Aspirin–Fisetin Combinatorial Treatment Exerts Cytotoxic and Anti-Migratory Activities in A375 Malignant Melanoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
2.3. In Vitro Models and Cell Culture
2.4. Cytotoxicity Assessments
2.5. Bright-Field Analysis of Cell Shape and Confluence
2.6. Immunofluorescence Visualization of Nuclei and Cytoskeletal Tubulin
2.7. Cell Migration Assay
2.8. In Ovo Hen’s Egg Test–Chorioallantoic Membrane (HET–CAM) Test
2.9. In Ovo Evaluation of Angiogenesis
2.10. Statistical Analysis
3. Results
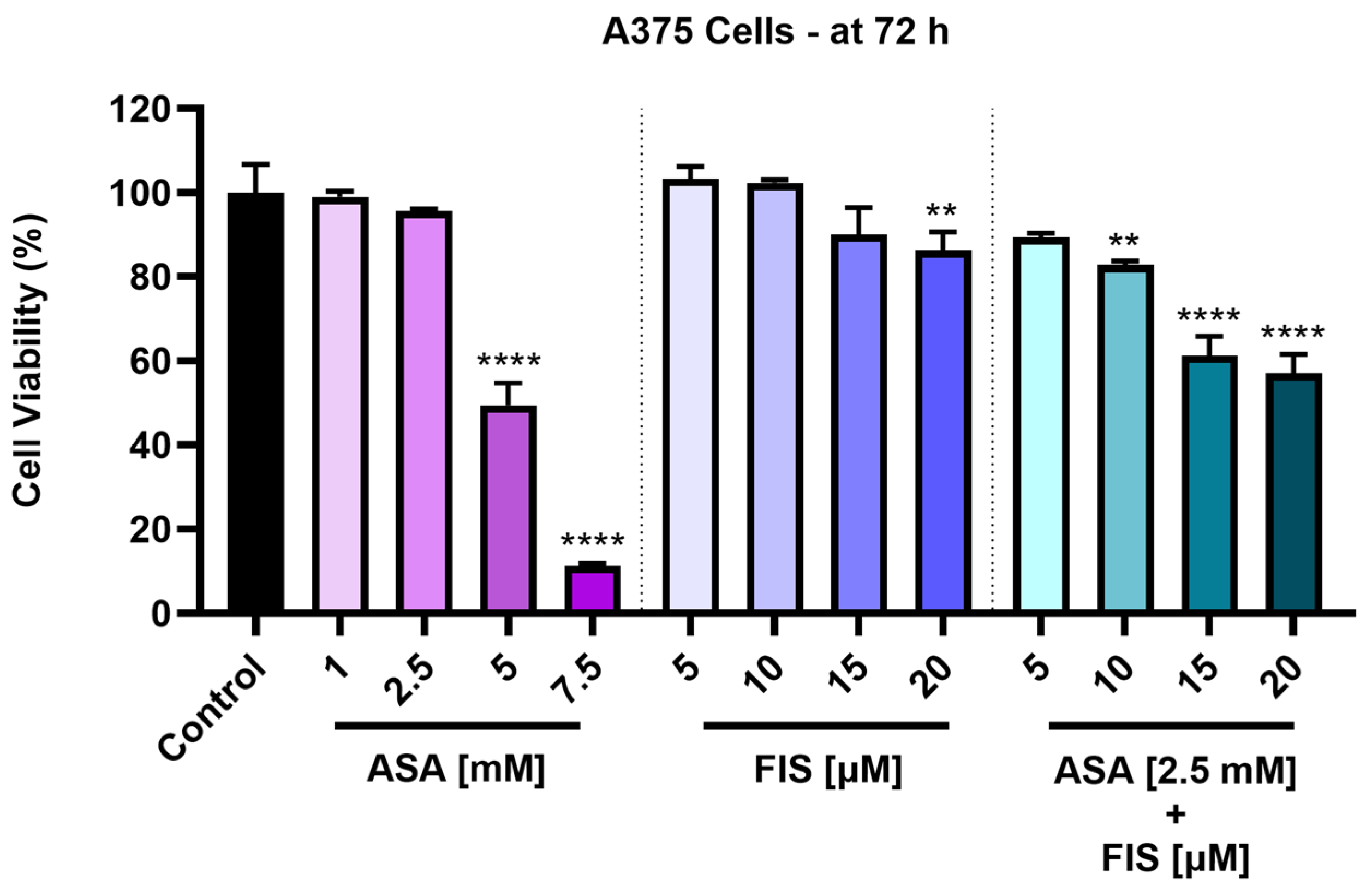
3.1. Cytotoxicity of ASA, FIS, and ASA + FIS
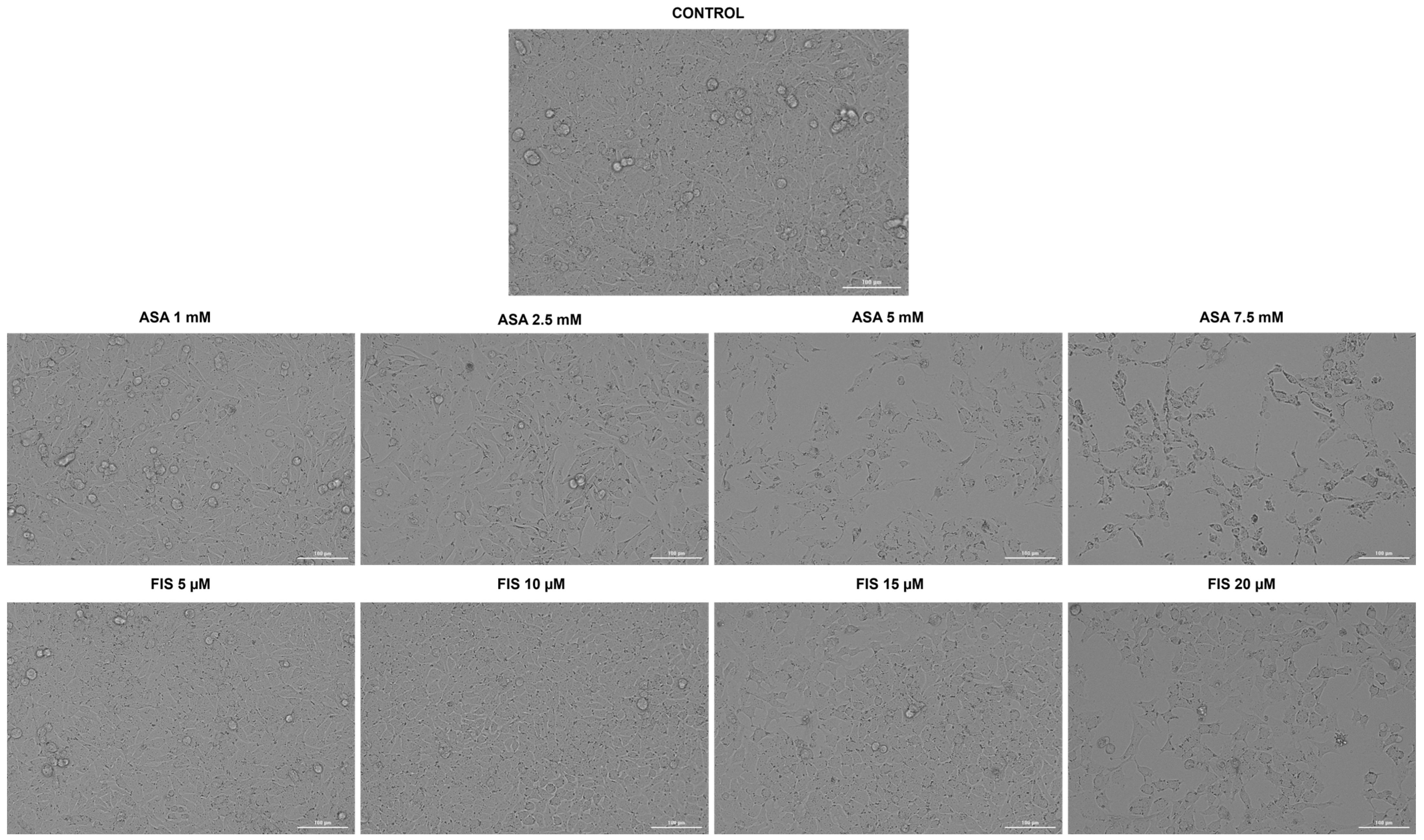
3.2. Influence of ASA, FIS, and ASA + FIS on Cellular Morphology and Confluence
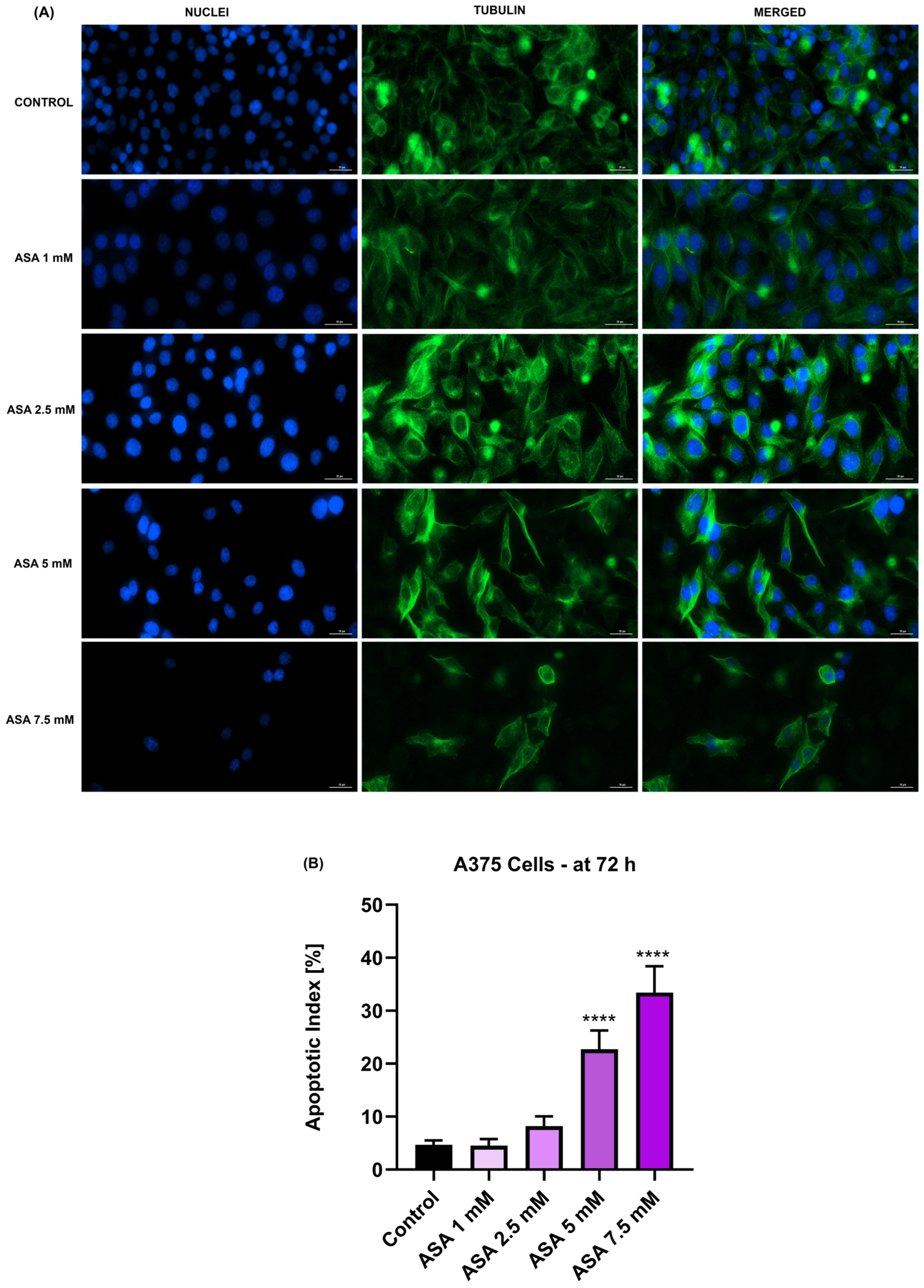
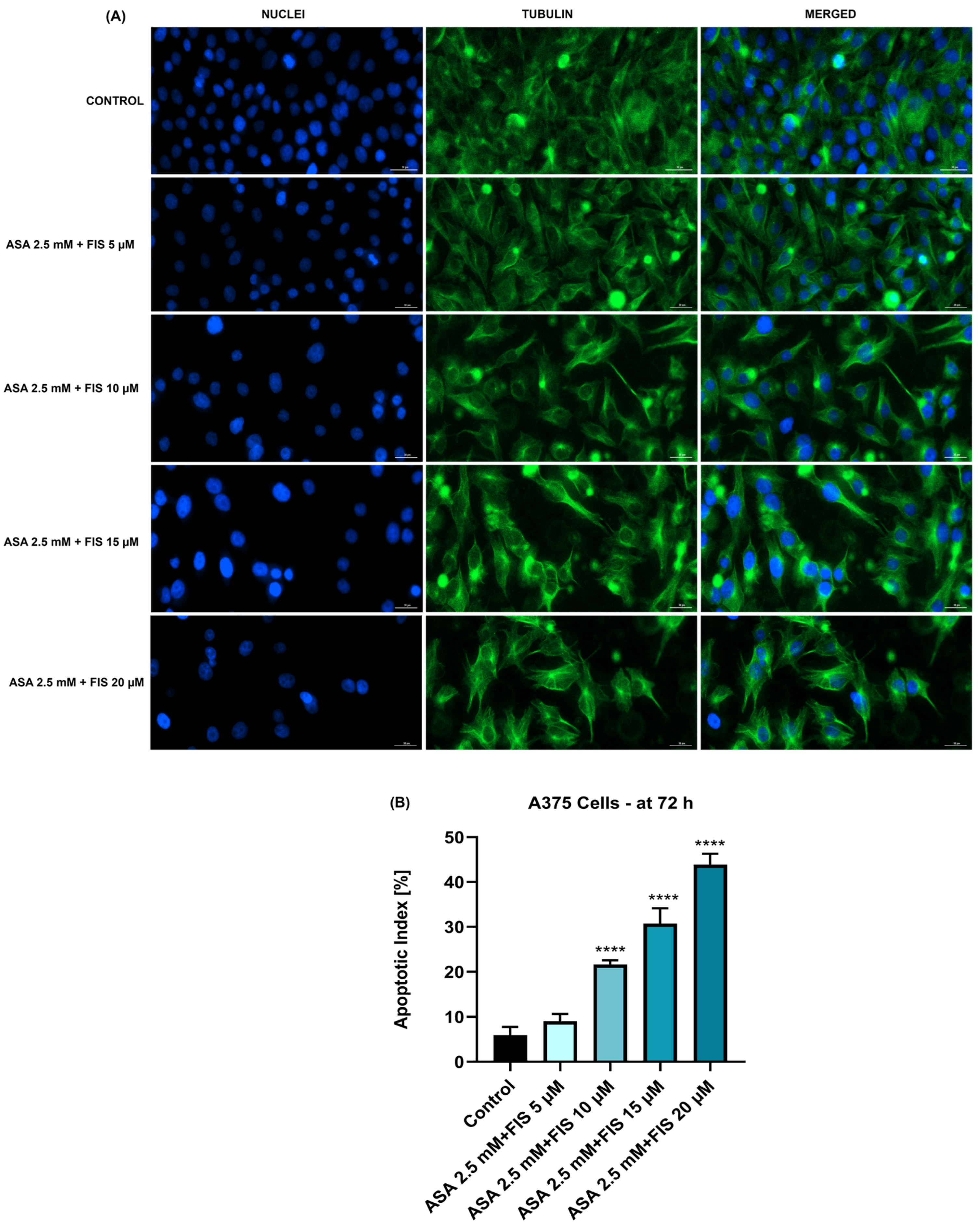
3.3. Influence of ASA, FIS, and ASA + FIS on Nuclear Shape and Tubulin Distribution
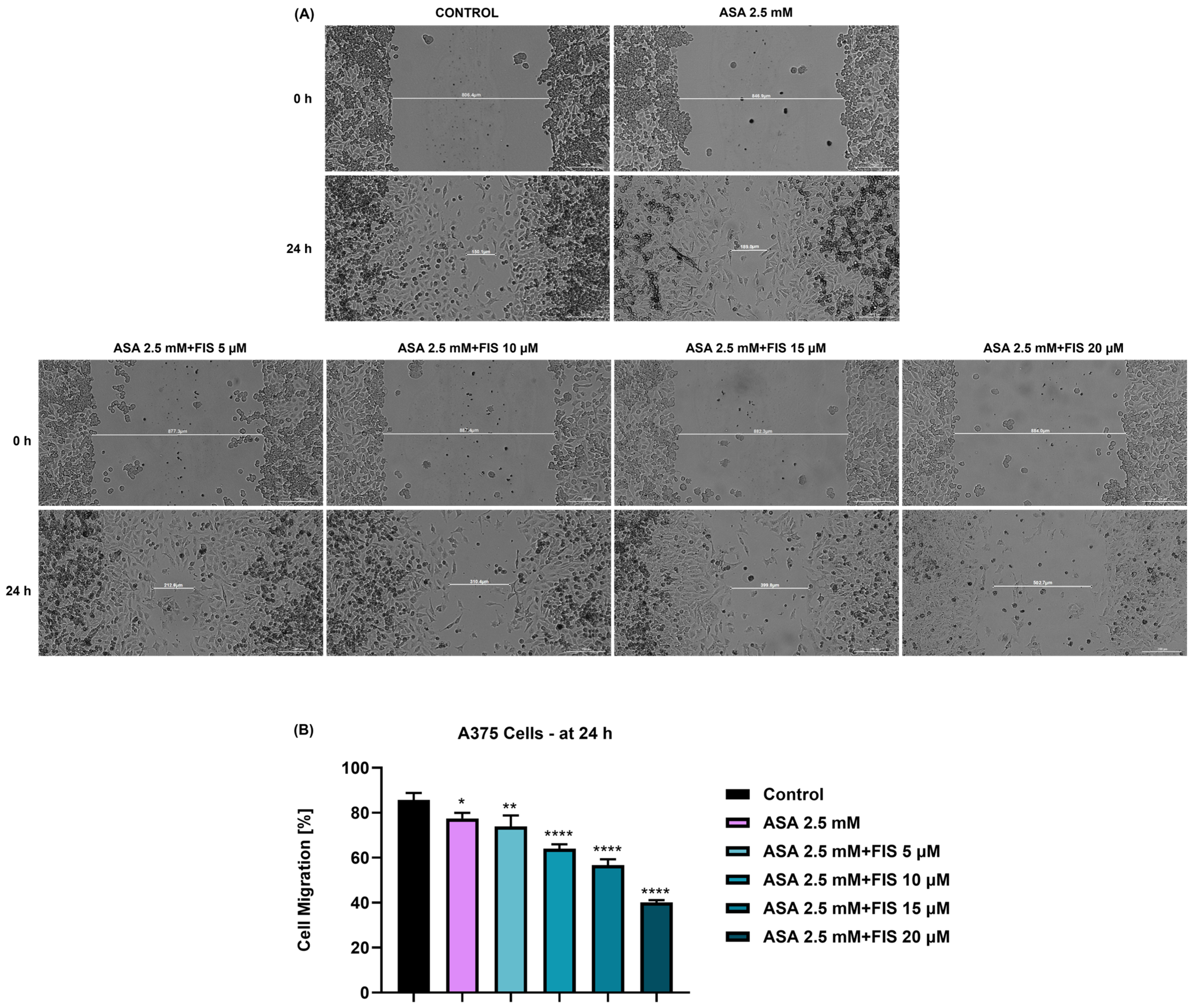
3.4. Influence of ASA, FIS, and ASA + FIS on Cell Migration
3.5. Irritant Potential of ASA, FIS, and ASA + FIS on the CAM
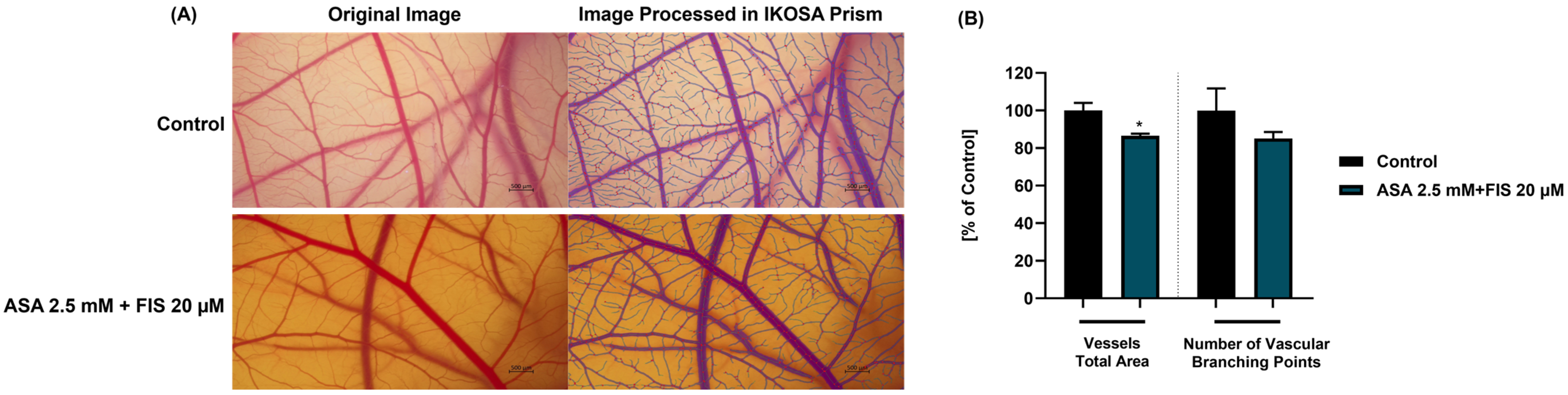
3.6. Influence of ASA, FIS, and ASA + FIS on In Ovo Angiogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, S.M. Epidemiology of Cancer. Clin. Chem. 2024, 70, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Perera, E.; Gnaneswaran, N.; Jennens, R.; Sinclair, R. Malignant melanoma. Healthcare 2014, 2, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sheikh, M.S. Melanoma: Molecular pathogenesis and therapeutic management. Mol. Cell. Pharmacol. 2014, 6, 31–44. [Google Scholar]
- Lukáčová, E.; Pös, O.; Túryová, E.; Hurtová, T.; Hanzlíková, Z.; Szemes, T.; Burjanivová, T. Copy number variations in malignant melanoma: Genomic regions, biomarkers, and therapeutic targets. Neoplasma 2013, 60, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Heistein, J.B.; Acharya, U.; Mukkamalla, S.K.R. Malignant Melanoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024; Volume 481. [Google Scholar]
- Domingues, B.; Lopes, J.; Soares, P.; Populo, H. Melanoma treatment in review. Immunotargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Bociort, F.; Macasoi, I.G.; Marcovici, I.; Motoc, A.; Grosu, C.; Pinzaru, I.; Petean, C.; Avram, S.; Dehelean, C.A. Investigation of lupeol as anti-melanoma agent: An in vitro-in ovo perspective. Curr. Oncol. 2021, 28, 5054–5066. [Google Scholar] [CrossRef] [PubMed]
- Coricovac, D.; Dehelean, C.A.; Pinzaru, I.; Mioc, A.; Aburel, O.M.; Macasoi, I.; Draghici, G.A.; Petean, C.; Soica, C.; Boruga, M.; et al. Assessment of betulinic acid cytotoxicity and mitochondrial metabolism impairment in a human melanoma cell line. Int. J. Mol. Sci. 2021, 22, 4870. [Google Scholar] [CrossRef] [PubMed]
- Chunarkar-Patil, P.; Kaleem, M.; Mishra, R.; Ray, S.; Ahmad, A.; Verma, D.; Bhayye, S.; Dubey, R.; Singh, H.N.; Kumar, S. Anticancer Drug Discovery Based on Natural Products: From Computational Approaches to Clinical Studies. Biomedicines 2024, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Natural compounds as anticancer agents: Experimental evidence. World J. Exp. Med. 2012, 2, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Iftode, C.; Iurciuc, S.; Marcovici, I.; Macasoi, I.; Coricovac, D.; Dehelean, C.; Ursoniu, S.; Rusu, A.; Ardelean, S. Genistein–Aspirin Combination Exerts Cytotoxic and Anti-Migratory Effects in Human Colorectal Cancer Cells. Life 2024, 14, 606. [Google Scholar] [CrossRef]
- Mohammed, A.; Fox, J.T.; Miller, M.S. Cancer Chemoprevention: Preclinical in Vivo Alternate Dosing Strategies to Reduce Drug Toxicities. Toxicol. Sci. 2019, 170, 251–259. [Google Scholar] [CrossRef]
- Danciu, C.; Soica, C.; Antal, D.; Popescu, A.; Ghiulai, R.; Pavel, I.Z.; Avram, S.; Daliana, M.; Dehelean, C. An Update on Natural Compounds and Their Modern Formulations for the Management of Malignant Melanoma. In Natural Products and Cancer Drug Discovery; InTech: London, UK, 2017. [Google Scholar]
- Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Khan, A.A.; Almatroodi, S.A. The Potential Role of Fisetin, a Flavonoid in Cancer Prevention and Treatment. Molecules 2022, 27, 9009. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Antoszczak, M.; Kacprzak, K.; Markowska, J.; Huczyński, A. Role of Fisetin in Selected Malignant Neoplasms in Women. Nutrients 2023, 15, 4686. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Huang, Y.; Nie, S.; Zhou, S.; Gao, X.; Chen, G. Biological effects and mechanisms of fisetin in cancer: A promising anti-cancer agent. Eur. J. Med. Res. 2023, 28, 297. [Google Scholar] [CrossRef]
- Pal, H.C.; Sharma, S.; Strickland, L.R.; Katiyar, S.K.; Ballestas, M.E.; Athar, M.; Elmets, C.A.; Afaq, F. Fisetin inhibits human melanoma cell invasion through promotion of mesenchymal to epithelial transition and by targeting MAPK and NFκB signaling pathways. PLoS ONE 2014, 9, e86338. [Google Scholar] [CrossRef]
- Syed, D.N.; Chamcheu, J.C.; Khan, M.I.; Sechi, M.; Lall, R.K.; Adhami, V.M.; Mukhtar, H. Fisetin inhibits human melanoma cell growth through direct binding to p70S6K and mTOR: Findings from 3-D melanoma skin equivalents and computational modeling. Biochem. Pharmacol. 2014, 89, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Jayaram, B.; Goliaei, B.; Masoudi-Nejad, A. Active repurposing of drug candidates for melanoma based on GWAS, PheWAS and a wide range of omics data. Mol. Med. 2019, 25, 30. [Google Scholar] [CrossRef]
- Abdelsayed, M.; Kort, E.J.; Jovinge, S.; Mercola, M. Repurposing drugs to treat cardiovascular disease in the era of precision medicine. Nat. Rev. Cardiol. 2022, 19, 751–764. [Google Scholar] [CrossRef]
- Cadavid, A.P. Aspirin: The mechanism of action revisited in the context of pregnancy complications. Front. Immunol. 2017, 8, 261. [Google Scholar] [CrossRef]
- Elwood, P.; Protty, M.; Morgan, G.; Pickering, J.; Delon, C.; Watkins, J. Aspirin and cancer: Biological mechanisms and clinical outcomes. Open Biol. 2022, 12, 2–8. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, Y.; Luo, R.C.; Li, A.M. Aspirin for the primary prevention of skin cancer: A meta-analysis. Oncol. Lett. 2015, 9, 1073–1080. [Google Scholar] [CrossRef]
- Fujikawa, I.; Ando, T.; Suzuki-Karasaki, M.; Suzuki-Karasaki, M.; Ochiai, T.; Suzuki-Karasaki, Y. Aspirin induces mitochon-drial Ca2+ remodeling in tumor cells via ROS-depolarization-voltage-gated Ca2+ entry. Int. J. Mol. Sci. 2020, 21, 4771. [Google Scholar] [CrossRef]
- Iftode, C.; Olaru, I.; Chioran, D.; Coricovac, D.; Geamantan, A.; Borza, C.; Ursoniu, S.; Ardelean, S. Non-Steroidal Anti-In-flammatory Drugs (Nsaids) As Repurposed Anticancer Drugs in Skin Cancer. Farmacia 2024, 72, 28–43. [Google Scholar]
- Hu, L.X.; Du, Y.Y.; Zhang, Y.; Pan, Y.Y. Synergistic effects of exemestane and aspirin on MCF-7 human breast cancer cells. Asian Pacific J. Cancer Prev. 2012, 13, 5903–5908. [Google Scholar] [CrossRef]
- Susan, M.; Macasoi, I.; Pinzaru, I.; Dehelean, C.; Ilia, I.; Susan, R.; Ionita, I. In Vitro Assessment of the Synergistic Effect of Aspirin and 5-Fluorouracil in Colorectal Adenocarcinoma Cells. Curr. Oncol. 2023, 30, 6197–6219. [Google Scholar] [CrossRef]
- Jiang, W.; Yan, Y.; Chen, M.; Luo, G.; Hao, J.; Pan, J.; Hu, S.; Guo, P.; Li, W.; Wang, R.; et al. Aspirin enhances the sensitivity of colon cancer cells to cisplatin by abrogating the binding of NF-κB to the COX-2 promoter. Aging 2020, 12, 611–627. [Google Scholar] [CrossRef]
- Marcovici, I.; Vlad, D.; Buzatu, R.; Popovici, R.A.; Cosoroaba, R.M.; Chioibas, R.; Geamantan, A.; Dehelean, C. Rutin Linoleate Triggers Oxidative Stress-Mediated Cytoplasmic Vacuolation in Non-Small Cell Lung Cancer Cells. Life 2024, 14, 215. [Google Scholar] [CrossRef]
- Rednic, R.; Macasoi, I.; Pinzaru, I.; Dehelean, C.A.; Tomescu, M.C.; Susan, M.; Feier, H. Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells. Life 2022, 12, 1855. [Google Scholar] [CrossRef]
- Aventurado, C.A.; Billones, J.B.; Vasquez, R.D.; Castillo, A.L. In ovo and in silico evaluation of the anti-angiogenic potential of syringin. Drug Des. Dev. Ther. 2020, 14, 5189–5204. [Google Scholar] [CrossRef]
- Parveen, S.; Nadumane, V.K. Anti-angiogenesis and apoptogenic potential of the brown marine alga, Chnoospora minima. Future J. Pharm. Sci. 2020, 6, 19. [Google Scholar] [CrossRef]
- Papa, V.; Li Pomi, F.; Borgia, F.; Vaccaro, M.; Pioggia, G.; Gangemi, S. Alarmins in cutaneous malignant melanoma: An up-dated overview of emerging evidence on their pathogenetic, diagnostic, prognostic, and therapeutic role. J. Dermatol. 2024, 51, 927–938. [Google Scholar] [CrossRef]
- Ombra, M.N.; Paliogiannis, P.; Stucci, L.S.; Colombino, M.; Casula, M.; Sini, M.C.; Manca, A.; Palomba, G.; Stanganelli, I.; Mandalà, M.; et al. Dietary compounds and cutaneous malignant melanoma: Recent advances from a biological perspective. Nutr. Metab. 2019, 16, 33. [Google Scholar] [CrossRef]
- Munhoz, R.R.; Postow, M.A. Combinatorial Approaches to the Treatment of Advanced Melanoma. Hematol. Oncol. Clin. N. Am. 2021, 35, 145–158. [Google Scholar] [CrossRef]
- Grimm, J.; Hufnagel, A.; Wobser, M.; Borst, A.; Haferkamp, S.; Houben, R.; Meierjohann, S. BRAF inhibition causes resilience of melanoma cell lines by inducing the secretion of FGF1. Oncogenesis. 2018, 7, 71. [Google Scholar] [CrossRef]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT Cells as a Reliable in Vitro Differentiation Model to Dissect the Inflammatory/Repair Response of Human Keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Khusro, F. Dual inhibition of PI3K/AKT and mTOR signaling in human non-small cell lung cancer cells by a dietary flavonoid fisetin. Int. J. Cancer. 2012, 130, 1695–1705. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Y.; Yang, Y.; Liu, Y.; Ma, L.; Zhang, Y. Aspirin suppresses chemoresistance and enhances antitumor activity of 5-Fu in 5-Fu-resistant colorectal cancer by abolishing 5-Fu-induced NF-κB activation. Sci. Rep. 2019, 9, 16937. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, J.; Mao, S.Y.; Xu, Y.S.; Zhang, D.; Feng, L.Y.; Zhang, B.; Yan, Y.Y.; Wang, S.C.; Pan, J.P.; et al. Role of p38 MAPK in enhanced human cancer cells killing by the combination of aspirin and ABT-737. J. Cell. Mol. Med. 2015, 19, 408–417. [Google Scholar] [CrossRef]
- Imtiyaz, K.; Husain Rahmani, A.; Alsahli, M.A.; Almatroodi, S.A.; Rizvi, M.M.A. Fisetin induces apoptosis in human skin cancer cells through downregulating MTH1. J. Biomol. Struct. Dyn. 2023, 41, 7339–7353. [Google Scholar] [CrossRef]
- Syed, D.N.; Afaq, F.; Maddodi, N.; Johnson, J.J.; Sarfaraz, S.; Ahmad, A.; Setaluri, V.; Mukhtar, H. Inhibition of human mel-anoma cell growth by the dietary flavonoid fisetin is associated with disruption of Wnt/β-catenin signaling and decreased mitf levels. J. Investig. Dermatol. 2011, 131, 1291–1299. [Google Scholar] [CrossRef]
- Vad, N.M.; Kudugunti, S.K.; Wang, H.; Jayarama Bhat, G.; Moridani, M.Y. Efficacy of acetylsalicylic acid (aspirin) in skin B16-F0 melanoma tumor-bearing C57BL/6 mice. Tumor Biol. 2014, 35, 4967–4976. [Google Scholar] [CrossRef]
- Ray, S.D.; Krmic, M.; Hussain, A.; Marvilli, C.; Fabian, R.; Niha, A.; Danai, M.; Smith, Z.; Jalshgari, A.; Malik, N.; et al. Toxicity of Natural Products, 4th ed.; Academic Press: Oxford, UK, 2024; pp. 257–282. ISBN 978-0-323-85434-4. [Google Scholar]
- Kwok, C.S.; Loke, Y.K. Critical overview on the benefits and harms of aspirin. Pharmaceuticals 2010, 3, 1491–1506. [Google Scholar] [CrossRef]
- Doonan, F.; Cotter, T.G. Morphological assessment of apoptosis. Methods 2008, 44, 200–204. [Google Scholar] [CrossRef]
- Povea-Cabello, S.; Oropesa-Ávila, M.; de la Cruz-Ojeda, P.; Villanueva-Paz, M.; De La Mata, M.; Suárez-Rivero, J.M.; Álvarez-Córdoba, M.; Villalón-García, I.; Cotán, D.; Ybot-González, P.; et al. Dynamic reorganization of the cytoskeleton during apop-562 tosis: The two coffins hypothesis. Int. J. Mol. Sci. 2017, 18, 2393. [Google Scholar] [CrossRef]
- Pal, H.C.; Baxter, R.D.; Hunt, K.M.; Agarwal, J.; Elmets, C.A.; Athar, M.; Afaq, F. Fisetin, a phytochemical, potentiates soraf-enib-induced apoptosis and abrogates tumor growth in athymic nude mice implanted with BRAF-mutated melanoma cells. Oncotarget 2015, 6, 28296–28311. [Google Scholar] [CrossRef]
- Batista-Duharte, A.; Jorge Murillo, G.; Pérez, U.M.; Tur, E.N.; Portuondo, D.F.; Martínez, B.T.; Téllez-Martínez, D.; Betancourt, J.E.; Pérez, O. The Hen’s Egg Test on Chorioallantoic Membrane: An Alternative Assay for the Assessment of the Irritating Effect of Vaccine Adjuvants. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef]
- Naik, M.; Brahma, P.; Dixit, M. A cost-effective and efficient chick ex-ovo cam assay protocol to assess angiogenesis. Methods Protoc. 2018, 1, 19. [Google Scholar] [CrossRef]
- Bhat, T.A.; Nambiar, D.; Pal, A.; Agarwal, R.; Singh, R.P. Fisetin inhibits various attributes of angiogenesis in vitro and in vivo-implications for angioprevention. Carcinogenesis 2012, 33, 385–393. [Google Scholar] [CrossRef]
- Maity, G.; Chakraborty, J.; Ghosh, A.; Haque, I.; Banerjee, S.; Banerjee, S.K. Aspirin suppresses tumor cell-induced angiogen-esis and their incongruity. J. Cell Commun. Signal. 2019, 13, 491–502. [Google Scholar] [CrossRef]
- Xie, S.; Wang, Y.; Huang, Y.; Yang, B. Mechanisms of the antiangiogenic effects of aspirin in cancer. Eur. J. Pharmacol. 2021, 898, 173989. [Google Scholar] [CrossRef]




| Treatment | Calculated Irritation Score (IS) | Interpretation |
|---|---|---|
| H2O | 0.07 | Non-irritant |
| SDS 1% | 19.51 | Strong irritant |
| ASA 2.5 mM + FIS 20 µM | 0.82 | Non-irritant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iftode, C.; Minda, D.; Draghici, G.; Geamantan, A.; Ursoniu, S.; Enatescu, I. Aspirin–Fisetin Combinatorial Treatment Exerts Cytotoxic and Anti-Migratory Activities in A375 Malignant Melanoma Cells. Medicina 2024, 60, 1125. https://doi.org/10.3390/medicina60071125
Iftode C, Minda D, Draghici G, Geamantan A, Ursoniu S, Enatescu I. Aspirin–Fisetin Combinatorial Treatment Exerts Cytotoxic and Anti-Migratory Activities in A375 Malignant Melanoma Cells. Medicina. 2024; 60(7):1125. https://doi.org/10.3390/medicina60071125
Chicago/Turabian StyleIftode, Claudia, Daliana Minda, George Draghici, Andreea Geamantan, Sorin Ursoniu, and Ileana Enatescu. 2024. "Aspirin–Fisetin Combinatorial Treatment Exerts Cytotoxic and Anti-Migratory Activities in A375 Malignant Melanoma Cells" Medicina 60, no. 7: 1125. https://doi.org/10.3390/medicina60071125





