Antimalarial Drugs at the Intersection of SARS-CoV-2 and Rheumatic Diseases: What Are the Potential Opportunities?
Abstract
:1. Introduction
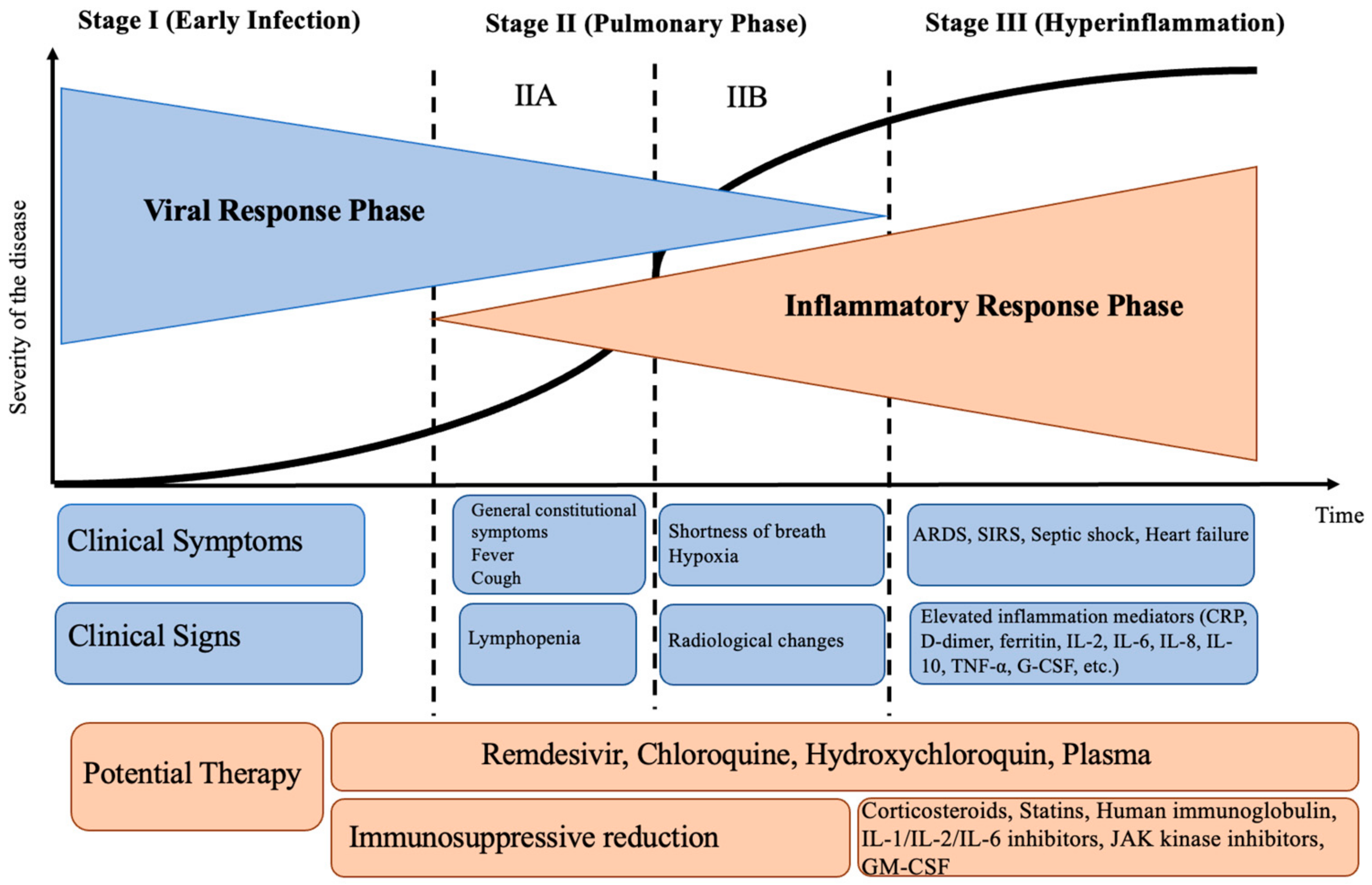
2. Pathogenetic Stages of SARS-CoV-2
3. Antimalarial Drugs in Rheumatology
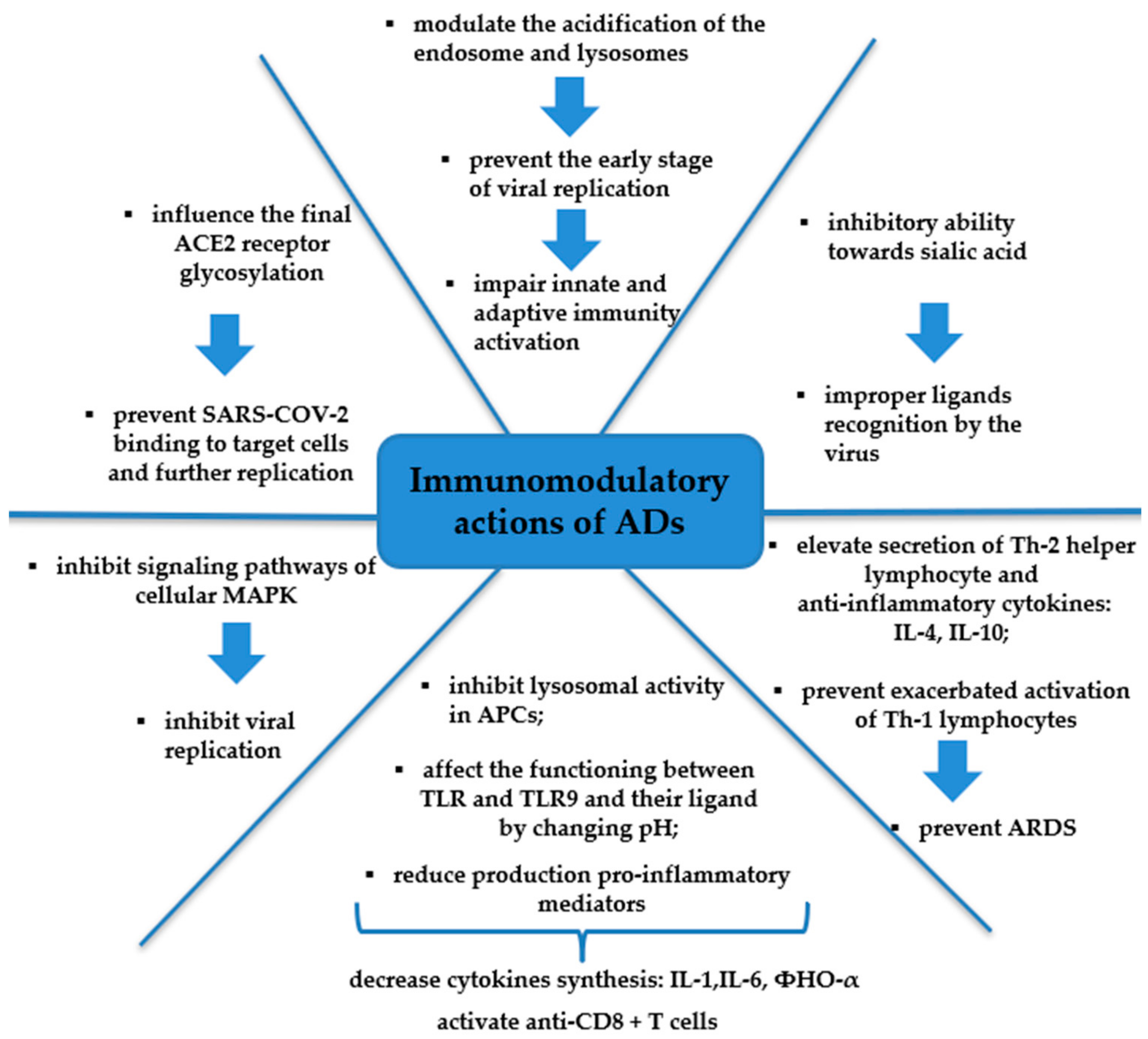
4. Mechanism of Action of the Aminoquinoline Series of Drugs against SARS-CoV-2
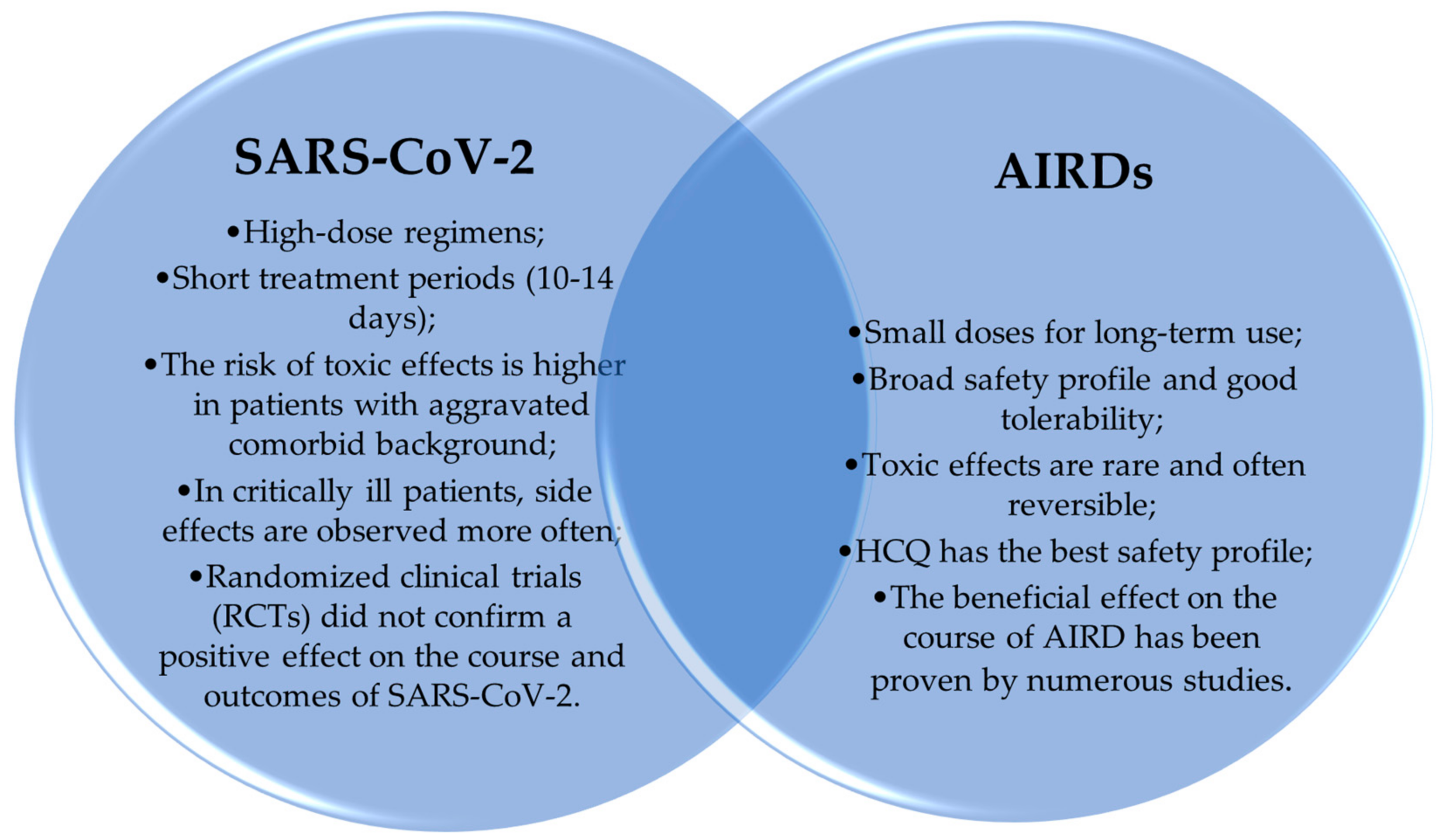
5. Experience in the Treatment of SARS-CoV-2
5.1. Research Contributing to the Successful HCQ Application in COVID-19 Treatment
5.2. Research Contributing to the Failure of HCQ Application in COVID-19 Treatment
6. Side Effects of ADs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rutskaya-Moroshan, K.; Abisheva, S.; Lila, A. Shared Features of Pathogenetic Aspects, Autoimmunity and Pharmacotherapy in Coronavirus Infection (COVID-19) and Immunoinflammatory Rheumatic Diseases. Mod. Rheumatol. J. 2022, 16, 82–87. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Rubin, L.; Peng, T.; Liu, L.; Xing, X.; Lazarovici, P.; Zheng, W. Cytokine storm in COVID-19: From viral infection to immune responses, diagnosis and therapy. Int. J. Biol. Sci. 2022, 18, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.U.; Parida, S.; Lingaraju, M.C.; Kesavan, M.; Kumar, D.; Singh, R.K. Drug Repurposing Approach to Fight COVID-19. Pharmacol. Rep. 2020, 72, 1479–1508. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, M.; Bachmann, S. Influence of the COVID-19 Pandemic on Medical Management and on Healthcare Delivery of Immune-Mediated Rheumatic and Musculoskeletal Diseases during the First Pandemic Period February to July 2020: A Systematic Review. Medicina 2024, 60, 596. [Google Scholar] [CrossRef] [PubMed]
- Jose, R.J.; Manuel, A. COVID-19 Cytokine Storm: The Interplay between Inflammation and Coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Fox, R. Anti-Malarial Drugs: Possible Mechanisms of Action in Autoimmune Disease and Prospects for Drug Development. Lupus 1996, 5 (Suppl. 1), S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.J. The history of antimalarials. Lupus 1996, 5 (Suppl. 1), S2–S3. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.J. Pharmacology of Chloroquine and Hydroxychloroquine. In Hydroxychloroquine and Chloroquine Retinopathy; Springer: New York, NY, USA, 2014; pp. 35–63. [Google Scholar] [CrossRef]
- Cairoli, E.; Rebella, M.; Danese, N.; Garra, V.; Borba, E.F. Hydroxychloroquine reduces low-density lipoprotein cholesterol levels in systemic lupus erythematosus: A longitudinal evaluation of the lipid-lowering effect. Lupus 2012, 21, 1178–1182. [Google Scholar] [CrossRef]
- Wozniacka, A.; Carter, A.; McCauliffe, D.P. Antimalarials in cutaneous lupus erythematosus: Mechanisms of therapeutic benefit. Lupus 2002, 11, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.; Hellmann, D.; Hochberg, M.; Meyerhoff, J.; Bell, W.; Goldman, D. Arterial thrombotic events (TE) in SLE: The Baltimore Lupus Cohort study. Arthr. Rheum. 1994, 37, S297. [Google Scholar]
- Ruiz-Irastorza, G.; Egurbide, M.V.; Pijoan, J.I.; Garmendia, M.; Villar, I.; Martinez-Berriotxoa, A.; Erdozain, J.G.; Aguirre, C. Effect of Antimalarials on Thrombosis and Survival in Patients with Systemic Lupus Erythematosus. Lupus 2006, 15, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.; Amissah-Arthur, M.-B.; Gayed, M.; Brown, S.; Bruce, I.N.; D’Cruz, D.; Empson, B.; Griffiths, B.; Jayne, D.; Khamashta, M.; et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology 2018, 57, e1–e45. [Google Scholar] [CrossRef]
- Götestam Skorpen, C.; Hoeltzenbein, M.; Tincani, A.; Fischer-Betz, R.; Elefant, E.; Chambers, C.; da Silva, J.; Nelson-Piercy, C.; Cetin, I.; Costedoat-Chalumeau, N.; et al. The EULAR Points to Consider for Use of Antirheumatic Drugs before Pregnancy, and during Pregnancy and Lactation. Ann. Rheum. Dis. 2016, 75, 795–810. [Google Scholar] [CrossRef]
- Flint, J.; Panchal, S.; Hurrell, A.; van de Venne, M.; Gayed, M.; Schreiber, K.; Arthanari, S.; Cunningham, J.; Flanders, L.; Moore, L.; et al. BSR and BHPR Guideline on Prescribing Drugs in Pregnancy and Breastfeeding-Part I: Standard and Biologic Disease Modifying Anti-Rheumatic Drugs and Corticosteroids. Rheumatology 2016, 55, 1693–1697. [Google Scholar] [CrossRef]
- Andreoli, L.; Bertsias, G.K.; Agmon-Levin, N.; Brown, S.; Cervera, R.; Costedoat-Chalumeau, N.; Doria, A.; Fischer-Betz, R.; Forger, F.; Moraes-Fontes, M.F.; et al. EULAR Recommendations for Women’s Health and the Management of Family Planning, Assisted Reproduction, Pregnancy and Menopause in Patients with Systemic Lupus Erythematosus and/or Antiphospholipid Syndrome. Ann. Rheum. Dis. 2017, 76, 476–485. [Google Scholar] [CrossRef]
- Sammaritano, L.R.; Bermas, B.L.; Chakravarty, E.E.; Chambers, C.; Clowse, M.E.B.; Lockshin, M.D.; Marder, W.; Guyatt, G.; Branch, D.W.; Buyon, J.; et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Care Res. 2020, 72, 461–488. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.O.; Cheetham, T.C.; Li, D.-K.; Stein, C.M.; Callahan, S.T.; Morgan, T.M.; Shintani, A.K.; Chen, N.; Griffin, M.R.; Ray, W.A. Brief Report: Risk of Adverse Fetal Outcomes Associated with Immunosuppressive Medications for Chronic Immune-Mediated Diseases in Pregnancy. Arthritis Rheumatol. 2014, 66, 444–450. [Google Scholar] [CrossRef]
- Diav-Citrin, O.; Blyakhman, S.; Shechtman, S.; Ornoy, A. Pregnancy Outcome Following in Utero Exposure to Hydroxychloroquine: A Prospective Comparative Observational Study. Reprod. Toxicol. 2013, 39, 58–62. [Google Scholar] [CrossRef]
- Clowse, M.E.B.; Eudy, A.M.; Balevic, S.; Sanders-Schmidler, G.; Kosinski, A.; Fischer-Betz, R.; Gladman, D.D.; Molad, Y.; Nalli, C.; Mokbel, A.; et al. Hydroxychloroquine in the Pregnancies of Women with Lupus: A Meta-Analysis of Individual Participant Data. Lupus Sci. Med. 2022, 9, e000651. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, V.; Bouhet, A.; Barnetche, T.; Richez, C.; Truchetet, M.-E.; Seneschal, J.; Duffau, P.; Lazaro, E. Fédération Hospitalo-Universitaire Acronim Hydroxychloroquine for the Prevention of Fetal Growth Restriction and Prematurity in Lupus Pregnancy: A Systematic Review and Meta-Analysis. Jt. Bone Spine 2018, 85, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Eudy, A.; Siega-Riz, A.M.; Engel, S.M.; Franceschini, N.; Howard, A.G.; Clowse, M.; Petri, M. Effect of Pregnancy on Disease Flares in Patients with Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2018, 77, 855–860. [Google Scholar] [CrossRef]
- Duan, J.; Ma, D.; Wen, X.; Guo, Q.; Gao, J.; Zhang, G.; Xu, K.; Zhang, L. Hydroxychloroquine Prophylaxis for Preeclampsia, Hypertension and Prematurity in Pregnant Patients with Systemic Lupus Erythematosus: A Meta-Analysis. Lupus 2021, 30, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Barsalou, J.; Jaeggi, E.; Laskin, C.A.; Brown, P.; Tian, S.Y.; Hamilton, R.M.; Silverman, E.D. Prenatal Exposure to Antimalarials Decreases the Risk of Cardiac but Not Non-Cardiac Neonatal Lupus: A Single-Centre Cohort Study. Rheumatology 2017, 56, 1552–1559. [Google Scholar] [CrossRef]
- Izmirly, P.M.; Costedoat-Chalumeau, N.; Pisoni, C.N.; Khamashta, M.A.; Kim, M.Y.; Saxena, A.; Friedman, D.; Llanos, C.; Piette, J.-C.; Buyon, J.P. Maternal Use of Hydroxychloroquine Is Associated with a Reduced Risk of Recurrent Anti-SSA/Ro-Antibody-Associated Cardiac Manifestations of Neonatal Lupus. Circulation 2012, 126, 76–82. [Google Scholar] [CrossRef]
- Izmirly, P.M.; Kim, M.Y.; Llanos, C.; Le, P.U.; Guerra, M.M.; Askanase, A.D.; Salmon, J.E.; Buyon, J.P. Evaluation of the Risk of Anti-SSA/Ro-SSB/La Antibody-Associated Cardiac Manifestations of Neonatal Lupus in Fetuses of Mothers with Systemic Lupus Erythematosus Exposed to Hydroxychloroquine. Ann. Rheum. Dis. 2010, 69, 1827–1830. [Google Scholar] [CrossRef]
- Martínez-Sánchez, N.; Pérez-Pinto, S.; Robles-Marhuenda, Á.; Arnalich-Fernández, F.; Martín Cameán, M.; Hueso Zalvide, E.; Bartha, J.L. Obstetric and Perinatal Outcome in Anti-Ro/SSA-Positive Pregnant Women: A Prospective Cohort Study. Immunol. Res. 2017, 65, 487–494. [Google Scholar] [CrossRef]
- Izmirly, P.; Kim, M.; Friedman, D.M.; Costedoat-Chalumeau, N.; Clancy, R.; Copel, J.A.; Phoon, C.K.L.; Cuneo, B.F.; Cohen, R.; Robins, K.; et al. Hydroxychloroquine to Prevent Recurrent Congenital Heart Block in Fetuses of Anti-SSA/Ro-Positive Mothers. J. Am. Coll. Cardiol. 2020, 76, 292. [Google Scholar] [CrossRef] [PubMed]
- Willis, R.; Seif, A.M.; McGwin, G.; Martinez-Martinez, L.A.; González, E.B.; Dang, N.; Papalardo, E.; Liu, J.; Vilá, L.M.; Reveille, J.D.; et al. Effect of Hydroxychloroquine Treatment on Pro-Inflammatory Cytokines and Disease Activity in SLE Patients: Data from LUMINA (LXXV), a Multiethnic US Cohort. Lupus 2012, 21, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Petri, M. Use of Hydroxychloroquine to Prevent Thrombosis in Systemic Lupus Erythematosus and in Antiphospholipid Antibody-Positive Patients. Curr. Rheumatol. Rep. 2011, 13, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Irastorza, G.; Ramos-Casals, M.; Brito-Zeron, P.; Khamashta, M.A. Clinical Efficacy and Side Effects of Antimalarials in Systemic Lupus Erythematosus: A Systematic Review. Ann. Rheum. Dis. 2010, 69, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Kyburz, D.; Brentano, F.; Gay, S. Mode of Action of Hydroxychloroquine in RA-Evidence of an Inhibitory Effect on Toll-like Receptor Signaling. Nat. Clin. Pract. Rheumatol. 2006, 2, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Clowse, M.E.B.; Magder, L.; Witter, F.; Petri, M. Hydroxychloroquine in Lupus Pregnancy. Arthritis Rheum. 2006, 54, 3640–3647. [Google Scholar] [CrossRef] [PubMed]
- James, J.A.; Kim-Howard, X.R.; Bruner, B.F.; Jonsson, M.K.; McClain, M.T.; Arbuckle, M.R.; Walker, C.; Dennis, G.J.; Merrill, J.T.; Harley, J.B. Hydroxychloroquine Sulfate Treatment Is Associated with Later Onset of Systemic Lupus Erythematosus. Lupus 2007, 16, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Carsons, S.E.; Vivino, F.B.; Parke, A.; Carteron, N.; Sankar, V.; Brasington, R.; Brennan, M.T.; Ehlers, W.; Fox, R.; Scofield, H.; et al. Treatment guidelines for rheumatologic manifestations of Sjogren’s syndrome: Use of biologic agents, management of fatigue, and inflammatory musculoskeletal pain. Arthritis Care Res. 2017, 69, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Price, E.J.; Benjamin, S.; Bombardieri, M.; Bowman, S.; Carty, S.; Ciurtin, C.; Crampton, B.; Dawson, A.; Fisher, B.A.; Giles, I.; et al. British Society for Rheumatology guideline on management of adult and juvenile onset Sjögren disease. Rheumatology 2024, keae152. [Google Scholar] [CrossRef]
- Mumcu, G.; Bicakcigil, M.; Yilmaz, N.; Ozay, H.; Karacayli, U.; Cimilli, H.; Yavuz, S. Salivary and serum B-cell activating factor (BAFF) levels after hydroxychloroquine treatment in primary Sjogren’s syndrome. Oral. Health Prev. Dent. 2013, 11, 229–234. [Google Scholar]
- Yavuz, S.; Asfuroglu, E.; Bicakcigil, M.; Toker, E. Hydroxychloroquine improves dry eye symptoms of patients with primary Sjogren’s syndrome. Rheumatol. Int. 2011, 31, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.; Dutz, J.P. New Concepts in Antimalarial Use and Mode of Action in Dermatology. Dermatol. Ther. 2007, 20, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.B.; Rosen, T. Evidence-Based Therapy for Cutaneous Sarcoidosis. Drugs 2008, 68, 1361–1383. [Google Scholar] [CrossRef] [PubMed]
- Hilderson, I.; Van Laecke, S.; Wauters, A.; Donck, J. Treatment of Renal Sarcoidosis: Is There a Guideline? Overview of the Different Treatment Options. Nephrol. Dial. Transplant. 2014, 29, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Ang, G.C.; Werth, V.P. Combination antimalarials in the treatment of cutaneous dermatomyositis. A retrospective study. Arch. Dermatol. 2005, 141, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.h.A.; Rolain, J.M.; Colson Ph Raoult, D. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int. J. Antimicrob. Agents 2020, 55, 105938. [Google Scholar] [CrossRef] [PubMed]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases? Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Tatsumi, K.; Imai, A.M.; Saito, K.; Kuriyama, T.; Shirasawa, H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: Involvement of p38 MAPK and ERK. Antivir. Res. 2008, 77, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, S.; Kumlin, U.; Dimock, K.; Arnberg, N. Avian influenza and sialic acid receptors: More than meets the eye? Lancet Infect. Dis. 2005, 5, 184–188. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.; Liu, K.; Guo, F.; Zhang, Y.; Zhang, G.; Jiang, C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008, 18, 290–301. [Google Scholar] [CrossRef]
- Burkard, C.; Verheije, M.H.; Wicht, O.; van Kasteren, S.I.; van Kuppeveld, F.J.; Haagmans, B.L.; Pelkmans, L.; Rottier, P.J.; Bosch, B.J.; de Haan, C.A. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014, 10, e1004502. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel Coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Simmons, G.; Bertram, S.; Glowacka, I.; Steffen, I.; Chaipan, C.; Agudelo, J.; Lu, K.; Rennekamp, A.J.; Hofmann, H.; Bates, P.; et al. Different Host Cell Proteases Activate the SARS-Coronavirus Spike-Protein for Cell–Cell and Virus–Cell Fusion. Virology 2011, 413, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine Is a Potent Inhibitor of SARS Coronavirus Infection and Spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.-W.; Cheng, Y.; Zhang, J.; Jiang, X.-M.; Wang, L.; Deng, J.; Wang, P.-H. Increasing Host Cellular Receptor-Angiotensin-Converting Enzyme 2 Expression by Coronavirus May Facilitate 2019-nCoV (or SARS-CoV-2) Infection. J. Med. Virol. 2020, 92, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L. The role of cell surface sialic acids for SARS-CoV-2 infection. Glycobiology 2021, 31, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.M.L.; Rabetafika, D.R.R.T.; Rindra, D.R.; Mihaja, R.N.; Cyris, D.H.J.; Léa, P.R. Evidence of SARS-CoV-2 Transmission through the Ocular Route. Clin. Ophthalmol. 2021, 15, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Cheng, Y. Increasing host cellular receptor—Angiotensin-converting enzyme 2 (ACE2) expression by coronavirus may facilitate 2019-nCoV infection. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zhou, D.; Dai, S.M.; Tong, Q. COVID-19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020, 75, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Mudgal, J.; Arora, D.; Kinra, M.; Mallik, S.B.; Rao, C.M.; Pai, K.S.; Nampoothiri, M. Cannabinoid receptor 2 activation mitigates lipopolysaccharide-induced neuroinflammation and sickness behavior in mice. Psychopharmacology 2019, 236, 1829–1838. [Google Scholar] [CrossRef]
- An, J.; Woodward, J.J.; Lai, W.; Minie, M.; Sun, X.; Tanaka, L.; Snyder, J.M.; Sasaki, T.; Elkon, K.B. Inhibition of Cyclic GMP-AMP Synthase Using a Novel Antimalarial Drug Derivative in Trex1-Deficient Mice. Arthritis Rheumatol. 2018, 70, 1807–1819. [Google Scholar] [CrossRef]
- Million, M.; Lagier, J.-C.; Gautret, P.; Colson, P.; Fournier, P.-E.; Amrane, S.; Hocquart, M.; Mailhe, M.; Esteves-Vieira, V.; Doudier, B.; et al. Early Treatment of COVID-19 Patients with Hydroxychloroquine and Azithromycin: A Retrospective Analysis of 1061 Cases in Marseille, France. Travel Med. Infect. Dis. 2020, 35, 101738. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Schones, D.E.; Oh, J.; Cui, Y.; Cui, K.; Roh, T.-Y.; Zhao, K.; Leonard, W.J. Priming for T Helper Type 2 Differentiation by Interleukin 2-Mediated Induction of Interleukin 4 Receptor Alpha-Chain Expression. Nat. Immunol. 2008, 9, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Alfano, G.; Mori, G.; Amurri, A.; Tei, L.; Ballestri, M.; Leonelli, M.; Facchini, F.; Damiano, F.; Magistroni, R.; et al. COVID-19 Pneumonia in a Kidney Transplant Recipient Successfully Treated with Tocilizumab and Hydroxychloroquine. Am. J. Transplant. 2020, 20, 1902–1906. [Google Scholar] [CrossRef] [PubMed]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and Azithromycin as a Treatment of COVID-19: Results of an Open-Label Non-Randomized Clinical Trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef] [PubMed]
- Ayerbe, L.; Risco-Risco, C.; Ayis, S. The association of treatment with hydroxychloroquine and hospital mortality in COVID-19 patients. Intern. Emerg. Med. 2020, 15, 1501–1506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arshad, S.; Kilgore, P.; Chaudhry, Z.S.; Jacobsen, G. COVID-19 Task Force. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020, 97, 396–403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furtado, R.H.; Berwanger, O.; Fonseca, H.A.; Corrêa, T.D.; Ferraz, L.R.; Lapa, M.G.; Zampieri, F.G.; Veiga, V.C.; Azevedo, L.C.; Rosa, R.G.; et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): A randomised linical trial. Lancet 2020, 396, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, A.B.; Zampieri, F.G.; Rosa, R.G.; Azevedo, L.C.; Veiga, V.C.; Avezum, A.; Damiani, L.P.; Marcadenti, A.; Kawano-Dourado, L.; Lisboa, T.; et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate COVID-19. N. Engl. J. Med. 2020, 383, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Geleris, J.; Sun, Y.; Platt, J.; Zucker, J.; Baldwin, M.; Hripcsak, G.; Labella, A.; Manson, D.K.; Kubin, C.; Barr, R.G.; et al. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N. Engl. J. Med. 2020, 382, 2411–2418. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Dufort, E.M.; Udo, T.; Wilberschied, L.A.; Kumar, J.; Tesoriero, J.; Weinberg, P.; Kirkwood, J.; Muse, A.; DeHovitz, J.; et al. Association of treatment with hydroxychloroquine or azithromycin with inhospital mortality in patients with COVID-19 in New York state. JAMA 2020, 323, 2493–2502. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Mafham, M.; Linsell, L.; Bell, J.L.; Staplin, N.; Emberson, J.R.; Wiselka, M.; Ustianowski, A.; Elmahi, E.; et al. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2020, 383, 2030–2040. [Google Scholar] [CrossRef] [PubMed]
- WHO Solidarity Trial Consortium; Pan, H.; Peto, R.; Henao-Restrepo, A.-M.; Preziosi, M.-P.; Sathiyamoorthy, V.; Abdool Karim, Q.; Alejandria, M.M.; Hernández García, C.; Kieny, M.-P.; et al. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Li, C.; Chen, P.; Zhou, N.; Wang, L.; Li, J.; Jiang, H.; Wang, D.W. Hydroxychloroquine Application Is Associated with a Decreased Mortality in Critically Ill Patients with COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Skipper, C.P.; Pastick, K.A.; Engen, N.W.; Bangdiwala, A.S.; Abassi, M.; Lofgren, S.M.; Williams, D.A.; Okafor, E.C.; Pullen, M.F.; Nicol, M.R.; et al. Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19: A Randomized Trial. Ann. Intern. Med. 2020, 173, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Jankelson, L.; Karam, G.; Becker, M.L.; Chinitz, L.A.; Tsai, M.-C. QT Prolongation, Torsades de Pointes, and Sudden Death with Short Courses of Chloroquine or Hydroxychloroquine as Used in COVID-19: A Systematic Review. Heart Rhythm. 2020, 17, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Hunt, B.; Stegemann, M.; Rochwerg, B.; Lamontagne, F.; Siemieniuk, R.A.; Agoritsas, T.; Askie, L.; Lytvyn, L.; Leo, Y.-S.; et al. A Living WHO Guideline on Drugs for COVID-19. BMJ 2020, 370, m3379. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, F.; Stegemann, M.; Agarwal, A.; Agoritsas, T.; Siemieniuk, R.; Rochwerg, B.; Bartoszko, J.; Askie, L.; Macdonald, H.; Al-Maslamani, M.; et al. A living WHO guideline on drugs to prevent COVID-19. BMJ 2021, 372, n526. [Google Scholar] [CrossRef] [PubMed]
- Karia, R.H.; Nagraj, S.; Gupta, I.; Barua, A.; Kaur, N.; Singh, H. Hydroxychloroquine: A Review of Its Safety and Efficacy in COVID-19. J. Fam. Med. Prim. Care 2021, 10, 1124–1133. [Google Scholar] [CrossRef]
- White, N.J.; Watson, J.A.; Hoglund, R.M.; Chan, X.H.S.; Cheah, P.Y.; Tarning, J. COVID-19 Prevention and Treatment: A Critical Analysis of Chloroquine and Hydroxychloroquine Clinical Pharmacology. PLoS Med. 2020, 17, e1003252. [Google Scholar] [CrossRef]
- Yusuf, I.H.; Sharma, S.; Luqmani, R.; Downes, S.M. Hydroxychloroquine Retinopathy. Eye 2017, 31, 828–845. [Google Scholar] [CrossRef]
- Jallouli, M.; Francès, C.; Piette, J.-C.; Huong, D.L.T.; Moguelet, P.; Factor, C.; Zahr, N.; Miyara, M.; Saadoun, D.; Mathian, A.; et al. Hydroxychloroquine-Induced Pigmentation in Patients with Systemic Lupus Erythematosus: A Case-Control Study. JAMA Dermatol. 2013, 149, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Assalie, N.A.; Durcan, R.; Durcan, L.; Petri, M.A. Hydroxychloroquine-Induced Erythema Multiforme. J. Clin. Rheumatol. 2017, 23, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Meyerowitz, E.A.; Vannier, A.G.L.; Friesen, M.G.N.; Schoenfeld, S.; Gelfand, J.A.; Callahan, M.V.; Kim, A.Y.; Reeves, P.M.; Poznansky, M.C. Rethinking the Role of Hydroxychloroquine in the Treatment of COVID-19. FASEB J. 2020, 34, 6027–6037. [Google Scholar] [CrossRef] [PubMed]
- Pastick, K.A.; Okafor, E.C.; Wang, F.; Lofgren, S.M.; Skipper, C.P.; Nicol, M.R.; Pullen, M.F.; Rajasingham, R.; McDonald, E.G.; Lee, T.C.; et al. Review: Hydroxychloroquine and Chloroquine for Treatment of SARS-CoV-2 (COVID-19). Open Forum Infect. Dis. 2020, 7, ofaa130. [Google Scholar] [CrossRef] [PubMed]
| Disease | Priority of Therapy | Mechanism of Action | Clinical Effects |
|---|---|---|---|
| Systemic lupus erythematosus | It is a first-line therapy drugs of choice for damage to the skin, joints, and constitutional disorders. There is evidence of the advisability of including ADs in combination therapies in patients with nephritis, vasculitis, and central nervous system damage. | Stabilizes cell membrane organelles and lysosomal enzymes; increases the endosomal pH => decreases interferon production => inhibits the stimulation of autoreactive B lymphocytes; inhibits antigen-presenting cells; inhibits mRNA expression => reduces the synthesis of pro-inflammatory mediators (IL-1 β, IL-6, and TNF-α); inhibits lipid oxidation processes [33]. | Reduces disease activity [33,34] and the risk of multiorgan damage and musculoskeletal and skin syndromes [33]; Reduces the frequency of serositis and prevents the exacerbations of SLE [34]; reduces fatigue and general weakness, reduces disease activity and infection risk [35]; increases long-term survival rates [18,19,20]; prevents multiorgan damage, photoprotective, thromboprotective, osteoprotective [33,34], nephroprotective [36]; preventive effect of neuro-lupus [37]; antithrombotic [34,38], cardioprotective due to hypoglycemic and hypolipidemic properties [13]; steroid-sparing effect [35]; reducing the risk of complications during pregnancy [36]. It can be used as part of a combination therapy if methotrexate is not sufficiently effective [38]. |
| Rheumatoid arthritis | It is a second-line therapy used as part of a combination or monotherapy for RA. Can be used in the case of intolerance to other basic anti-inflammatory drugs in the early stage of low activity and absence of an unfavorable prognosis. | Antagonism of Toll-like receptors (TLR) => suppression of the immune response [36]; interference with the processes of antigen presentation and lysosomal oxidation [36]; inhibition of the production of RF antibodies, proteases, and collagenases => preventing the destruction of cartilage, inhibition of phospholipase A2 [11]; decreased synthesis of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) [11]. | |
| Sjögren disease (SD) | It is a first-line therapy in combination with muscular-articular syndrome [39] recommended for patients with SD with significant fatigue and systemic symptoms for 6–12 months [40]. | Reduction of the factors that activate B cells in tear fluid [41,42]. | Reduces arthralgia, myalgia, and fatigue syndromes [40]; reduces disease activity, increases salivation, reduces dry eye syndrome’s impact on the integrity of the cornea [41,42]; Reduces cardiovascular risk due to the effect of lipidemia and glycemia [13]. |
| Sarcoidosis | It is a second-line therapy recommended for the cutaneous form of sarcoidosis in the case of the ineffectiveness of glucocorticoid local application [43]. | Decreased secretion of pro-inflammatory cytokines [44]; inhibition of the function of antigen-presenting cells => reduction in antigen processing and presentation through the major histocompatibility complex (MHC) system => reduction in granulomatous damage by T lymphocytes [45]. | Prevents sarcoid lesions in the skin in monotherapy or in combination with glucocorticoids [44]. Favorable effect on hypercalcemia and hypercalcemia in sarcoidosis (in combination with glucocorticoids) [45]. Promotes the regression of lung damage in the pulmonary form [45]. |
| Dermatomyositis | It is allowed to be prescribed with a minimal degree of activity to reduce the manifestations of skin syndromes in combination with glucocorticoids. | Inhibition of phospholipase A2; decreased synthesis of pro-inflammatory cytokines; decreased phagocytic and chemotactic activity of immune cells; inhibition of the formation of immune complexes; antioxidant effects [46]. | Reduces skin manifestations, including the juvenile form of dermatomyositis [46]. |
| Authors | Study Design | Quantity | Results |
|---|---|---|---|
| Gautret et al. [66] | Open-label and non-randomized | 80 | Viral load reduction/disappearance with HCQ in combination with azithromycin |
| Yu et al. [75] | Retrospective cohort | 568 | Significant association with decreased mortality in critically ill COVID-19 patients and attenuation of inflammatory cytokine IL-6 level. |
| Million et al. [63] | Retrospective analysis of a case series | 1061 | Improved clinical outcomes, mortality rates, and virological cure |
| Arshad et al. [68] | Multicenter retrospective observational study | 2541 | Association with reduced COVID-19-associated mortality in HCQ monotherapy or in combination with azithromycin |
| Furtado et al. [69] | Open-label and randomized | 447 | HCQ with azithromycin did not improve clinical outcomes |
| Skipper et al. [76] | Randomized, double-blind, and placebo-controlled | 491 | HCQ did not substantially reduce symptom severity in outpatients with early, mild COVID-19 |
| Pan et al. [74] | Randomized multicenter retrospective | 954 | Minimal or no effect of HCQ on hospitalized patients, as indicated by the overall mortality, initiation of ventilation, and duration of hospital stay |
| Rosenberg et al. [72] | Retrospective multicenter cohort study | 1438 | Monotherapy with HCQ or in combination with azithromycin was not associated with differences in in-hospital mortality |
| Jankelson et al. [77] | Systematic review | 1515 | QT prolongation was registered in approximately 10% of COVID-19 patients treated with HCQ, and there were two cases of ventricular arrhythmia |
| Horby et al. [73] | Randomized, controlled, open-label platform | 1561 | HCQ did not cause a lower incidence of death at 28 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abisheva, S.; Rutskaya-Moroshan, K.; Nuranova, G.; Batyrkhan, T.; Abisheva, A. Antimalarial Drugs at the Intersection of SARS-CoV-2 and Rheumatic Diseases: What Are the Potential Opportunities? Medicina 2024, 60, 1171. https://doi.org/10.3390/medicina60071171
Abisheva S, Rutskaya-Moroshan K, Nuranova G, Batyrkhan T, Abisheva A. Antimalarial Drugs at the Intersection of SARS-CoV-2 and Rheumatic Diseases: What Are the Potential Opportunities? Medicina. 2024; 60(7):1171. https://doi.org/10.3390/medicina60071171
Chicago/Turabian StyleAbisheva, Saule, Kristina Rutskaya-Moroshan, Gulnaz Nuranova, Tansholpan Batyrkhan, and Anilim Abisheva. 2024. "Antimalarial Drugs at the Intersection of SARS-CoV-2 and Rheumatic Diseases: What Are the Potential Opportunities?" Medicina 60, no. 7: 1171. https://doi.org/10.3390/medicina60071171
APA StyleAbisheva, S., Rutskaya-Moroshan, K., Nuranova, G., Batyrkhan, T., & Abisheva, A. (2024). Antimalarial Drugs at the Intersection of SARS-CoV-2 and Rheumatic Diseases: What Are the Potential Opportunities? Medicina, 60(7), 1171. https://doi.org/10.3390/medicina60071171







