Availability of Observational Pain Assessment Tools in Hospitalized Patients with Osteoporotic Vertebral Fractures
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval Statement
2.2. Participants
2.3. Assessment
2.3.1. Pain Assessment Using Self-Reported and Observational Assessment Tools
2.3.2. Assessment of Ambulatory Status and ADLs
2.3.3. Evaluation of Pain Assessment Tool Scores Using the Mini-Mental State Examination
2.4. Sample Size Determination
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
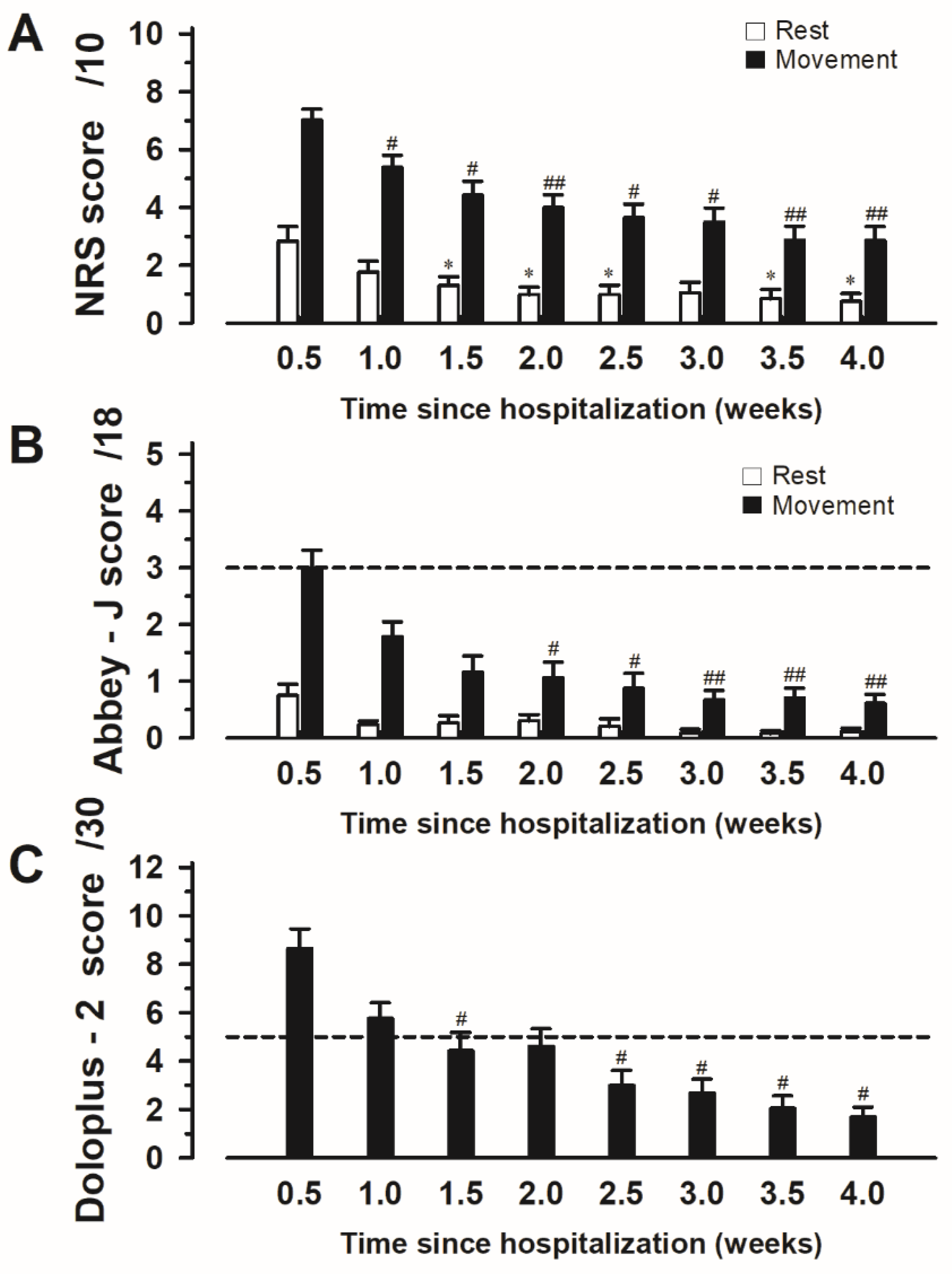
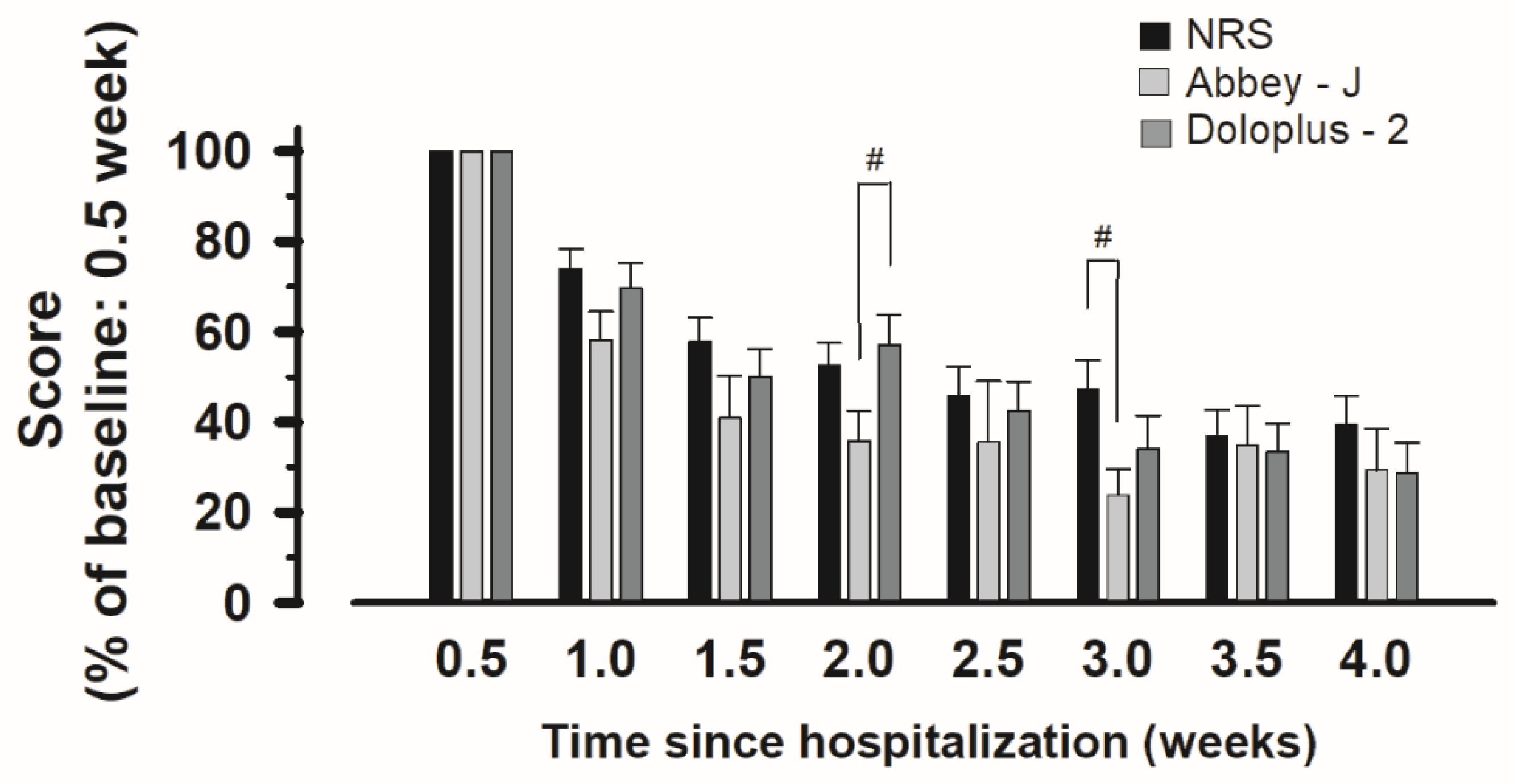
3.2. Changes in the Scores of Each Pain Assessment Tool over Time
3.3. Correlation between Pain Assessment Tools
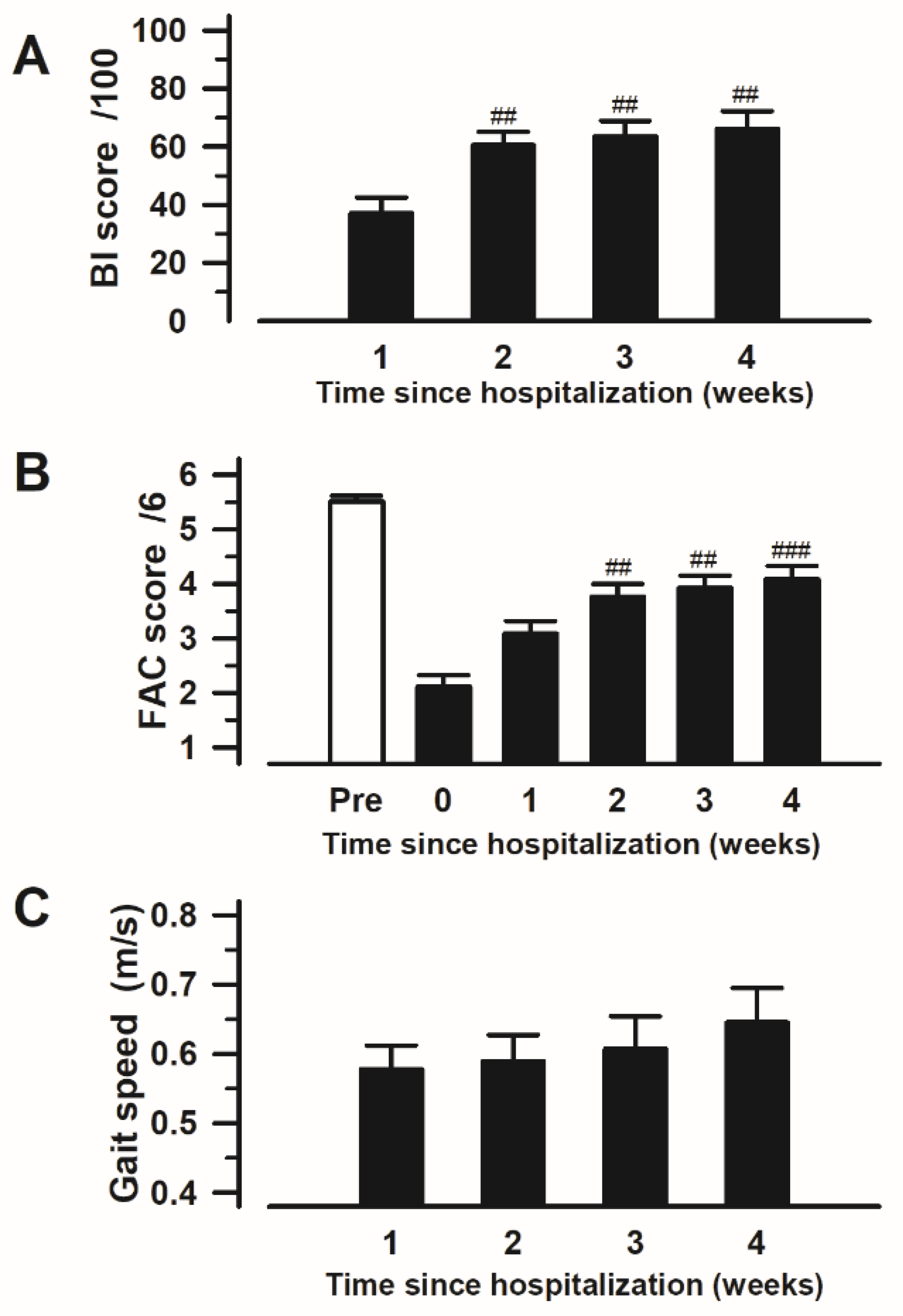
3.4. Changes in ADLs and Ambulatory Status over Time
3.5. Correlation between ADLs and Ambulatory Status
3.6. Correlation of Each Pain Assessment Tool with ADLs and Ambulatory Status
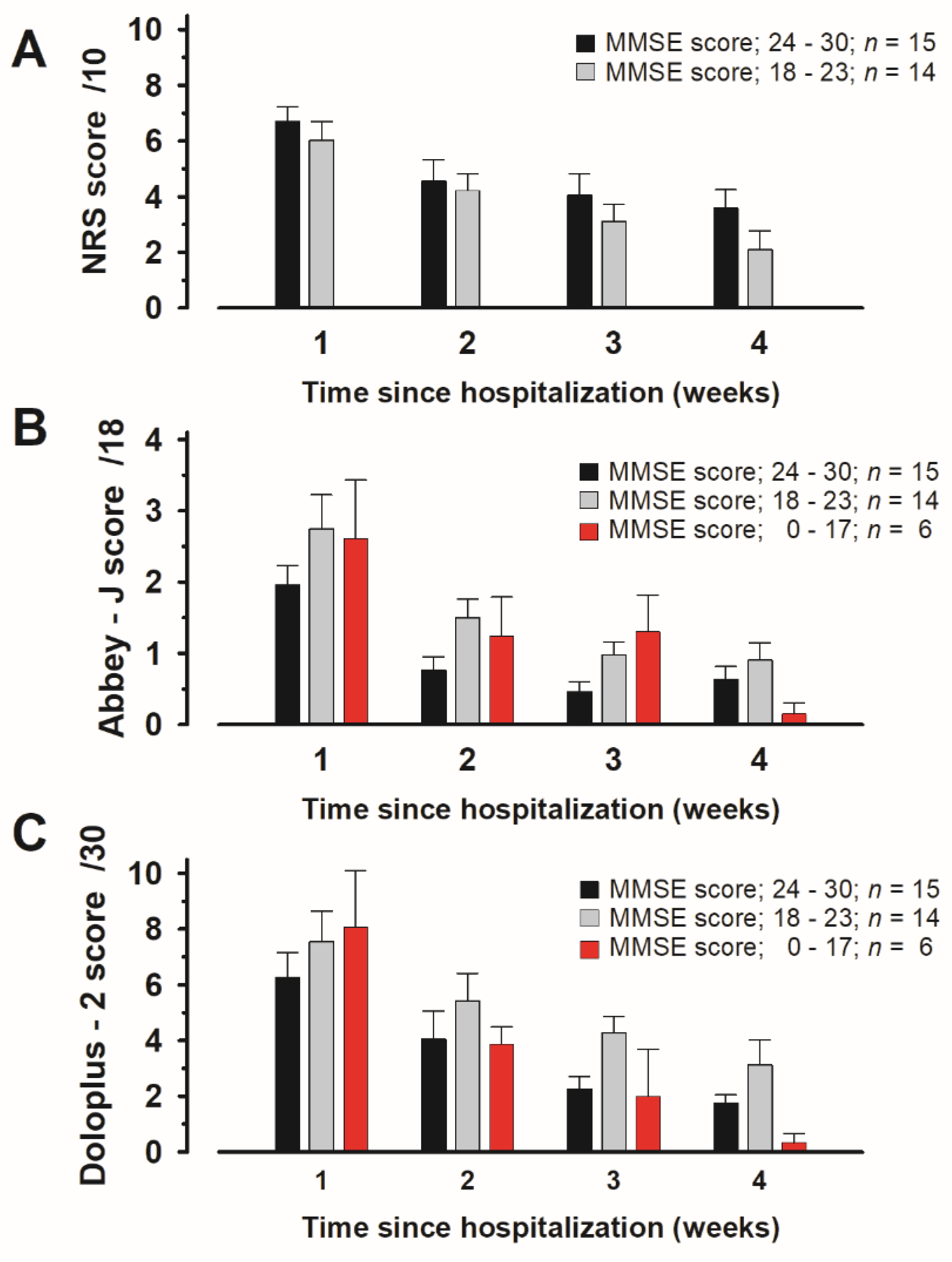
3.7. Evaluation of Scores Assigned to Items in Each Pain Assessment Tool According to MMSE Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Sari, U.A.; Tobias, J.; Clark, E. Health-related quality of life in older people with osteoporotic vertebral fractures: A systematic review and meta-analysis. Osteoporos. Int. 2016, 27, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Zhao, X.; Teoh, C.; Jaffe, D.H.; Taguchi, Y. Disease burden of fractures among patients with osteoporosis in Japan: Health-related quality of life, work productivity and activity impairment, healthcare resource utilization, and economic costs. J. Bone Miner. Metab. 2019, 37, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, I.; Rosenqvist, A.M.; Kartous, L.; Lofman, O.; Wahlstrom, O.; Toss, G. Health-related quality of life after osteoporotic fractures. Osteoporos. Int. 2004, 15, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Lyles, K.W.; Gold, D.T.; Shipp, K.M.; Pieper, C.F.; Martinez, S.; Mulhausen, P.L. Association of osteoporotic vertebral compression fractures with impaired functional status. Am. J. Med. 1993, 94, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, N.; Itoi, E.; Kobayashi, M.; Kodama, H. Impact of postural deformities and spinal mobility on quality of life in postmenopausal osteoporosis. Osteoporos. Int. 2003, 14, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K. A “super-aged” society and the “locomotive syndrome”. J. Orthop. Sci. 2008, 13, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Jonsson, B. Economic evaluation of interventions for osteoporosis. Osteoporos. Int. 2002, 13, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, T.; Okimoto, N.; Teramoto, H.; Shirakawa, T.; Nakagawa, T.; Mizuno, N.; Yamasaki, T.; Sasashige, Y.; Fujiwara, S. Incidence of clinical vertebral fractures and hip fractures of the elderly (65 years or over) population-large-scale data analysis using claim database in Kure City, Hiroshima, Japan. Arch. Osteoporos. 2020, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Hofler, R.C.; Jones, G.A. Bracing for Acute and Subacute Osteoporotic Compression Fractures: A Systematic Review of the Literature. World Neurosurg. 2020, 141, e453–e460. [Google Scholar] [CrossRef]
- Longo, U.G.; Loppini, M.; Denaro, L.; Maffulli, N.; Denaro, V. Conservative management of patients with an osteoporotic vertebral fracture: A review of the literature. J. Bone Jt. Surg. 2012, 94, 152–157. [Google Scholar] [CrossRef]
- Closs, S.J.; Barr, B.; Briggs, M.; Cash, K.; Seers, K. A comparison of five pain assessment scales for nursing home residents with varying degrees of cognitive impairment. J. Pain Symptom Manag. 2004, 27, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Stafseth, S.K. Documentation for Assessing Pain in Postoperative Pain Management Pre- and Post-intervention. J. Perianesthesia Nurs. 2023, 38, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Fry, M.; Arendts, G.; Chenoweth, L. Emergency nurses’ evaluation of observational pain assessment tools for older people with cognitive impairment. J. Clin. Nurs. 2017, 26, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Park, J.M.; Moon, Y.S.; Han, S.H. Assessment of pain in the elderly: A literature review. Natl. Med. J. India 2017, 30, 203–207. [Google Scholar] [PubMed]
- Warnky, D.M.; Diebolt, J.H.; Ho, B.V.; Brake, A.D.; French, E.L.; Villwock, M.R.; Sykes, K.J.; Villwock, J.A. Investigation of a Novel Activity-Based Checks (ABC) Functional Pain Scale in the Post-Operative Urologic Surgery Patient. Kans. J. Med. 2023, 16, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Herr, K.; Coyne, P.J.; Ely, E.; Gelinas, C.; Manworren, R.C.B. Pain Assessment in the Patient Unable to Self-Report: Clinical Practice Recommendations in Support of the ASPMN 2019 Position Statement. Pain Manag. Nurs. 2019, 20, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Wynne, C.F.; Ling, S.M.; Remsburg, R. Comparison of pain assessment instruments in cognitively intact and cognitively impaired nursing home residents. Geriatr. Nurs. 2000, 21, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Barzanji, A.; Zareiyan, A.; Nezamzadeh, M.; Mazhari, M.S. Evaluation of Observational and Behavioural Pain Assessment Tools in Nonverbal Intubated Critically Adult Patients after Open—Heart Surgery: A Systematic Review. Open Access Maced. J. Med. Sci. 2019, 7, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, S.; O’Conner-Von, S.; Tracy, M.F. Survey of Nurses’ Use of the Clinically Aligned Pain Assessment (CAPA) Tool. Pain Manag. Nurs. 2022, 23, 568–575. [Google Scholar] [CrossRef]
- Hughes, J.D.; Chivers, P.; Hoti, K. The Clinical Suitability of an Artificial Intelligence-Enabled Pain Assessment Tool for Use in Infants: Feasibility and Usability Evaluation Study. J. Med. Internet Res. 2023, 25, e41992. [Google Scholar] [CrossRef]
- Lichtner, V.; Dowding, D.; Esterhuizen, P.; Closs, S.J.; Long, A.F.; Corbett, A.; Briggs, M. Pain assessment for people with dementia: A systematic review of systematic reviews of pain assessment tools. BMC Geriatr. 2014, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Lukas, A.; Niederecker, T.; Gunther, I.; Mayer, B.; Nikolaus, T. Self- and proxy report for the assessment of pain in patients with and without cognitive impairment: Experiences gained in a geriatric hospital. Z. Gerontol. Geriatr. 2013, 46, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Ngu, S.S.; Tan, M.P.; Subramanian, P.; Abdul Rahman, R.; Kamaruzzaman, S.; Chin, A.V.; Tan, K.M.; Poi, P.J. Pain Assessment Using Self-reported, Nurse-reported, and Observational Pain Assessment Tools among Older Individuals with Cognitive Impairment. Pain Manag. Nurs. 2015, 16, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Hartrick, C.T.; Kovan, J.P.; Shapiro, S. The numeric rating scale for clinical pain measurement: A ratio measure? Pain Pract. Off. J. World Inst. Pain 2003, 3, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Yamamoto-Mitani, N.; Chiba, Y.; Nishikawa, Y.; Hayashi, K.; Sugai, Y. Abbey Pain Scale: Development and validation of the Japanese version. Geriatr. Gerontol. Int. 2010, 10, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wary, B.; Doloplus, C. Doloplus-2, a scale for pain measurement. Soins Gerontol. 1999, 19, 25–27. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G. Measures of reliability in sports medicine and science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hjermstad, M.J.; Gibbins, J.; Haugen, D.F.; Caraceni, A.; Loge, J.H.; Kaasa, S.; Epcrc, E.P.C.R.C. Pain assessment tools in palliative care: An urgent need for consensus. Palliat. Med. 2008, 22, 895–903. [Google Scholar] [CrossRef]
- Hadjistavropoulos, T.; Herr, K.; Prkachin, K.M.; Craig, K.D.; Gibson, S.J.; Lukas, A.; Smith, J.H. Pain assessment in elderly adults with dementia. Lancet. Neurol. 2014, 13, 1216–1227. [Google Scholar] [CrossRef]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of four pain intensity rating scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef] [PubMed]
- Turk, D.C.; Melzack, R. Handbook of Pain Assessment, 3rd ed.; Guilford Press: New York, NY, USA, 2011. [Google Scholar]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S.; European Palliative Care Research, C. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: A systematic literature review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef] [PubMed]
- Chanques, G.; Viel, E.; Constantin, J.M.; Jung, B.; de Lattre, S.; Carr, J.; Cisse, M.; Lefrant, J.Y.; Jaber, S. The measurement of pain in intensive care unit: Comparison of 5 self-report intensity scales. Pain 2010, 151, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Abbey, J.; Piller, N.; De Bellis, A.; Esterman, A.; Parker, D.; Giles, L.; Lowcay, B. The Abbey pain scale: A 1-minute numerical indicator for people with end-stage dementia. Int. J. Palliat. Nurs. 2004, 10, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Krulewitch, H.; London, M.R.; Skakel, V.J.; Lundstedt, G.J.; Thomason, H.; Brummel-Smith, K. Assessment of pain in cognitively impaired older adults: A comparison of pain assessment tools and their use by nonprofessional caregivers. J. Am. Geriatr. Soc. 2000, 48, 1607–1611. [Google Scholar] [CrossRef]
- Vitullo, M.; Holloway, D.; Tellson, A.; Nguyen, H.; Estimon, K.; Linthicum, J.; Viejo, H.; Coffee, A.; Huddleston, P. *Surgical patients’ and registered nurses’ satisfaction and Perception of Using the Clinically Aligned Pain Assessment (CAPA(c)) Tool for Pain Assessment. J. Vasc. Nurs. 2020, 38, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Atee, M.; Hoti, K.; Parsons, R.; Hughes, J.D. A novel pain assessment tool incorporating automated facial analysis: Interrater reliability in advanced dementia. Clin. Interv. Aging 2018, 13, 1245–1258. [Google Scholar] [CrossRef]
- Ando, C.; Ito, Y.; Amemiya, S.; Tamura, K.; Kako, K.; Tsuzura, S.; Yoshida, R.; Hishinuma, M. Effectiveness of the Japanese DOLOPLUS-2: A pain assessment scale for patients with moderate-to-severe dementia. Psychogeriatr. Off. J. Jpn. Psychogeriatr. Soc. 2016, 16, 315–322. [Google Scholar] [CrossRef]
| Characteristic | Value |
|---|---|
| Age (year) | 84.4 ± 6.65 |
| Sex; male: female | 4(11.4%): 31 (88.6%) |
| Height (cm) | 147.3 ± 7.97 |
| Weight (kg) | 46.3 ± 9.46 |
| Body mass index (kg/m2) | 21.3 ± 4.02 |
| Fracture (number) (multiple fractute included) | T7 (1), T8 (2), T9 (1), T10 (2), T11 (2), T12 (12), L1 (6), L2 (7), L3 (3), L5 (1) |
| 2 Weeks | NRS | Abbey | Doloplus-2 | BI | FAC |
|---|---|---|---|---|---|
| Abbey | 0.476 ### | ||||
| Doloplus-2 | 0.663 ### | 0.587 ### | |||
| BI | −0.101 | −0.542 ### | −0.258 | ||
| FAC | −0.101 | −0.406 # | −0.299 | 0.796 ### | |
| Gait Speed | −0.461 # | −0.372 | −0.537 ## | 0.576 ## | 0.608 ### |
| 3 Weeks | NRS | Abbey | Doloplus-2 | BI | FAC |
| Abbey | 0.608 ## | ||||
| Doloplus-2 | 0.484 # | 0.585 ## | |||
| BI | −0.272 | −0.44 # | −0.443 # | ||
| FAC | −0.244 | −0.359 | −0.371 | 0.816 ### | |
| Gait Speed | −0.245 | −0.386 | −0.585 ### | 0.653 ### | 0.605 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimi, Y.; Matsuura, T.; Miyazato, K.; Takahashi, S.; Tanaka, N.; Morinaga, H.; Hayata, A.; Onishi, M.; Nagano, Y.; Ohnishi, H. Availability of Observational Pain Assessment Tools in Hospitalized Patients with Osteoporotic Vertebral Fractures. Medicina 2024, 60, 1217. https://doi.org/10.3390/medicina60081217
Yoshimi Y, Matsuura T, Miyazato K, Takahashi S, Tanaka N, Morinaga H, Hayata A, Onishi M, Nagano Y, Ohnishi H. Availability of Observational Pain Assessment Tools in Hospitalized Patients with Osteoporotic Vertebral Fractures. Medicina. 2024; 60(8):1217. https://doi.org/10.3390/medicina60081217
Chicago/Turabian StyleYoshimi, Youhei, Takanori Matsuura, Kazuaki Miyazato, Shiho Takahashi, Nami Tanaka, Hanae Morinaga, Asuka Hayata, Minami Onishi, Yousuke Nagano, and Hideo Ohnishi. 2024. "Availability of Observational Pain Assessment Tools in Hospitalized Patients with Osteoporotic Vertebral Fractures" Medicina 60, no. 8: 1217. https://doi.org/10.3390/medicina60081217
APA StyleYoshimi, Y., Matsuura, T., Miyazato, K., Takahashi, S., Tanaka, N., Morinaga, H., Hayata, A., Onishi, M., Nagano, Y., & Ohnishi, H. (2024). Availability of Observational Pain Assessment Tools in Hospitalized Patients with Osteoporotic Vertebral Fractures. Medicina, 60(8), 1217. https://doi.org/10.3390/medicina60081217







