clinical parameters. Thorax 2007, 62, 595–601. [Google Scholar] [CrossRef]
- Sathish, V.; Vanoosten, S.K.; Miller, B.S.; Aravamudan, B.; Thompson, M.A.; Pabelick, C.M.; Vassallo, R.; Prakash, Y.S. Brain-derived neurotrophic factor in cigarette smoke-induced airway hyperreactivity. Am. J. Respir. Cell Mol. Biol. 2013, 48, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Sköld, C.M. Remodeling in asthma and COPD-differences and similarities. Clin. Respir. J. 2010, 4 (Suppl. S1), 20–27. [Google Scholar] [CrossRef]
- Porter, G.A.; O’Connor, J.C. Brain-derived neurotrophic factor and inflammation in depression: Pathogenic partners in crime? World J. Psychiatry 2022, 12, 77–97. [Google Scholar] [CrossRef]
- Bath, K.G.; Schilit, A.; Lee, F.S. Stress effects on BDNF expression: Effects of age, sex, and form of stress. Neuroscience 2013, 239, 149–156. [Google Scholar] [CrossRef]
- Hung, J.; Lam, J.Y.; Lacoste, L.; Letchacovski, G. Cigarette Smoking Acutely Increases Platelet Thrombus Formation in Patients with Coronary Artery Disease Taking Aspirin. Circulation 1995, 92, 2432–24361. [Google Scholar] [CrossRef]
- Neves, C.D.C.; Lacerda, A.C.R.; Lima, L.P.; Lage, V.K.S.; Balthazar, C.H.; Leite, H.R.; Mendonça, V.A. Different levels of brain-derived neurotrophic factor and cortisol in healthy heavy smokers. Braz. J. Med. Biol. Res. 2017, 50, e6424. [Google Scholar] [CrossRef]
- Jamal, M.; Van der Does, W.; Elzinga, B.M.; Molendijk, M.L.; Penninx, B.W. Association between smoking, nicotine dependence, and BDNF Val66Met polymorphism with BDNF concentrations in serum. Nicotine Tob. Res. 2015, 17, 323–329. [Google Scholar] [CrossRef]
- Singh, S.; Verma, S.K.; Kumar, S.; Ahmad, M.K.; Nischal, A.; Singh, S.K.; Dixit, R.K. Evaluation of Oxidative Stress and Antioxidant Status in Chronic Obstructive Pulmonary Disease. Scand. J. Immunol. 2017, 85, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Suzuki, S.; Kumamaru, E.; Adachi, N.; Richards, M.; Kunugi, H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010, 25, 237–258. [Google Scholar] [PubMed]
- Piedimonte, G. Contribution of neuroimmune mechanisms to airway inflammation and remodeling during and after respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 2003, 22 (Suppl. S2), S66–S75. [Google Scholar] [CrossRef]
- Papp, C.; Pak, K.; Erdei, T.; Juhasz, B.; Seres, I.; Szentpéteri, A.; Kardos, L.; Szilasi, M.; Gesztelyi, R.; Zsuga, J. Alteration of the irisin-brain-derived neurotrophic factor axis contributes to disturbance of mood in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.S.; Spira, A. State of the art. Chronic obstructive pulmonary disease, inflammation, and lung cancer. Proc. Am. Thorac. Soc. 2006, 3, 535–537. [Google Scholar] [CrossRef]
- Cha, S.-R.; Jang, J.; Park, S.-M.; Ryu, S.M.; Cho, S.-J.; Yang, S.-R. Cigarette Smoke-Induced Respiratory Response: Insights into Cellular Processes and Biomarkers. Antioxidants 2023, 12, 1210. [Google Scholar] [CrossRef]


| Data | Patients with COPD n (%) | Controls n (%) |
|---|---|---|
| Number | (n = 57) | (n = 19) |
| Male | 42 (73.7) | 8 (42.1) |
| Female | 15 (26.3) | 11 (57.9) |
| Age at inclusion in this study | ||
| Mean ± SEM (years) | 67.14 ± 1.31 | 55.89 ± 2.51 |
| Median (range) (years) | 66.00 (40–88) | 56.00 (35–80) |
| Age at diagnosis | ||
| Mean ± SEM (years) | 62.47 ± 1.44 | |
| Median (range) (years) | 64.00 (30–86) | |
| Duration of the disease | ||
| Mean ± SEM (years) | 4.69 ± 0.81 | |
| Median (range) (years) | 2.00 (0–30) | |
| Smoking status | ||
| Non-smokers | 16 (28.1) | 11 (57.9) |
| Ex-smokers | 32 (56.1) | 3 (15.8) |
| Current smokers | 9 (15.8) | 5 (26.3) |
| Smoking habits (packs/year) | ||
| Mean ± SD (years) | 31.90 ± 2.75 | 14.17 ± 3.96 |
| Median (range) | 30.00 (5–88) | 12.50 (5–30) |
| COPD stage | ||
| GOLD II | 36 (63.1) | |
| GOLD III | 16 (28.1) | |
| GOLD IV | 5 (8.8) | |
| FEV1% predicted | ||
| Mean ± SEM | 53.30 (±2.16) | |
| FEV1/FVC% | ||
| Mean ± SEM | 63.32 (±0.95) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandrova, E.; Dimov, D.; Tacheva, T.; Petrova, H.; Celik, K.; Vlaykova, T. Smoking-Dependent Association of Serum Brain-Derived Neurotrophic Factor with Pulmonary Function Parameters in Chronic Obstructive Pulmonary Disease. Medicina 2024, 60, 1111. https://doi.org/10.3390/medicina60071111
Aleksandrova E, Dimov D, Tacheva T, Petrova H, Celik K, Vlaykova T. Smoking-Dependent Association of Serum Brain-Derived Neurotrophic Factor with Pulmonary Function Parameters in Chronic Obstructive Pulmonary Disease. Medicina. 2024; 60(7):1111. https://doi.org/10.3390/medicina60071111
Chicago/Turabian StyleAleksandrova, Elina, Dimo Dimov, Tanya Tacheva, Hristina Petrova, Kahan Celik, and Tatyana Vlaykova. 2024. "Smoking-Dependent Association of Serum Brain-Derived Neurotrophic Factor with Pulmonary Function Parameters in Chronic Obstructive Pulmonary Disease" Medicina 60, no. 7: 1111. https://doi.org/10.3390/medicina60071111





