Obesity and Colorectal Cancer: A Narrative Review
Abstract
1. Introduction
1.1. Background
1.2. Rationale and Knowledge Gap
1.3. Objective
2. Methods
3. Results
3.1. Included Publications
3.2. Obesity and Incidence of Colorectal Cancer
3.3. Obesity and Colorectal Cancer Prognosis
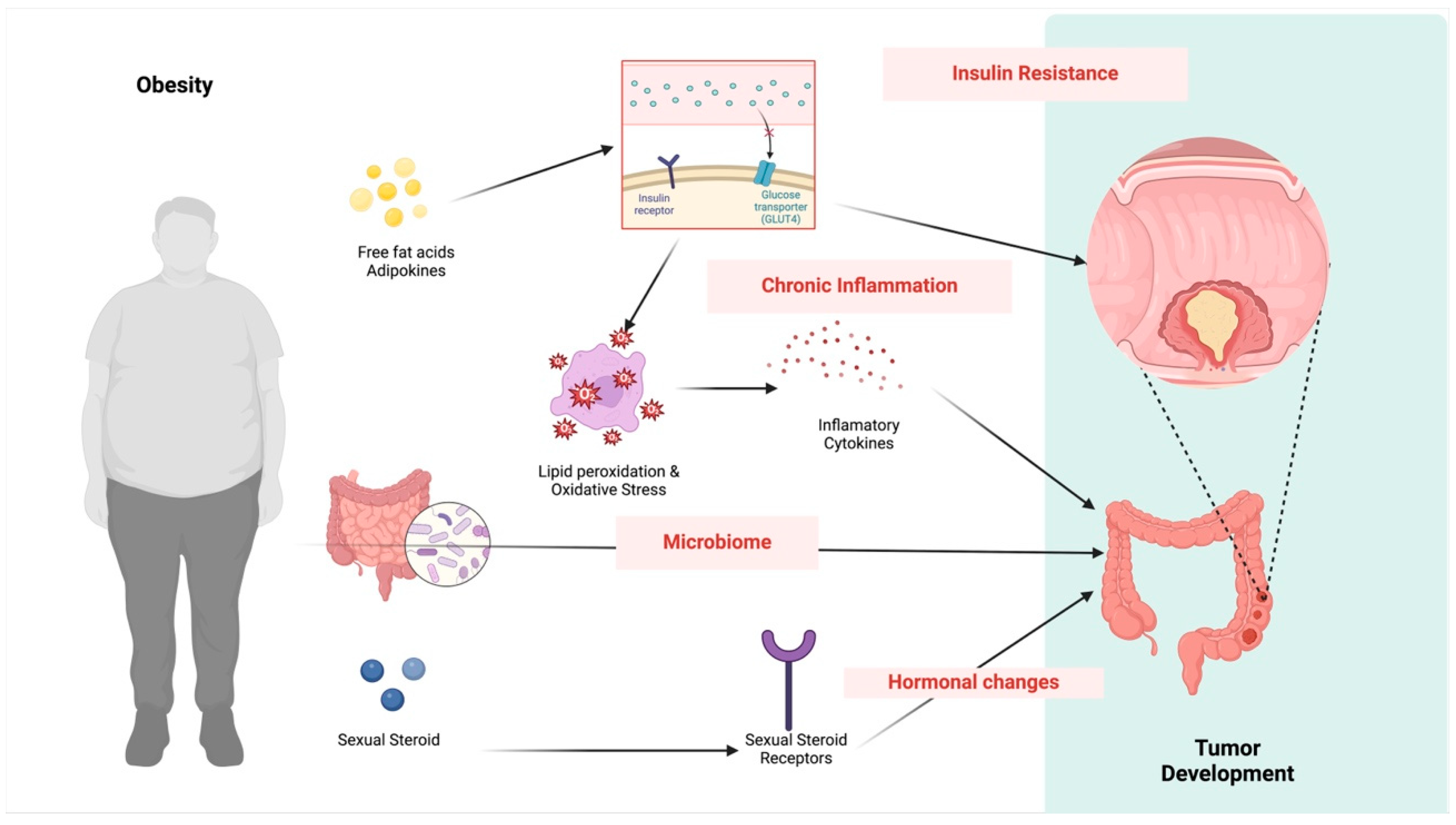
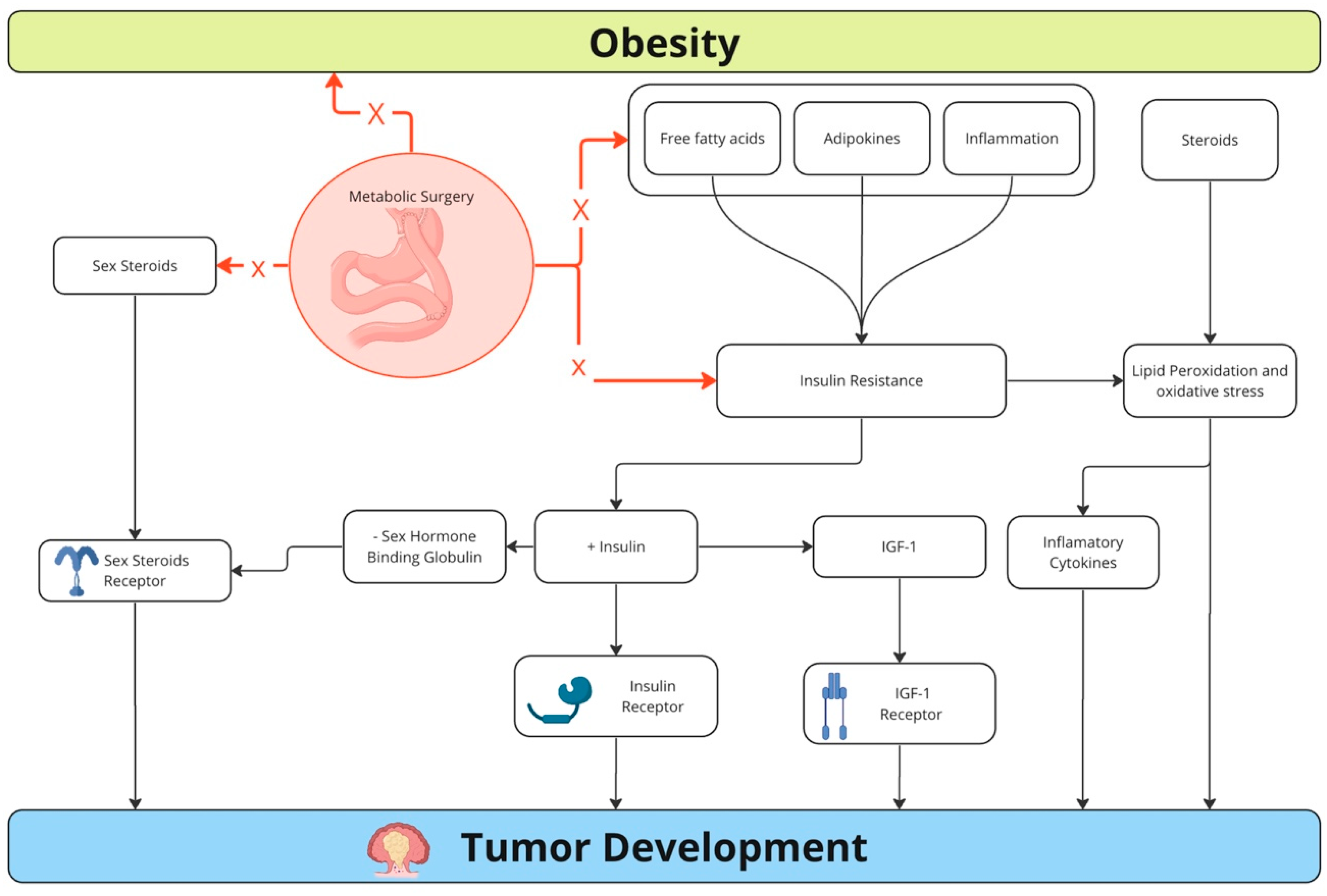
3.4. Mechanisms for the Association between Colorectal Cancer and Obesity
3.5. Challenges of Cancer Therapy in Patients with Obesity
3.6. Obesity Treatment and Reduction in Colorectal Cancer Incidence
3.6.1. Diet Therapy
3.6.2. Physical Exercises
3.6.3. Medical Therapy
3.6.4. Bariatric Surgery
3.7. Treating Patients with Obesity and Colorectal Cancer
3.8. Role of Future Studies
4. Conclusions
Funding
Conflicts of Interest
References
- Francisco, P.M.S.B.; Friestino, J.K.O.; Ferraz, R.O.; Bacurau, A.G.M.; Stopa, S.R.; Moreira Filho, D.C. Prevalence of diagnosis and types of cancer in the elderly: Data from National Health Survey 2013. Rev. Bras. Geriatr. Gerontol. 2020, 23, e200023. [Google Scholar] [CrossRef]
- Estimativa 2023: Incidência de câncer no Brasil; INCA (Instituto Nacional de Câncer José Alencar Gomes da Silva): Rio de Janeiro, Brazil, 2022.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Zhou, E.; Rifkin, S. Colorectal Cancer and Diet: Risk Versus Prevention, Is Diet an Intervention? Gastroenterol. Clin. N. Am. 2021, 50, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, A. The genesis and growth of tumors. II. Effects of caloric restriction per se. Cancer Res. 1942, 2, 460–467. [Google Scholar]
- Ashrafian, H.; Ahmed, K.; Rowland, S.P.; Patel, V.M.; Gooderham, N.J.; Holmes, E.; Darzi, A.; Athanasiou, T. Metabolic surgery and cancer: Protective effects of bariatric procedures. Cancer 2011, 117, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, H.; Markar, S.R.; Askari, A.; Faiz, O.; Hull, M.; Purkayastha, S.; Møller, H.; Lagergren, J. Obesity surgery and risk of cancer. Br. J. Surg. 2018, 105, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, Y.; Wang, F.; Zhang, P.; Shi, C.; Zou, Y.; Qin, H. Obesity and risk of colorectal cancer: A systematic review of prospective studies. PLoS ONE 2013, 8, e53916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ezzati, M.; Lopez, A.D.; Rodgers, A.; Vander Hoorn, S.; Murray, C.J. Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet 2002, 360, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Meyerhardt, J.A.; Catalano, P.J.; Haller, D.G.; Mayer, R.J.; Benson, A.B.; Macdonald, J.S.; Fuchs, C.S. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer 2003, 98, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Popovici, D.; Stanisav, C.; Saftescu, S.; Negru, S.; Dragomir, R.; Ciurescu, D.; Diaconescu, R. Exploring the Influence of Age, Gender and Body Mass Index on Colorectal Cancer Location. Medicina 2023, 59, 1399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, Y.; Kartsonaki, C.; Guo, Y.; Chen, Y.; Yang, L.; Bian, Z.; Bragg, F.; Millwood, I.Y.; Mao, E.; Li, Y.; et al. Adiposity and risks of colorectal and small intestine cancer in Chinese adults: A prospective study of 0.5 million people. Br. J. Cancer 2018, 119, 248–250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Li, C.; Wu, G.; Yang, W.; Wang, X.; Duan, L.; Niu, L.; Chen, J.; Zhang, Y.; Zhou, W.; et al. The obesity paradox in patients with colorectal cancer: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Stocks, T.; Lukanova, A.; Bjørge, T.; Ulmer, H.; Manjer, J.; Almquist, M.; Concin, H.; Engeland, A.; Hallmans, G.; Nagel, G.; et al. Metabolic Syndrome Cancer Project Me-Can Group. Metabolic factors and the risk of colorectal cancer in 580,000 men and women in the metabolic syndrome and cancer project (Me-Can). Cancer 2011, 117, 2398–2407. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Ducreux, M.; Faroux, R.; Barbier, E.; Manfredi, S.; Lecomte, T.; Etienne, P.L.; Bedenne, L.; Bennouna, J.; Phelip, J.M.; et al. Overweight is associated to a better prognosis in metastatic colorectal cancer: A pooled analysis of FFCD trials. Eur. J. Cancer 2018, 98, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Meyerhardt, J.A.; Giovannucci, E.; Jeon, J.Y. Association between body mass index and prognosis of colorectal cancer: A meta-analysis of prospective cohort studies. PLoS ONE 2015, 10, e0120706, Erratum in PLoS ONE 2016, 11, e0147456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrer, M.; Mourikis, N.; Davidson, E.E.; Kleeman, S.O.; Zaccaria, M.; Habel, J.; Rubino, R.; Flint, T.R.; Connell, C.M.; Lukey, M.J.; et al. Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia. bioRxiv 2023. Update in Cell Metab. 2023, 35, 1147–1162.e7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, S.; Brown, J.L.; Washington, T.A.; Greene, N.P. Development and progression of cancer cachexia: Perspectives from bench to bedside. Sports Med. Health Sci. 2020, 2, 177–185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines. 2023, 11, 1290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, I.; Shaker, M.E.; Mehal, W.Z. Therapeutic Opportunities in Damage-Associated Molecular Pattern-Driven Metabolic Diseases. Antioxid. Redox Signal. 2015, 23, 1305–1315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fain, J.N.; Leffler, C.W.; Cowan GSJr Buffington, C.; Pouncey, L.; Bahouth, S.W. Stimulation of leptin release by arachidonic acid and prostaglandin E(2) in adipose tissue from obese humans. Metabolism 2001, 50, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.C.; Hsiao, F.C.; Chang, H.M.; Wabitsch, M.; Hsieh, P.S. Importance of adipocyte cyclooxygenase-2 and prostaglandin E2-prostaglandin E receptor 3 signaling in the development of obesity-induced adipose tissue inflammation and insulin resistance. FASEB J. 2016, 30, 2282–2297. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, S.; Trivedi, D.B.; Graf, G.A.; Loftin, C.D. Cyclooxygenase-2 deficiency attenuates adipose tissue differentiation and inflammation in mice. J. Biol. Chem. 2011, 286, 889–898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, X.; Nishimura, K.; Jisaka, M.; Nagaya, T.; Shono, F.; Yokota, K. Up-regulation of adipogenesis in adipocytes expressing stably cyclooxygenase-2 in the antisense direction. Prostaglandins Other Lipid Mediat. 2010, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tustumi, F.; de Moura, D.T.H.; Waisberg, J.; Herbella, F.A.M. Editorial: Premalignant conditions in the esophagus and stomach. Front. Oncol. 2022, 12, 1091911. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Rahilly, S. 20 years of leptin: What we know and what the future holds. J. Endocrinol. 2014, 223, E1–E3. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Magkos, F.; Brinkoetter, M.; Sienkiewicz, E.; Dardeno, T.A.; Kim, S.Y.; Hamnvik, O.P.; Koniaris, A. Leptin in human physiology and pathophysiology. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E567–E584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duan, X.F.; Tang, P.; Li, Q.; Yu, Z.T. Obesity, adipokines and hepatocellular carcinoma. Int. J. Cancer 2013, 133, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Xu, X.; Du, L.; Yang, Y.; Cheng, H.; Zhang, X.; Li, Z.; Wang, L.; Li, J.; Liu, H.; et al. Leptin-mediated regulation of MT1-MMP localization is KIF1B dependent and enhances gastric cancer cell invasion. Carcinogenesis 2013, 34, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Stępień, S.; Olczyk, P.; Gola, J.; Komosińska-Vassev, K.; Mielczarek-Palacz, A. The Role of Selected Adipocytokines in Ovarian Cancer and Endometrial Cancer. Cells 2023, 12, 1118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghasemi, A.; Hashemy, S.I.; Aghaei, M.; Panjehpour, M. Leptin induces matrix metalloproteinase 7 expression to promote ovarian cancer cell invasion by activating ERK and JNK pathways. J. Cell Biochem. 2018, 119, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Wang, F.Y.; Kuo, Y.H.; Tang, F.Y. Cancer chemopreventive effects of lycopene: Suppression of MMP-7 expression and cell invasion in human colon cancer cells. J. Agric. Food Chem. 2011, 59, 11304–11318. [Google Scholar] [CrossRef] [PubMed]
- Màrmol, J.M.; Carlsson, M.; Raun, S.H.; Grand, M.K.; Sørensen, J.; Lang Lehrskov, L.; Richter, E.A.; Norgaard, O.; Sylow, L. Insulin resistance in patients with cancer: A systematic review and meta-analysis. Acta Oncol. 2023, 62, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Insulin Resistance: The Increased Risk of Cancers. Curr. Oncol. 2024, 31, 998–1027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Q.; Li, Z.; Luo, T.; Shi, H. Targeting the PI3K/AKT/mTOR and RAF/MEK/ERK pathways for cancer therapy. Mol. Biomed. 2022, 3, 47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Frystyk, J.; Flyvbjerg, A. Obesity and cancer risk: The role of the insulin-IGF axis. Trends Endocrinol. Metab. 2006, 17, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Stefani, C.; Miricescu, D.; Stanescu-Spinu, I.I.; Nica, R.I.; Greabu, M.; Totan, A.R.; Jinga, M. Growth Factors, PI3K/AKT/mTOR and MAPK Signaling Pathways in Colorectal Cancer Pathogenesis: Where Are We Now? Int. J. Mol. Sci. 2021, 22, 10260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diaz-Arjonilla, M.; Schwarcz, M.; Swerdloff, R.S.; Wang, C. Obesity, low testosterone levels and erectile dysfunction. Int. J. Impot. Res. 2009, 21, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, C.H.; Kim, N.; Nam, R.H.; Choi, S.I.; Yu, J.E.; Nho, H.; Shin, E.; Lee, H.N.; Surh, Y.J. Testosterone strongly enhances azoxymethane/dextran sulfate sodium-induced colorectal cancer development in C57BL/6 mice. Am. J. Cancer Res. 2021, 11, 3145–3162. [Google Scholar] [PubMed] [PubMed Central]
- Dignam, J.J.; Polite, B.N.; Yothers, G.; Raich, P.; Colangelo, L.; O’Connell, M.J.; Wolmark, N. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J. Natl. Cancer Inst. 2006, 98, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ağagündüz, D.; Cocozza, E.; Cemali, Ö.; Bayazıt, A.D.; Nanì, M.F.; Cerqua, I.; Morgillo, F.; Saygılı, S.K.; Berni Canani, R.; Amero, P.; et al. Understanding the role of the gut microbiome in gastrointestinal cancer: A review. Front. Pharmacol. 2023, 14, 1130562. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poglio, S.; Galvani, S.; Bour, S.; André, M.; Prunet-Marcassus, B.; Pénicaud, L.; Casteilla, L.; Cousin, B. Adipose tissue sensitivity to radiation exposure. Am. J. Pathol. 2009, 174, 44–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lyman, G.H.; Sparreboom, A. Chemotherapy dosing in overweight and obese patients with cancer. Nat. Rev. Clin. Oncol. 2013, 10, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Lashinger, L.M.; Rossi, E.L.; Hursting, S.D. Obesity and Resistance to Cancer Chemotherapy: Interacting Roles of Inflammation and Metabolic Dysregulation. Clin. Pharmacol. Ther. 2014, 96, 458–463. [Google Scholar] [CrossRef]
- Khan, M.T.; Nieuwdorp, M.; Bäckhed, F. Microbial modulation of insulin sensitivity. Cell Metab. 2014, 20, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.J.; Chen, L.M.; Gu, M.E.; Xu, H.X.; Li, J.; Wu, L.Y. Body mass index-based predictions and personalized clinical strategies for colorectal cancer in the context of PPPM. EPMA J. 2022, 13, 615–632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, R.; Grimm, S.A.; Chrysovergis, K.; Kosak, J.; Wang, X.; Du, Y.; Burkholder, A.; Janardhan, K.; Mav, D.; Shah, R.; et al. Obesity, rather than diet, drives epigenomic alterations in colonic epithelium resembling cancer progression. Cell Metab. 2014, 19, 702–711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koutoukidis, D.A.; Jebb, S.A.; Foster, C.; Wheatstone, P.; Horne, A.; Hill, T.M.; Taylor, A.; Realpe, A.; Achana, F.; Buczacki, S.J. CARE: Protocol of a randomised trial evaluating the feasibility of preoperative intentional weight loss to support postoperative recovery in patients with excess weight and colorectal cancer. Colorectal Dis. 2023, 25, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, E.R.; Fenger, M.; Henriksen, T.; Kjaer, L.K.; Worm, D.; Hansen, D.L.; Madsbad, S.; Poulsen, H.E. Reduction of oxidative stress on DNA and RNA in obese patients after Roux-en-Y gastric bypass surgery-An observational cohort study of changes in urinary markers. PLoS ONE 2020, 15, e0243918. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rychter, A.M.; Łykowska-Szuber, L.; Zawada, A.; Szymczak-Tomczak, A.; Ratajczak, A.E.; Skoracka, K.; Kolan, M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Why Does Obesity as an Inflammatory Condition Predispose to Colorectal Cancer? J. Clin. Med. 2023, 12, 2451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehta, R.S.; Song, M.; Nishihara, R.; Drew, D.A.; Wu, K.; Qian, Z.R.; Fung, T.T.; Hamada, T.; Masugi, Y.; da Silva, A.; et al. Dietary Patterns and Risk of Colorectal Cancer: Analysis by Tumor Location and Molecular Subtypes. Gastroenterology 2017, 152, 1944–1953. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwak, M.; Mehaffey, J.H.; Hawkins, R.B.; Hedrick, T.L.; Slingluff, C.L., Jr.; Schirmer, B.; Hallowell, P.T.; Friel, C.M. Bariatric surgery is independently associated with a decrease in the development of colorectal lesions. Surgery 2019, 166, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Moțățăianu, A.; Șerban, G.; Andone, S. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Cross-Talk with a Focus on Amyotrophic Lateral Sclerosis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 15094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guiu, B.; Petit, J.M.; Bonnetain, F.; Ladoire, S.; Guiu, S.; Cercueil, J.P.; Krausé, D.; Hillon, P.; Borg, C.; Chauffert, B.; et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut 2010, 59, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Fontana, L. Calorie restriction and cancer prevention: Metabolic and molecular mechanisms. Trends Pharmacol. Sci. 2010, 31, 89–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vidoni, C.; Ferraresi, A.; Esposito, A.; Maheshwari, C.; Dhanasekaran, D.N.; Mollace, V.; Isidoro, C. Calorie Restriction for Cancer Prevention and Therapy: Mechanisms, Expectations, and Efficacy. J. Cancer Prev. 2021, 26, 224–236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cangemi, A.; Fanale, D.; Rinaldi, G.; Bazan, V.; Galvano, A.; Perez, A.; Barraco, N.; Massihnia, D.; Castiglia, M.; Vieni, S.; et al. Dietary restriction: Could it be considered as speed bump on tumor progression road? Tumour Biol. 2016, 37, 7109–7118. [Google Scholar] [CrossRef] [PubMed]
- Bagherniya, M.; Butler, A.E.; Barreto, G.E.; Sahebkar, A. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res. Rev. 2018, 47, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhu, X.; Wang, H.; Wang, F.; Guan, W. Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: A systematic review and meta-analysis. PLoS ONE 2014, 9, e115147. [Google Scholar] [CrossRef]
- Mittelman, S.D. The Role of Diet in Cancer Prevention and Chemotherapy Efficacy. Annu. Rev. Nutr. 2020, 40, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Slattery, M.L. Physical activity and colorectal cancer. Sports Med. 2004, 34, 239–252. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Yasui, Y.; Sorensen, B.; Irwin, M.L.; Morgan, A.; Rudolph, R.E.; Surawicz, C.; Lampe, J.W.; Ayub, K.; Potter, J.D.; et al. Effect of a 12-month exercise intervention on patterns of cellular proliferation in colonic crypts: A randomized controlled trial. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Rines, A.K.; Sharabi, K.; Tavares, C.D.; Puigserver, P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat. Rev. Drug Discov. 2016, 15, 786–804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calderon, G.; Gonzalez-Izundegui, D.; Shan, K.L.; Garcia-Valencia, O.A.; Cifuentes, L.; Campos, A.; Collazo-Clavell, M.L.; Shah, M.; Hurley, D.L.; Abu Lebdeh, H.S.; et al. Effectiveness of anti-obesity medications approved for long-term use in a multidisciplinary weight management program: A multi-center clinical experience. Int. J. Obes. 2022, 46, 555–563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bailly, L.; Fabre, R.; Pradier, C.; Iannelli, A. Colorectal Cancer Risk Following Bariatric Surgery in a Nationwide Study of French Individuals With Obesity. JAMA Surg. 2020, 155, 395–402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Abreu Sesconetto, L.; da Silva, R.B.R.; Galletti, R.P.; Agareno, G.A.; Colonno, B.B.; de Sousa, J.H.B.; Tustumi, F. Scores for Predicting Diabetes Remission in Bariatric Surgery: A Systematic Review and Meta-analysis. Obes. Surg. 2023, 33, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B.; O’Brien, P.E.; Playfair, J.; Chapman, L.; Schachter, L.M.; Skinner, S.; Proietto, J.; Bailey, M.; Anderson, M. Adjustable gastric banding and conventional therapy for type 2 diabetes: A randomized controlled trial. JAMA 2008, 299, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A.; Toth, C.; Hoffmeister, M.; Roth, W.; Herpel, E.; Schirmacher, P.; Brenner, H.; Chang-Claude, J. Colorectal cancer risk associated with hormone use varies by expression of estrogen receptor-β. Cancer Res. 2013, 73, 3306–3315. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Gan, Y.; Shen, Y.; Cai, X.; Song, Y.; Zhao, F.; Yao, M.; Gu, J.; Tu, H. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget 2015, 6, 16120–16134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Himbert, C.; Ose, J.; Gigic, B.; Viskochil, R.; Santuci, K.; Lin, T.; Ashworth, A.; Cohan, J.N.; Scaife, C.L.; Jedrzkiewicz, J.; et al. Associations of combined physical activity and body mass index groups with colorectal cancer survival outcomes. BMC Cancer 2023, 23, 300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Blarigan, E.L.; Meyerhardt, J.A. Role of physical activity and diet after colorectal cancer diagnosis. J. Clin. Oncol. 2015, 33, 1825–1834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakagawa, H.; Tanaka, K.; Sasai, H.; Nishizawa, Y. Providing Weight Loss Support to Patients Who Are Obese in Preparation for Colorectal Cancer Surgery to Reduce Surgical Site Infection Risk: A Mixed-methods Study. Wound Manag. Prev. 2020, 66, 23–32. [Google Scholar] [CrossRef] [PubMed]
| Items | Specification |
|---|---|
| Date of search | Last search was conducted on 29 February 2024. |
| Databases and other sources searched | The search encompassed databases such as PubMed, Embase, Lilacs/BVS, Cochrane Central, and Google Scholar. |
| Search terms used | Search terms included “cancer”, “neoplasm”, “tumor”, “oncogenesis”, “oncology”, “obesity”, “obese”, “overweight”, “insulin resistance”, “metabolic syndrome”, “colorectal”, “colonic”, “colon”, “rectal”, and “rectum”. |
| Timeframe | Articles were considered for inclusion from inception of these databases until February 2024. |
| Inclusion and exclusion criteria | Only English and Portuguese studies were considered for inclusion. The review encompassed observational and experimental studies, including human studies, in vivo and in vitro studies, and animal models. |
| Selection process | A non-systematic study selection was independently conducted by two authors (B.C.J.M. and E.T.N.). Any disagreement regarding inclusion was solved by a third experienced author (F.T.). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, B.C.J.; Tustumi, F.; Nakamura, E.T.; Shimanoe, V.H.; Kikawa, D.; Waisberg, J. Obesity and Colorectal Cancer: A Narrative Review. Medicina 2024, 60, 1218. https://doi.org/10.3390/medicina60081218
Miranda BCJ, Tustumi F, Nakamura ET, Shimanoe VH, Kikawa D, Waisberg J. Obesity and Colorectal Cancer: A Narrative Review. Medicina. 2024; 60(8):1218. https://doi.org/10.3390/medicina60081218
Chicago/Turabian StyleMiranda, Bárbara Cristina Jardim, Francisco Tustumi, Eric Toshiyuki Nakamura, Victor Haruo Shimanoe, Daniel Kikawa, and Jaques Waisberg. 2024. "Obesity and Colorectal Cancer: A Narrative Review" Medicina 60, no. 8: 1218. https://doi.org/10.3390/medicina60081218
APA StyleMiranda, B. C. J., Tustumi, F., Nakamura, E. T., Shimanoe, V. H., Kikawa, D., & Waisberg, J. (2024). Obesity and Colorectal Cancer: A Narrative Review. Medicina, 60(8), 1218. https://doi.org/10.3390/medicina60081218







