The Influence of Circulating Immune Cell and CA125 Dynamics on Neoadjuvant Therapy Selection for Advanced Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
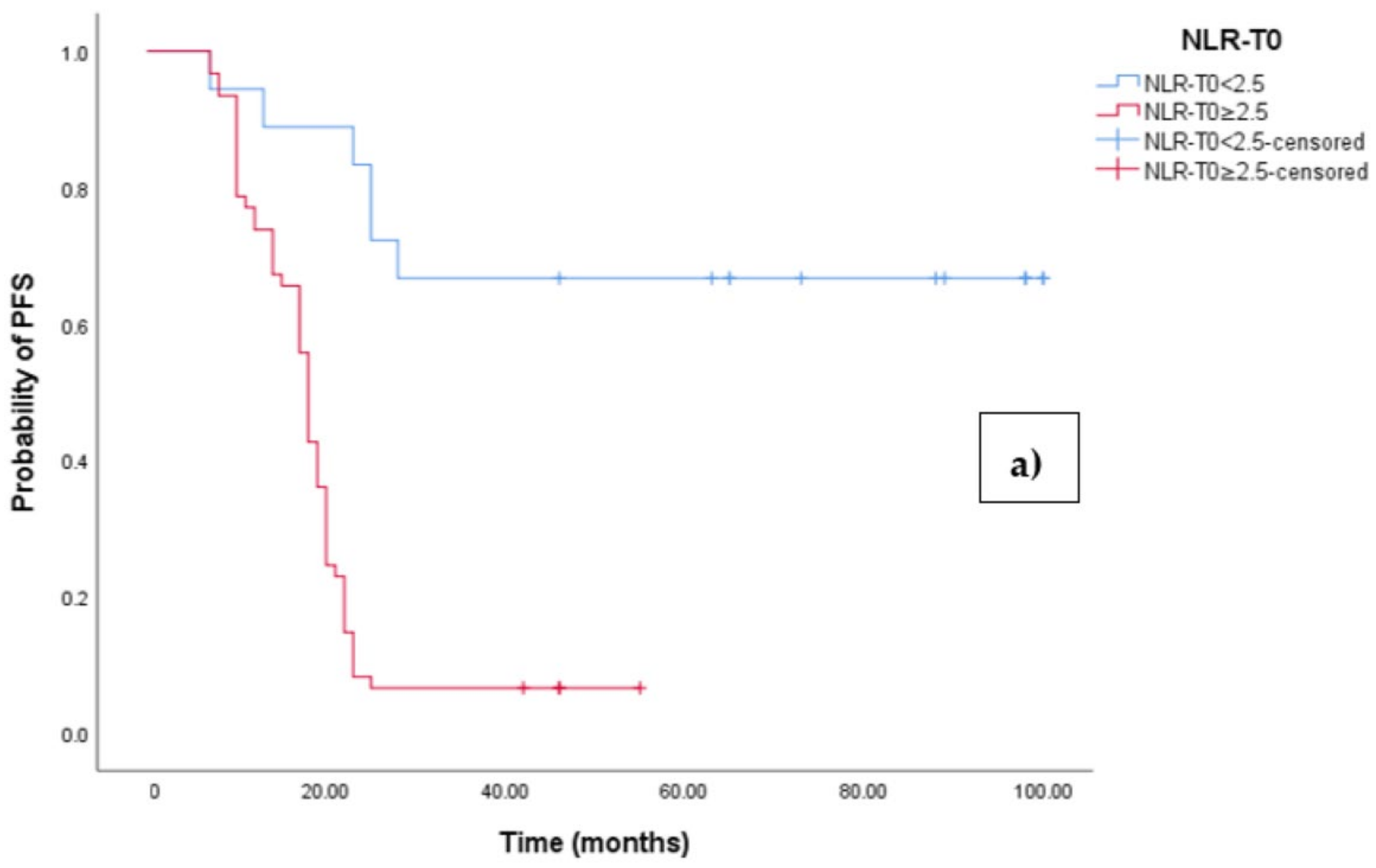
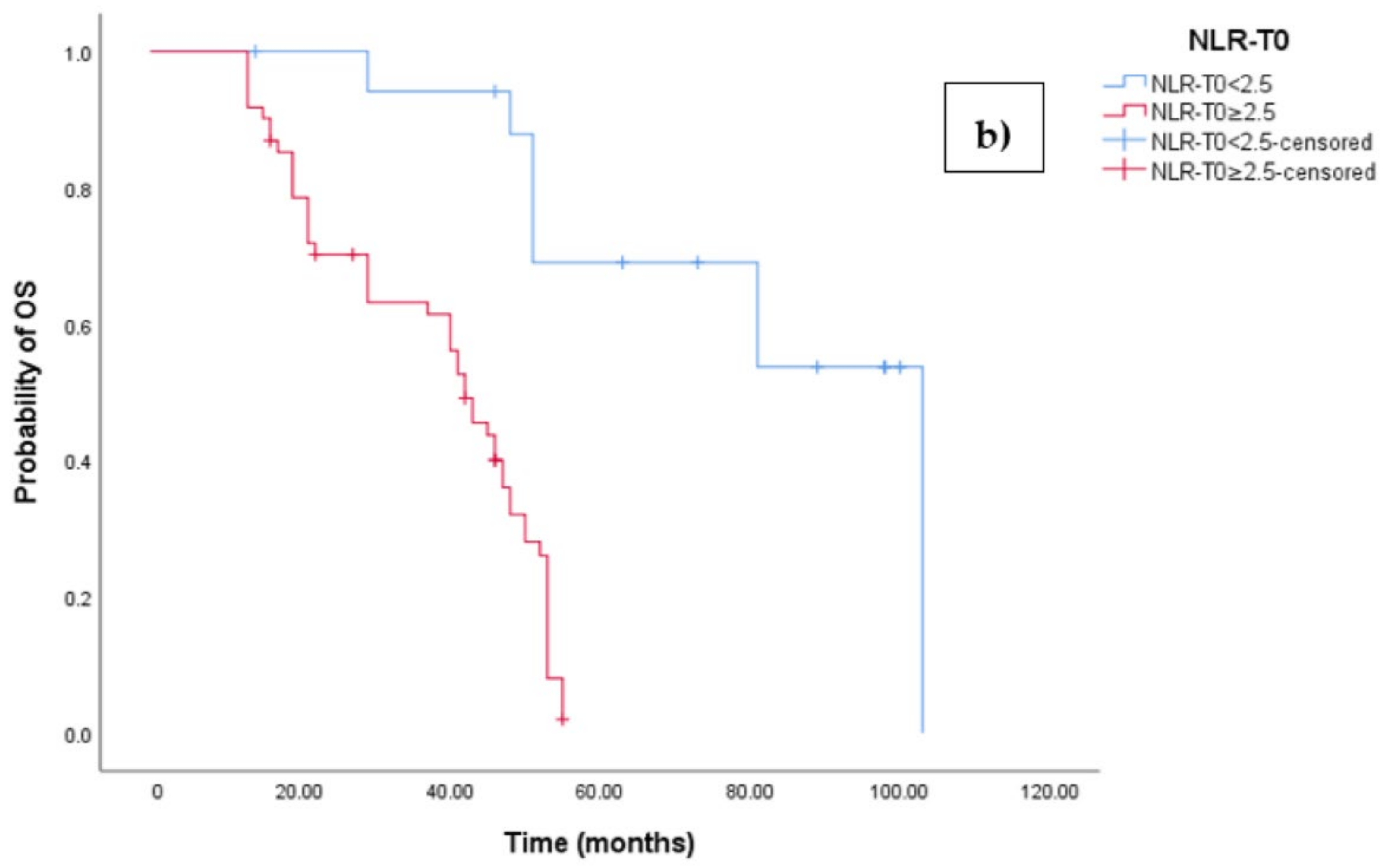
3.2. Clinical Outcome According to Baseline NLR-ROC Analysis
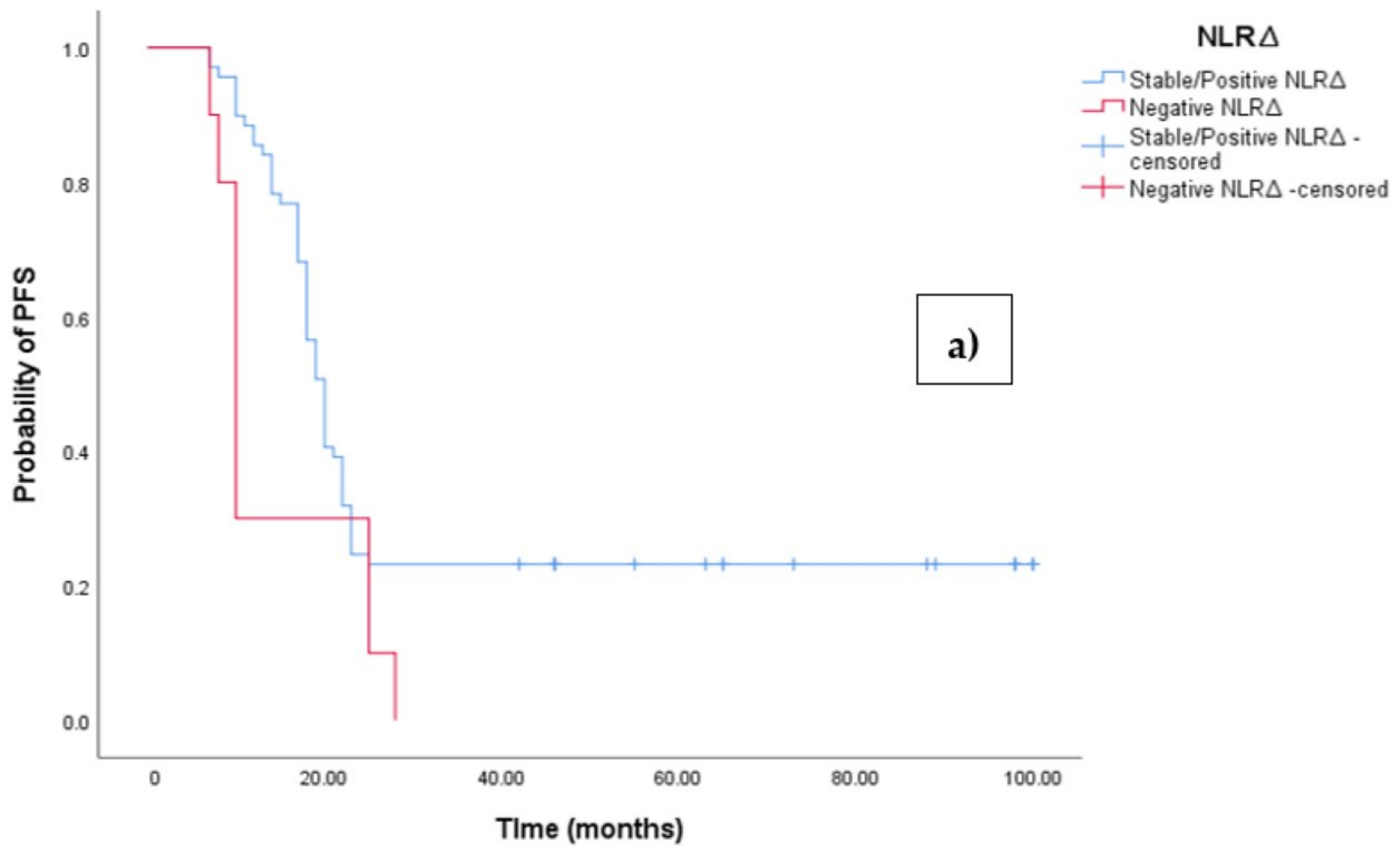
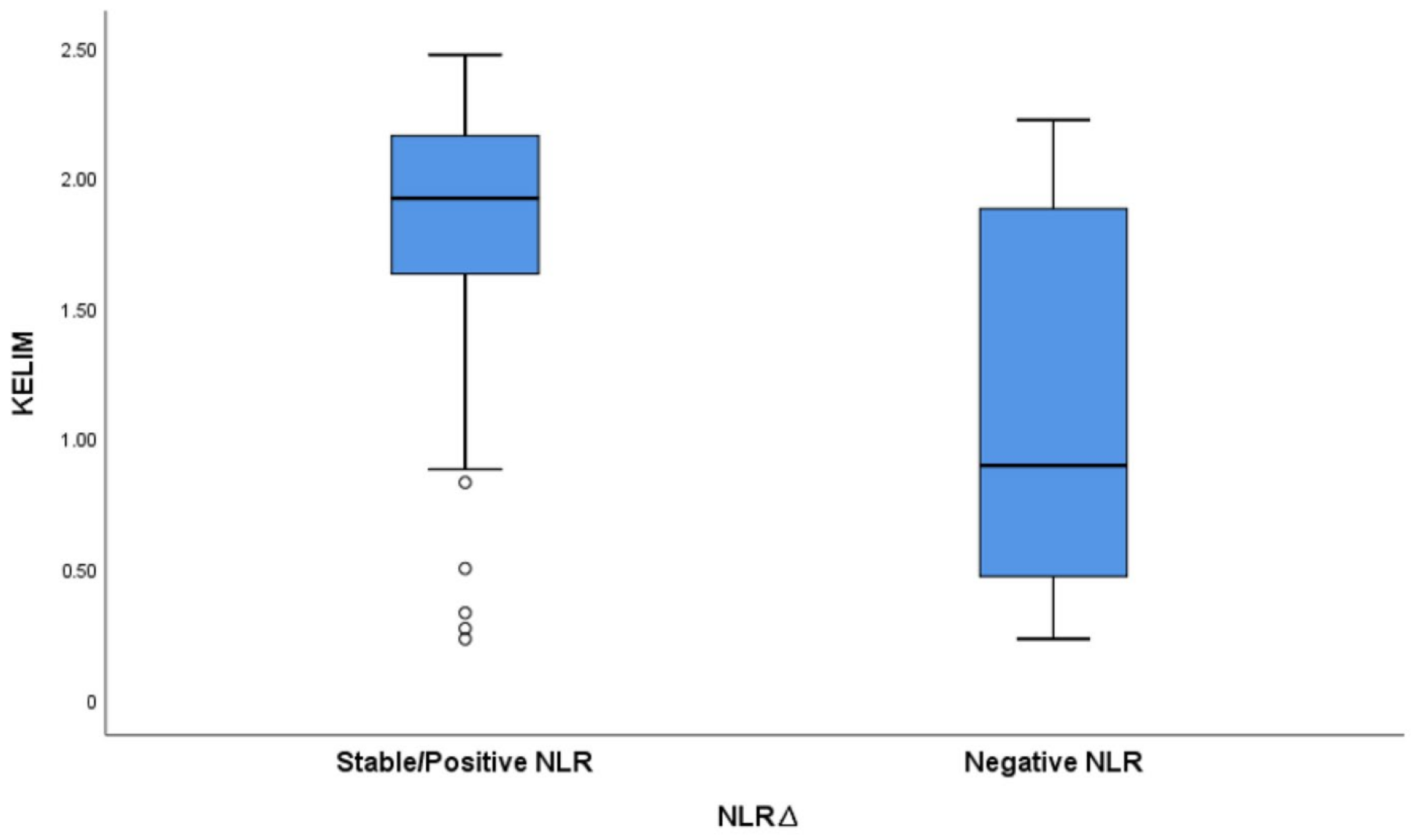
3.3. Clinical Outcome According to NLRΔ
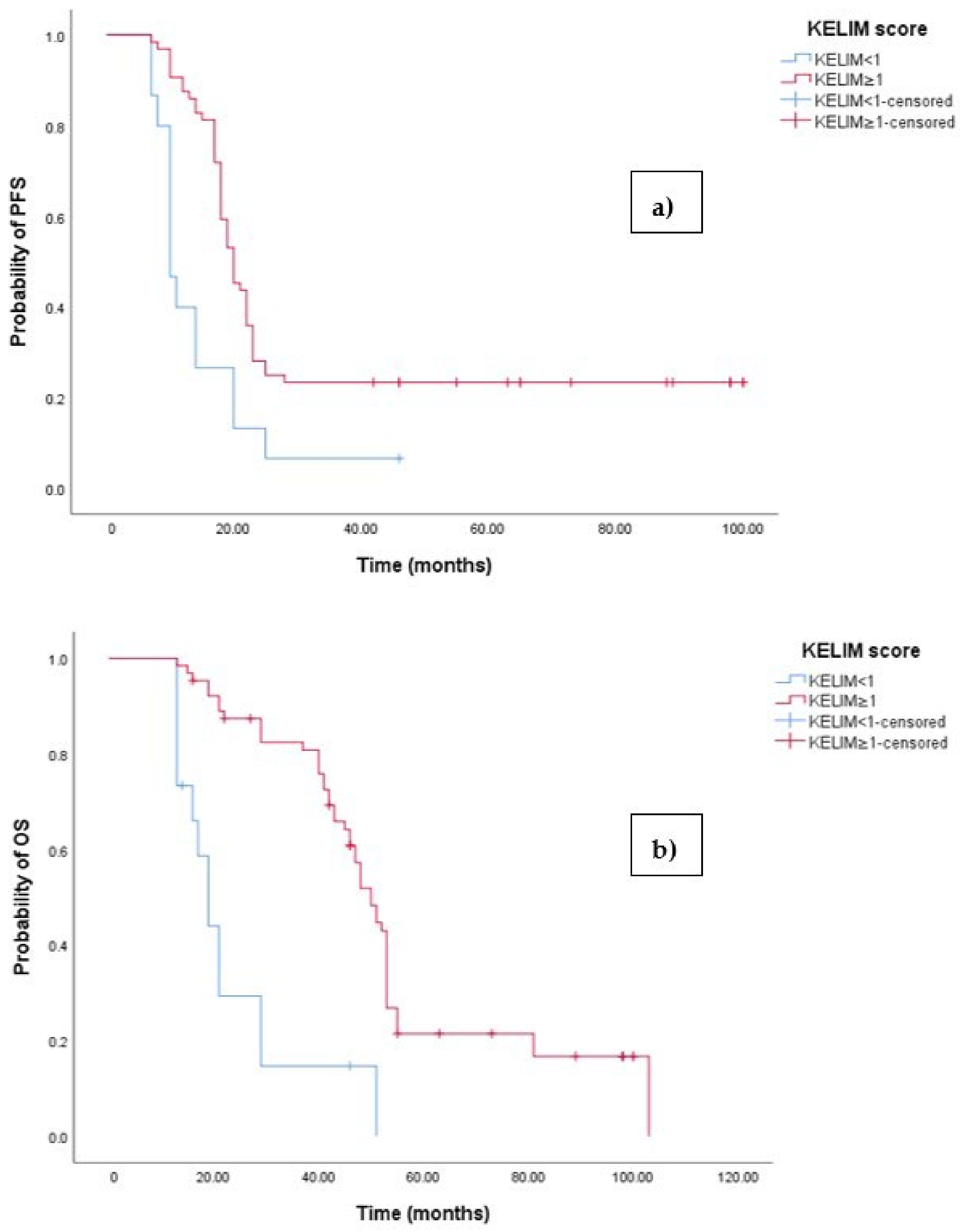
3.4. Clinical Outcome According to KELIM Score
3.5. Multivariate Analysis to Identify Predictors for Increased Risk of Death and Tumor Progression
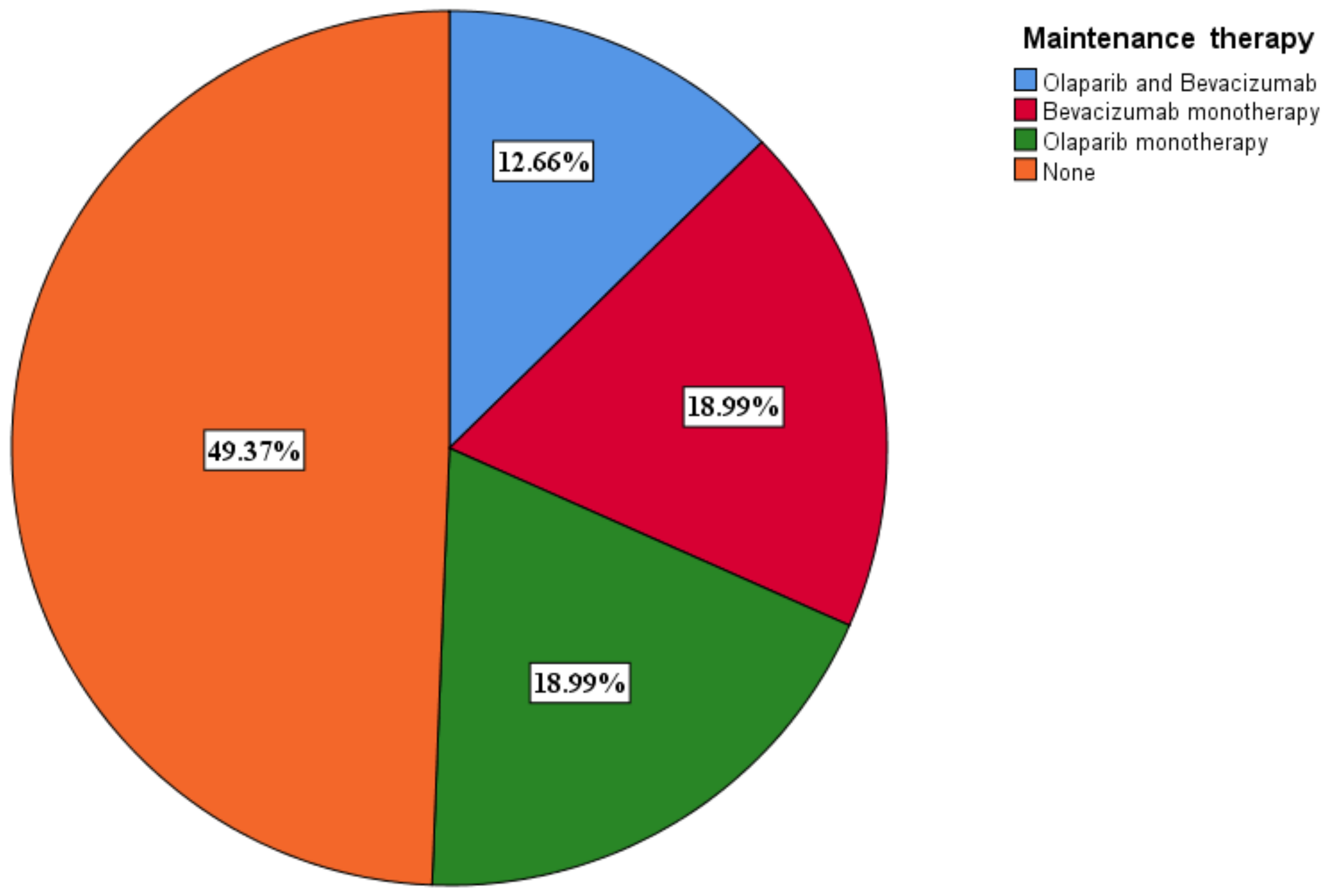
3.6. Selection of Maintenance Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/25-ovary-fact-sheet.pdf (accessed on 15 May 2024).
- Azangou-Khyavy, M.; Ghasemi, E.; Rezaei, N.; Khanali, J.; Kolahi, A.-A.; Malekpour, M.-R.; Heidari-Foroozan, M.; Nasserinejad, M.; Mohammadi, E.; Abbasi-Kangevari, M.; et al. Global, regional, and national quality of care index of cervical and ovarian cancer: A systematic analysis for the global burden of disease study 1990–2019. BMC Women’s Health 2024, 24, 69. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G. Morphological subtypes of ovarian carcinoma: A review with emphasis on new developments and pathogenesis. Pathology 2011, 43, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Köbel, M.; Kalloger, S.E.; Huntsman, D.G.; Santos, J.L.; Swenerton, K.D.; Seidman, J.D.; Gilks, C.B. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. International journal of gynecological pathology: Official journal of the International Society of Gynecological. Pathologists 2010, 29, 203–211. [Google Scholar] [CrossRef]
- Seidman, J.D.; Horkayne-Szakaly, I.; Haiba, M.; Boice, C.R.; Kurman, R.J.; Ronnett, B.M. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int. J. Gynecol. Pathol. 2004, 23, 41–44. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Peres, L.C.; Cushing-Haugen, K.L.; Köbel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J. Natl. Cancer Inst. 2019, 111, 60–68. [Google Scholar] [CrossRef]
- Park, H.K.; Ruterbusch, J.J.; Cote, M.L. Recent Trends in Ovarian Cancer Incidence and Relative Survival in the United States by Race/Ethnicity and Histologic Subtypes. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1511–1518. [Google Scholar] [CrossRef]
- Available online: https://seer.cancer.gov/archive/csr/1975_2014/results_merged/sect_21_ovary.pdf (accessed on 15 May 2024).
- Stewart, S.L.; Harewood, R.; Matz, M.; Rim, S.H.; Sabatino, S.A.; Ward, K.C.; Weir, H.K. Disparities in ovarian cancer survival in the United States (2001-2009): Findings from the CONCORD-2 study. Cancer 2017, 123 (Suppl. 24), 5138–5159. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Ferrandina, G.; Vizzielli, G.; Fanfani, F.; Gallotta, V.; Chiantera, V.; Costantini, B.; Margariti, P.A.; Gueli Alletti, S.; Cosentino, F.; et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur. J. Cancer 2016, 59, 22–33. [Google Scholar] [CrossRef]
- Worzfeld, T.; Pogge von Strandmann, E.; Huber, M.; Adhikary, T.; Wagner, U.; Reinartz, S.; Müller, R. The Unique Molecular and Cellular Microenvironment of Ovarian Cancer. Front. Oncol. 2017, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Iaciu, C.I.; Emilescu, R.A.; Cotan, H.T.; Nitipir, C. Systemic Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Colon Cancer. Chirurgia 2023, 118, 260–271. [Google Scholar] [CrossRef]
- Pirlog, C.F.; Cotan, H.T.; Parosanu, A.; Orlov Slavu, C.; Popa, A.M.; Iaciu, C.; Olaru, M.; Oprita, A.V.; Nita, I.; Nitipir, C. Correlation Between Pretreatment Neutrophil-to-Lymphocyte Ratio and Programmed Death-Ligand 1 Expression as Prognostic Markers in Non-Small Cell Lung Cancer. Cureus 2022, 14, e26843. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wu, L.; Yang, H.; Yang, H. Prognostic significance of neutrophil-lymphocyte ratio (NLR) in patients with ovarian cancer: A systematic review and meta-analysis. Medicine 2019, 9, e17475. [Google Scholar] [CrossRef] [PubMed]
- Sanna, E.; Tanca, L.; Cherchi, C.; Gramignano, G.; Oppi, S.; Chiai, M.G.; Macciò, A.; Madeddu, C. Decrease in Neutrophil-to-Lymphocyte Ratio during Neoadjuvant Chemotherapy as a Predictive and Prognostic Marker in Advanced Ovarian Cancer. Diagnostics 2021, 11, 1298. [Google Scholar] [CrossRef]
- Roy, H.K.; Khandekar, J.D. Biomarkers for the early detection of cancer: An inflammatory concept. Arch. Intern. Med. 2007, 167, 1822–1824. [Google Scholar] [CrossRef] [PubMed]
- Suidan, R.S.; Ramirez, P.T.; Sarasohn, D.M.; Teitcher, J.B.; Mironov, S.; Iyer, R.B.; Zhou, Q.; Iasonos, A.; Paul, H.; Hosaka, M.; et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol. Oncol. 2014, 134, 455–461. [Google Scholar] [CrossRef]
- You, B.; Purdy, C.; Copeland, L.J.; Swisher, E.M.; Bookman, M.A.; Fleming, G.; Coleman, R.; Randall, L.M.; Tewari, K.S.; Monk, B.J.; et al. Identification of Patients with Ovarian Cancer Experiencing the Highest Benefit from Bevacizumab in the First-Line Setting on the Basis of Their Tumor-Intrinsic Chemosensitivity (KELIM): The GOG-0218 Validation Study. J. Clin. Oncol. 2022, 40, 3965–3974. [Google Scholar] [CrossRef]
- Piedimonte, S.; Kim, R.; Bernardini, M.Q.; Atenafu, E.G.; Clark, M.; Lheureux, S.; May, T. Validation of the KELIM score as a predictor of response to neoadjuvant treatment in patients with advanced high grade serous ovarian cancer. Gynecol. Oncol. 2022, 167, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Oufkir, N.; Rouzier, R.; Paoletti, X.; Bonneau, C. External validation of standardized KELIM and platinum resistant recurrence score in patients with advanced epithelial ovarian cancer. J. Ovarian Res. 2024, 17, 152. [Google Scholar] [CrossRef] [PubMed]
- Modeled CA-125 KELIM™ in Patients with Stage III-IV High Grade Serous Ovarian Carcinomas Treated in First-Line Setting with Neo-Adjuvant Chemotherapy. Available online: https://www.biomarker-kinetics.org/CA-125-neo (accessed on 25 June 2024).
- Macciò, A.; Madeddu, C. Inflammation and ovarian cancer. Cytokine 2012, 58, 133–147. [Google Scholar] [CrossRef]
- Li, Z.; Hong, N.; Robertson, M.; Wang, C.; Jiang, G. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci. Rep. 2017, 7, 43001. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Hur, H.W.; Kim, S.W.; Kim, S.H.; Kim, J.H.; Kim, Y.T.; Lee, K. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol. Immunother. 2009, 58, 15–23. [Google Scholar] [CrossRef] [PubMed]
- du Bois, A.; Baert, T.; Vergote, I. Role of Neoadjuvant Chemotherapy in Advanced Epithelial Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Peiretti, M.; Parma, G.; Lapresa, M.; Mancari, R.; Carinelli, S.; Sessa, C.; Castiglione, M.; ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21 (Suppl. 5), v23–v30. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, J.R.; Nederby, L.; Donskov, F.; Waldstrøm, M.; Adimi, P.; Jakobsen, A.; Steffensen, K.D. Prognostic significance of baseline T cells, B cells and neutrophil-lymphocyte ratio (NLR) in recurrent ovarian cancer treated with chemotherapy. J. Ovarian. Res. 2020, 13, 59. [Google Scholar] [CrossRef]
- Feng, Z.; Wen, H.; Bi, R.; Ju, X.; Chen, X.; Yang, W.; Wu, X. Preoperative Neutrophil-to-Lymphocyte Ratio as a Predictive and Prognostic Factor for High-Grade Serous Ovarian Cancer. PLoS ONE 2016, 11, e0156101. [Google Scholar] [CrossRef]
- You, B.; Robelin, P.; Tod, M.; Louvet, C.; Lotz, J.-P.; Abadie-Lacourtoisie, S.; Fabbro, M.; Desauw, C.; Bonichon-Lamichhane, N.; Kurtz, J.-E.; et al. CA-125 ELIMination Rate Constant K (KELIM) Is a Marker of Chemosensitivity in Patients with Ovarian Cancer: Results from the Phase II CHIVA Trial. Clin. Cancer Res. 2020, 26, 4625–4632. [Google Scholar] [CrossRef]
- You, B.; Purdy, C.; Swisher, E.M.; Bookman, M.A.; Fleming, G.F.; Coleman, R.L.; Randall, L.M.; Tewari, K.S.; Monk, B.J.; Mannel, R.S.; et al. Identification of patients with ovarian cancer who are experiencing the highest benefit from bevacizumab in first-line setting based on their tumor intrinsic chemosensitivity (KELIM): GOG-0218 validation study. J. Clin. Oncol. 2022, 40, 5553. [Google Scholar] [CrossRef]
- van Wagensveld, L.; Colomban, O.; van der Aa, M.A.; Freyer, G.; Sonke, G.S.; Kruitwagen, R.F.; You, B. Confirmation of the utility of the CA-125 elimination rate (KELIM) as an indicator of the chemosensitivity in advanced-stage ovarian cancer in a “real-life setting”. J. Gynecol. Oncol. 2024, 35, e34. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Labidi-Galy, S.I.; Terry, K.L.; Vitonis, A.F.; Welch, W.R.; Goodman, A.; Cramer, D.W. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol. Oncol. 2014, 132, 542–550. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Patients Diagnosed with EOC (n = 79) |
|---|---|
| Age, years, mean (SD) | 65.4 (±5.43) |
| Clinical stage, n (%) | |
| Stage IIIA | 10 (2.5%) |
| Stage IIIB | 12 (6.3%) |
| Stage IIIC | 34 (43%) |
| Stage IV | 27 (34.2%) |
| ECOG, n (%) | |
| 0 | 21 (26.5%) |
| 1 | 43 (54.4%) |
| ≥2 | 15 (19%) |
| KELIM score, n (%) | |
| KELIM ≥ 1 | 64 (81%) |
| KELIM < 1 | 15 (19%) |
| BRCA 1/2 mutational status, n (%) | |
| Wild-type | 61 (77.2%) |
| Mutant | 18 (22.8%) |
| Homologous recombination status, n (%) | |
| Homologous recombination deficient (HRD) | 13 (16.5%) |
| Homologous recombination proficient (HRP) | 66 (83.5%) |
| Resction status | |
| R0 | 51 (64.6%) |
| R1 | 12 (15.2%) |
| Unresectable | 16 (20.3%) |
| Bevacizumab treatment | |
| Bevacizumab + | 12 (15.2%) |
| Bevacizumab - | 67 (84.8%) |
| Number of NACT cycles | |
| 3 cycles | 26 (32.9%) |
| 4 cycles | 6 (7.6%) |
| 5 cycles | 1 (1.3%) |
| 6 cycles | 30 (38%) |
| Baseline NLR | |
| ≥2.5 | 18 (22.8%) |
| <2.5 | 61 (77.2%) |
| NLR∆ | |
| Positive/Stationary | 69 (87.3%) |
| Negative | 10 (12.7%) |
| Median PFS (months) | 19 months (95%CI 17.626–20.374) |
| Median OS (months) | 47 months (95% CI 43.660–50.340) |
| Test Variables | OS HR (95% CI) | p-Value | PFS HR (95% CI) | p-Value |
|---|---|---|---|---|
| NLR∆ positive/stable (ref.)/negative | 3.750 (2.220–6.421) | <0.001 | 3.177 (2.225–7.671) | <0.001 |
| BRCA1/2 mutational status mutant (ref.)/wild-type | 1.352 (1.120–1.631) | 0.003 | 1.478 (1.234–1.763) | 0.001 |
| HRD (ref.)/HRP | 1.523 (1.293–1.842) | 0.006 | 1.411 (1.192–1.682) | 0.006 |
| ECOG 0-1 (ref.)/2 | 2.124 (1.753–2.573) | 0.072 | 1.589 (1.345–1.922) | 0.143 |
| KELIM score ≥ 1 (ref.)/KELIM score < 1 | 1.910 (0.852–4.282) | 0.006 | 2.185 (1.865–6.722) | 0.006 |
| Stage III (ref.)/Stage IV | 2.301 (1.904–2.792) | 0.257 | 1.519 (1.284–1.830) | 0.226 |
| R0 resection (ref.)/R1 resection | 2.456 (2.004–3.002) | <0.001 | 1.772 (1.504–2.223) | 0.003 |
| Cancer antigen 125 (CA 125) low (ref.)/high | 1.392 (1.182–1.643) | 0.186 | 1.576 (1.314–1.889) | 0.247 |
| Age < 65 years (ref.)/>65 years | 1.382 (1.157–1.652) | 0.935 | 1.198 (0.537–1.517) | 0.653 |
| No. of NACT: 3 cycles (ref.)/6 cycles | 1.653 (1.389–1.967) | 0.893 | 1.548 (1.293–1.856) | 0.785 |
| Baseline NLR low (ref.)/baseline NLR high | 2.173 (1.810–2.610) | 0.454 | 1.927 (1.602–2.319) | 0.321 |
| ORR | KELIM Score ≥ 1 | KELIM Score < 1 | NLR∆ Positive/Stable | NLR∆ Negative |
|---|---|---|---|---|
| Favorable response (CR + PR + SD) (n, %) | 64 (100%) | 8 (53.3%) | 68 (98.5%) | 4 (40%) |
| Unfavorable response (PD) (n, %) | 0 (0%) | 7 (46.6%) | 1 (1.5%) | 6 (60%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazar, A.; Popa, A.M.; Orlov-Slavu, C.; Cotan, H.-T.; Iaciu, C.I.; Olaru, C.M.; Schreiner, O.D.; Ciobanu, R.C.; Nitipir, C. The Influence of Circulating Immune Cell and CA125 Dynamics on Neoadjuvant Therapy Selection for Advanced Ovarian Cancer. Medicina 2024, 60, 1290. https://doi.org/10.3390/medicina60081290
Lazar A, Popa AM, Orlov-Slavu C, Cotan H-T, Iaciu CI, Olaru CM, Schreiner OD, Ciobanu RC, Nitipir C. The Influence of Circulating Immune Cell and CA125 Dynamics on Neoadjuvant Therapy Selection for Advanced Ovarian Cancer. Medicina. 2024; 60(8):1290. https://doi.org/10.3390/medicina60081290
Chicago/Turabian StyleLazar, Alexandra, Ana Maria Popa, Cristina Orlov-Slavu, Horia-Teodor Cotan, Cristian Ion Iaciu, Cristina Mihaela Olaru, Oliver Daniel Schreiner, Romeo Cristian Ciobanu, and Cornelia Nitipir. 2024. "The Influence of Circulating Immune Cell and CA125 Dynamics on Neoadjuvant Therapy Selection for Advanced Ovarian Cancer" Medicina 60, no. 8: 1290. https://doi.org/10.3390/medicina60081290
APA StyleLazar, A., Popa, A. M., Orlov-Slavu, C., Cotan, H.-T., Iaciu, C. I., Olaru, C. M., Schreiner, O. D., Ciobanu, R. C., & Nitipir, C. (2024). The Influence of Circulating Immune Cell and CA125 Dynamics on Neoadjuvant Therapy Selection for Advanced Ovarian Cancer. Medicina, 60(8), 1290. https://doi.org/10.3390/medicina60081290




