Impact of preS1 Evaluation in the Management of Chronic Hepatitis B Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Monoclonal Antibodies
2.2. Binding Activity of Anti-preS1 mAbs in Different Genotypes
2.3. Epitope Mapping
2.4. Binding Activity of Anti-preS1 mAbs for an Amino Acid-Substituted Peptide
2.5. Sample Collection
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Western Blotting
2.8. Statistical Analyses
3. Results
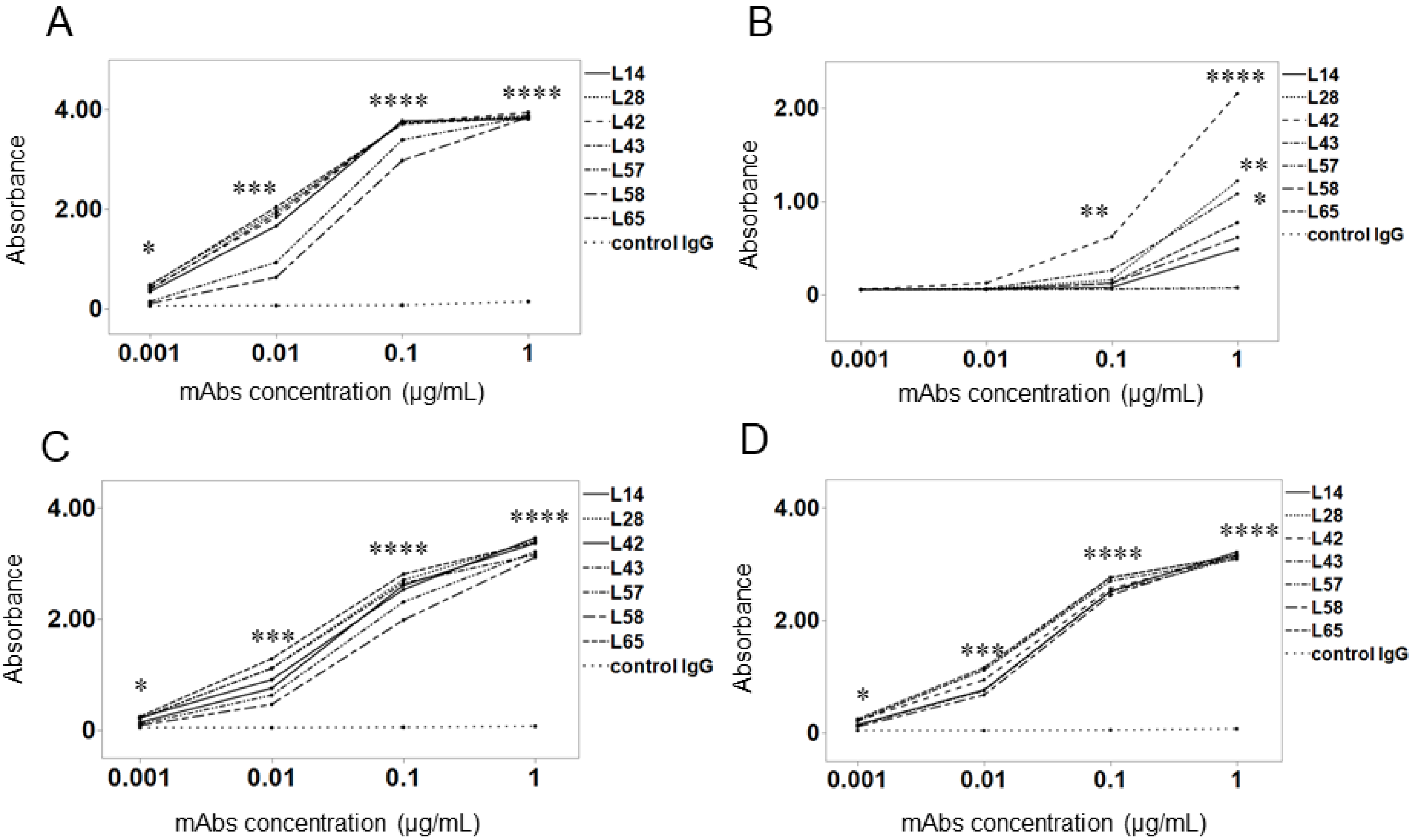
3.1. Binding Activity of Anti-preS1 mAbs against preS1 Peptides with Genotypes A–D
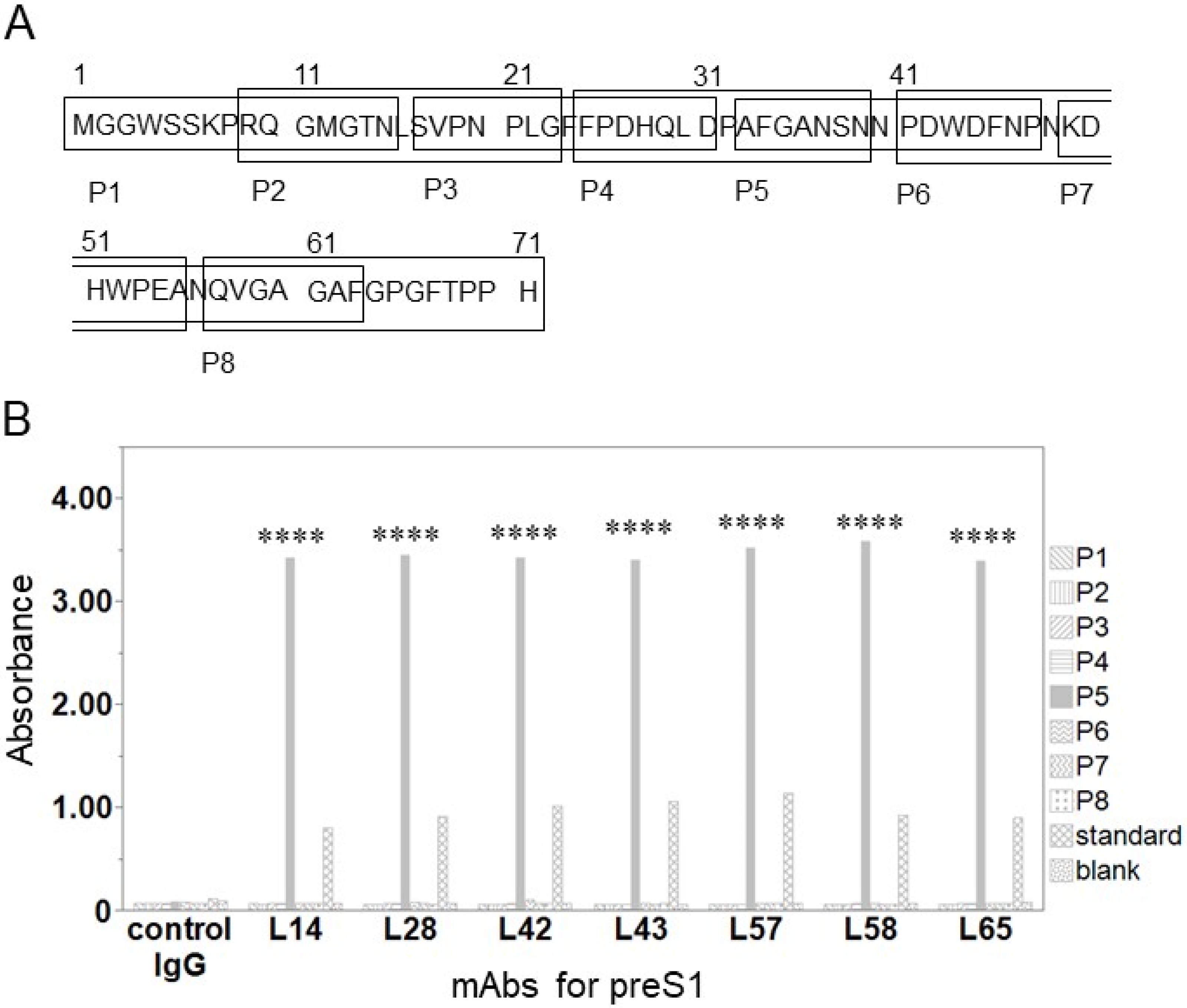
3.2. Epitope Analyses of Anti-preS1 mAbs
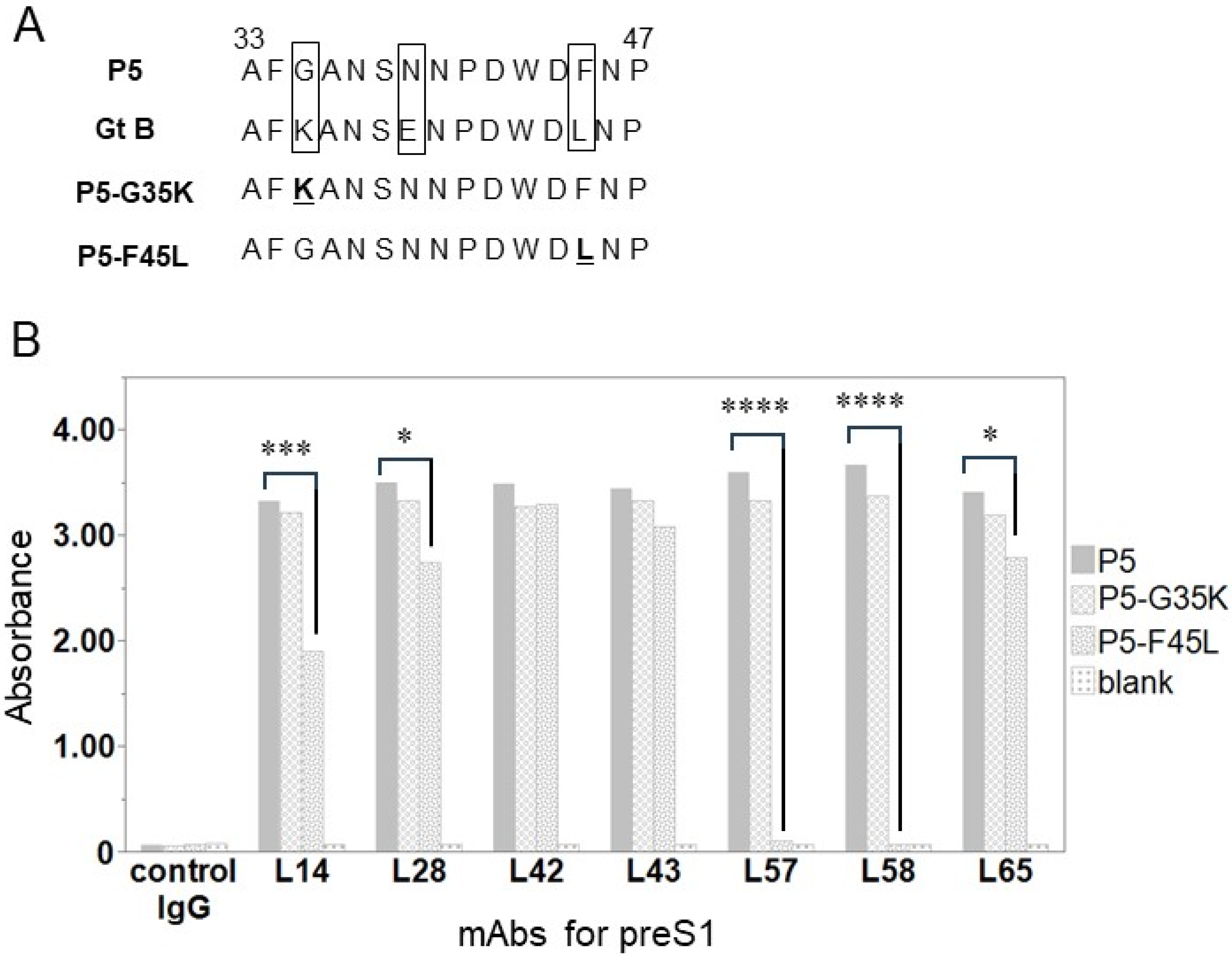
3.3. Identification of Single Amino Acid Causing Weak Binding between Anti-preS1 mAbs and preS1 of Gt B
3.4. Patient Characteristics
3.5. Correlation between Each HB Surface Protein and Total HBsAg
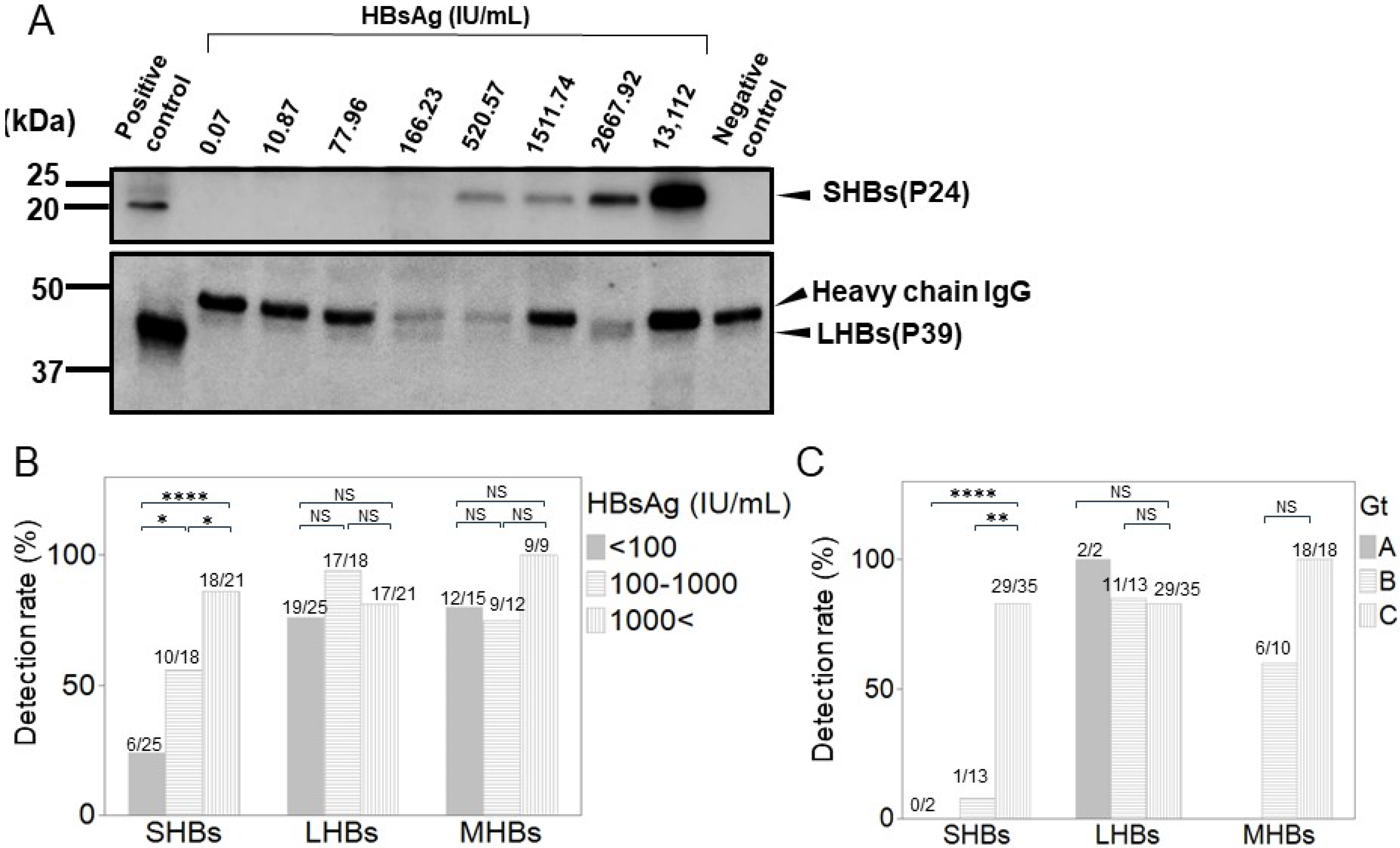
3.6. Detection of Each HB Surface Protein Using WB in Various CHB Patients
3.7. Each HB Surface Protein Levels According to HBeAg Status and HBV-DNA
3.8. Changes in HB Surface Proteins During Switch from ETV to TAF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, M.; Bhadoria, A.S.; Cui, F.; Tan, A.; Van Holten, J.; Easterbrook, P.; Ford, N.; Han, Q.; Lu, Y.; Bulterys, M.; et al. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 106–119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanto, T. Messages from Japan policy for viral hepatitis. Glob. Health Med. 2021, 3, 249–252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trepo, C.; Chan, H.L.; Lok, A. Hepatitis B virus infection. Lancet 2014, 384, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Hendrickx, G.; Vorsters, A.; Van Damme, P. Hepatitis B Vaccines. J. Infect. Dis. 2021, 224, S343–S351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Z.; Zhang, Y.; Xu, M.; Li, X.; Zhang, Z. Distribution of hepatitis B virus genotypes and subgenotypes: A meta-analysis. Medicine 2021, 100, e27941. [Google Scholar] [CrossRef] [PubMed]
- Orito, E.; Ichida, T.; Sakugawa, H.; Sata, M.; Horiike, N.; Hino, K.; Okita, K.; Okanoue, T.; Iino, S.; Tanaka, E.; et al. Geographic distribution of hepatitis B virus (HBV) genotype in patients with chronic HBV infection in Japan. Hepatology 2001, 34, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Ahn, S.H. Quantification of HBsAg: Basic virology for clinical practice. World J. Gastroenterol. 2011, 17, 283–289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamontagne, R.J.; Bagga, S.; Bouchard, M.J. Hepatitis B virus molecular biology and pathogenesis. Hepatoma Res. 2016, 2, 163–186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrscher, C.; Roingeard, P.; Blanchard, E. Hepatitis B Virus Entry into Cells. Cells 2020, 9, 1486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Glebe, D.; Urban, S. Viral and cellular determinants involved in hepadnaviral entry. World J. Gastroenterol. 2007, 13, 22–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schulze, A.; Schieck, A.; Ni, Y.; Mier, W.; Urban, S. Fine mapping of pre-S sequence requirements for hepatitis B virus large envelope protein-mediated receptor interaction. J. Virol. 2010, 84, 1989–2000. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, Y.; Zhai, J.; Deng, Q.; Tiollais, P.; Wang, Y.; Zhao, M. Entry of hepatitis B virus: Mechanism and new therapeutic target. Pathol. Biol. 2010, 58, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mondal, R.K.; Banerjee, P.; Nandi, M.; Sarkar, S.; Das, K.; Santra, A.; Banerjee, S.; Chowdhury, A.; Datta, S. Tracking the naturally occurring mutations across the full-length genome of hepatitis B virus of genotype D in different phases of chronic e-antigen-negative infection. Clin. Microbiol. Infect. 2012, 18, E412–E418. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.; Mehrle, S.; Weiss, T.S.; Mier, W.; Urban, S. Myristoylated PreS1-domain of the hepatitis B virus L-protein mediates specific binding to differentiated hepatocytes. Hepatology 2013, 58, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.S.; Patwa, S.M.; Tan, Y.J. Development of monoclonal antibodies to target the large surface protein of hepatitis B virus and their use in therapeutic and diagnostic applications. J. Viral Hepat. 2023, 30, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.; Zoulim, F.; Dusheiko, G.; Chan, H.L.Y.; Buti, M.; Ghany, M.G.; Gaggar, A.; Yang, J.C.; Wu, G.; Flaherty, J.F.; et al. Durability of Hepatitis B Surface Antigen Loss with Nucleotide Analogue and Peginterferon Therapy in Patients with Chronic Hepatitis B. Hepatol. Commun. 2020, 4, 8–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yip, T.C.; Wong, G.L.; Chan, H.L.; Tse, Y.K.; Lam, K.L.; Lui, G.C.; Wong, V.W. HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. J. Hepatol. 2019, 70, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Cornberg, M.; Lok, A.S.; Terrault, N.A.; Zoulim, F.; the 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B—Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference(double dagger). J. Hepatol. 2020, 72, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Dusheiko, G.; Wang, B. Hepatitis B Surface Antigen Loss: Too Little, Too Late and the Challenge for the Future. Gastroenterology 2019, 156, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, M.; Kagita, M.; Yoshioka, N.; Tsukamoto, H.; Takao, M.; Tahara, K.; Maeda, I.; Hidaka, Y.; Yamauchi, S.; Kaneko, A.; et al. Evaluation of the highly sensitive chemiluminescent enzyme immunoassay “Lumipulse HBsAg-HQ” for hepatitis B virus screening. J. Clin. Lab. Anal. 2018, 32, e22334. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, W.; Shang, H.; Lin, S.; Xun, Z.; Huang, E.; Lin, J.; Yang, B.; Ou, Q. Prediction value of serum HBV large surface protein in different phases of HBV infection and virological response of chronic hepatitis B patients. Clin. Chim. Acta 2018, 481, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, M.; Bohm, S.; Schott, T.; Deichsel, D.; Bremer, C.M.; Schroder, K.; Gerlich, W.H.; Glebe, D.; Berg, T.; van Bommel, F. Quantification of large and middle proteins of hepatitis B virus surface antigen (HBsAg) as a novel tool for the identification of inactive HBV carriers. Gut 2018, 67, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, M.; Schott, T.; Bohm, S.; Deichsel, D.; Felkel, C.; Gerlich, W.H.; Glebe, D.; Wat, C.; Pavlovic, V.; Heyne, R.; et al. Composition of HBsAg is predictive of HBsAg loss during treatment in patients with HBeAg-positive chronic hepatitis B. J. Hepatol. 2021, 74, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Imamura, M.; Teraoka, Y.; Morio, K.; Fujino, H.; Ono, A.; Nakahara, T.; Murakami, E.; Yamauchi, M.; Kawaoka, T.; et al. Serum PreS1 and HBsAg ratio reflects liver fibrosis and predicts the development of hepatocellular carcinoma in chronic hepatitis B patients. J. Viral Hepat. 2021, 28, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, K.H.; Kuhnhenn, L.; Jiang, B.; Mondorf, A.; Vermehren, J.; Knop, V.; Susser, S.; Walter, D.; Dietz, J.; Carra, G.; et al. Divergent preS Sequences in Virion-Associated Hepatitis B Virus Genomes and Subviral HBV Surface Antigen Particles From HBV e Antigen-Negative Patients. J. Infect. Dis. 2018, 218, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.T.; Tanaka, Y.; Tateno, Y.; Mizokami, M. Development and public release of a comprehensive hepatitis virus database. Hepatol. Res. 2008, 38, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.; Ozawa, T.; Tajiri, K.; Obata, T.; Kondo, S.; Kinoshita, K.; Kadowaki, S.; Takahashi, K.; Sugiyama, T.; Kishi, H.; et al. A rapid and efficient single-cell manipulation method for screening antigen-specific antibody-secreting cells from human peripheral blood. Nat. Med. 2009, 15, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Kishi, H.; Tokimitsu, Y.; Kondo, S.; Ozawa, T.; Kinoshita, K.; Jin, A.; Kadowaki, S.; Sugiyama, T.; Muraguchi, A. Cell-microarray analysis of antigen-specific B-cells: Single cell analysis of antigen receptor expression and specificity. Cytom. A 2007, 71, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Ozawa, T.; Jin, A.; Tokimitsu, Y.; Minemura, M.; Kishi, H.; Sugiyama, T.; Muraguchi, A. Analysis of the epitope and neutralizing capacity of human monoclonal antibodies induced by hepatitis B vaccine. Antiviral. Res. 2010, 87, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Tokimitsu, Y.; Kishi, H.; Kondo, S.; Honda, R.; Tajiri, K.; Motoki, K.; Ozawa, T.; Kadowaki, S.; Obata, T.; Fujiki, S.; et al. Single lymphocyte analysis with a microwell array chip. Cytom. A 2007, 71, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Kishi, H.; Muraguchi, A. Amplification and analysis of cDNA generated from a single cell by 5′-RACE: Application to isolation of antibody heavy and light chain variable gene sequences from single B cells. Biotechniques 2006, 40, 469–470+472+474 passim. [Google Scholar] [CrossRef] [PubMed]
- Angata, K.; Wagatsuma, T.; Togayachi, A.; Sato, T.; Sogabe, M.; Tajiri, K.; Ozawa, T.; Nagashima, I.; Shimizu, H.; Iijima, S.; et al. O-glycosylated HBsAg peptide can induce specific antibody neutralizing HBV infection. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130020. [Google Scholar] [CrossRef] [PubMed]
- Drafting Committee for Hepatitis Management Guidelines, tJSoH. Japan Society of Hepatology Guidelines for the Management of Hepatitis B Virus Infection: 2019 update. Hepatol. Res. 2020, 50, 892–923. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Iwamoto, M.; Yun, J.H.; Uchikubo-Kamo, T.; Son, D.; Jin, Z.; Yoshida, H.; Ohki, M.; Ishimoto, N.; Mizutani, K.; et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 2022, 606, 1027–1031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sausen, D.G.; Shechter, O.; Bietsch, W.; Shi, Z.; Miller, S.M.; Gallo, E.S.; Dahari, H.; Borenstein, R. Hepatitis B and Hepatitis D Viruses: A Comprehensive Update with an Immunological Focus. Int. J. Mol. Sci. 2022, 23, 15973. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takemori, T.; Sugimoto-Ishige, A.; Nishitsuji, H.; Futamura, Y.; Harada, M.; Kimura-Someya, T.; Matsumoto, T.; Honma, T.; Tanaka, M.; Yaguchi, M.; et al. Establishment of a Monoclonal Antibody against Human NTCP That Blocks Hepatitis B Virus Infection. J. Virol. 2022, 96, e0168621. [Google Scholar] [CrossRef] [PubMed]
- Hatooka, H.; Shimomura, Y.; Imamura, M.; Teraoka, Y.; Morio, K.; Fujino, H.; Ono, A.; Nakahara, T.; Murakami, E.; Yamauchi, M.; et al. Construction of an anti-hepatitis B virus preS1 antibody and usefulness of preS1 measurement for chronic hepatitis B patients: Anti-HBV PreS1 antibody. J. Infect. 2022, 84, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Sankhyan, A.; Sharma, C.; Dutta, D.; Sharma, T.; Chosdol, K.; Wakita, T.; Watashi, K.; Awasthi, A.; Acharya, S.K.; Khanna, N.; et al. Inhibition of preS1-hepatocyte interaction by an array of recombinant human antibodies from naturally recovered individuals. Sci. Rep. 2016, 6, 21240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsai, H.W.; Lee, Y.P.; Yen, C.J.; Cheng, K.H.; Huang, C.J.; Huang, W. The Serum Hepatitis B Virus Large Surface Protein as High-Risk Recurrence Biomarker for Hepatoma after Curative Surgery. Int. J. Mol. Sci. 2022, 23, 5376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryu, C.J.; Kim, Y.K.; Hur, H.; Kim, H.S.; Oh, J.M.; Kang, Y.J.; Hong, H.J. Mouse monoclonal antibodies to hepatitis B virus preS1 produced after immunization with recombinant preS1 peptide. Hybridoma 2000, 19, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.F.; Wu, H.C.; Su, I.J.; Jeng, L.B. Hepatitis B Virus Pre-S Mutants as Biomarkers and Targets for the Development and Recurrence of Hepatocellular Carcinoma. Viruses 2020, 12, 945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, X.; Zheng, Y.; Li, H.; Lu, J.; Ren, S.; Liu, Y.; Wang, X.; Zheng, S.; Ma, L.; Cao, Z.; et al. Serum hepatitis B virus large and medium surface proteins as novel tools for predicting HBsAg clearance. Front. Immunol. 2022, 13, 1028921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lazarevic, I.; Banko, A.; Miljanovic, D.; Cupic, M. Hepatitis B Surface Antigen Isoforms: Their Clinical Implications, Utilisation in Diagnosis, Prevention and New Antiviral Strategies. Pathogens 2024, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Glebe, D.; Bremer, C.M. The molecular virology of hepatitis B virus. Semin. Liver Dis. 2013, 33, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Rinker, F.; Bremer, C.M.; Schroder, K.; Wiegand, S.B.; Bremer, B.; Manns, M.P.; Kraft, A.R.; Wedemeyer, H.; Yang, L.; Pavlovic, V.; et al. Quantitation of large, middle and small hepatitis B surface proteins in HBeAg-positive patients treated with peginterferon alfa-2a. Liver Int. 2020, 40, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Langley, J.M.; Spaans, J.N.; Petrov, I.; Popovic, V.; Yassin-Rajkumar, B.; Anderson, D.E.; Diaz-Mitoma, F. The persistence of seroprotective levels of antibodies after vaccination with PreHevbrio, a 3-antigen hepatitis B vaccine. Vaccine 2023, 41, 3584–3588. [Google Scholar] [CrossRef] [PubMed]
- Dishlers, A.; Petrovskis, I.; Skrastina, D.; Zarina, I.; Lieknina, I.; Jansons, J.; Akopjana, I.; Zakova, J.; Ose, V.; Sominskaya, I. PreS1 Containing HBc VLPs for the Development of a Combined Therapeutic/Prophylactic Hepatitis B Vaccine. Microorganisms 2023, 11, 972. [Google Scholar] [CrossRef] [PubMed]
- Sanada, T.; Oda, Y.; Ohashi, C.; Isotani, K.; Goh, Y.; Kohara, M. Hybrid large hepatitis B surface protein composed of two viral genotypes C and D induces strongly cross-neutralizing antibodies. Vaccine 2023, 41, 6514–6521. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Jin, X.; Wu, S.; Liu, J.; Zhang, H.; Cai, X.; Yang, Y.; Zhang, X.; Wei, J.; Luo, M.; et al. Release of hepatitis B virions is positively regulated by glucose-regulated protein 78 through direct interaction with preS1. J. Med. Virol. 2023, 95, e28271. [Google Scholar] [CrossRef] [PubMed]
- Wagatsuma, T.; Kuno, A.; Angata, K.; Tajiri, K.; Takahashi, J.; Korenaga, M.; Mizokami, M.; Narimatsu, H. Highly Sensitive Glycan Profiling of Hepatitis B Viral Particles and a Simple Method for Dane Particle Enrichment. Anal. Chem. 2018, 90, 10196–10203. [Google Scholar] [CrossRef] [PubMed]
- Murata, A.; Angata, K.; Sogabe, M.; Sato, S.; Ichida, T.; Narimatsu, H.; Genda, T. Serum O-glycosylated hepatitis B surface antigen levels in patients with chronic hepatitis B during nucleos(t)ide analog therapy. BMC Gastroenterol. 2022, 22, 270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chien, R.N.; Liaw, Y.F. Current Trend in Antiviral Therapy for Chronic Hepatitis B. Viruses 2022, 14, 434. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, H.J.; Lee, J.; Cho, S.; Ko, M.J.; Lim, Y.S. Risk of Hepatocellular Carcinoma in Patients Treated with Entecavir vs Tenofovir for Chronic Hepatitis B: A Korean Nationwide Cohort Study. JAMA Oncol. 2019, 5, 30–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hagiwara, S.; Nishida, N.; Ida, H.; Ueshima, K.; Minami, Y.; Takita, M.; Komeda, Y.; Kudo, M. Switching from entecavir to tenofovir alafenamide versus maintaining entecavir for chronic hepatitis B. J. Med. Virol. 2019, 91, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Nakao, M.; Yamada, S.; Tsuji, S.; Uemura, H.; Kouyama, J.I.; Naiki, K.; Sugawara, K.; Nakayama, N.; Imai, Y.; et al. Superiority of tenofovir alafenamide fumarate over entecavir for serum HBsAg level reduction in patients with chronic HBV infection: A 144-week outcome study after switching of the nucleos(t)ide analog. PLoS ONE 2022, 17, e0262764. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hagiwara, S.; Nishida, N.; Ueshima, K.; Yoshida, A.; Minami, Y.; Kudo, M. Comparison of efficacy and safety of entecavir and switching from entecavir to tenofovir alafenamide fumarate in chronic hepatitis B: Long-term effects from a prospective study. Hepatol. Res. 2021, 51, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Inoue, J.; Akahane, T.; Kobayashi, T.; Sato, S.; Kisara, N.; Ninomiya, M.; Iwata, T.; Sano, A.; Tsuruoka, M.; et al. Switching to tenofovir alafenamide versus continued therapy in chronic hepatitis B patients who were treated with entecavir: A prospective, multicenter, randomized controlled study. Medicine 2022, 101, e30630. [Google Scholar] [CrossRef] [PubMed]


| Variables | Number or Median (Range) |
|---|---|
| Patients, n | 64 |
| Age (years), median (range) | 66 (30–87) |
| Sex, n | |
| Male | 36 |
| Female | 28 |
| CHB status, n | |
| Chronic hepatitis | 28 |
| Cirrhosis | 11 |
| Inactive carrier | 25 |
| HBsAg (IU/mL), n | |
| <100 | 25 |
| 100–1000 | 18 |
| 1000< | 21 |
| Genotype, n | |
| A | 2 |
| B | 13 |
| C | 35 |
| Not determined | 14 |
| HBV-DNA (Log IU/mL), n | |
| Undetectable | 31 |
| Detectable (<1.3) | 8 |
| Detectable (range: 1.3–9.0) | 25 |
| HBeAg, n | |
| Positive | 12 |
| Negative | 49 |
| Not examined | 3 |
| Antiviral therapy, n | |
| ETV | 31 |
| TAF | 8 |
| TDF | 1 |
| LAM | 1 |
| Combined regimen | 7 |
| Treatment-naïve | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, Y.; Tajiri, K.; Ozawa, T.; Angata, K.; Sato, T.; Togayachi, A.; Nagashima, I.; Shimizu, H.; Murayama, A.; Muraishi, N.; et al. Impact of preS1 Evaluation in the Management of Chronic Hepatitis B Virus Infection. Medicina 2024, 60, 1334. https://doi.org/10.3390/medicina60081334
Hayashi Y, Tajiri K, Ozawa T, Angata K, Sato T, Togayachi A, Nagashima I, Shimizu H, Murayama A, Muraishi N, et al. Impact of preS1 Evaluation in the Management of Chronic Hepatitis B Virus Infection. Medicina. 2024; 60(8):1334. https://doi.org/10.3390/medicina60081334
Chicago/Turabian StyleHayashi, Yuka, Kazuto Tajiri, Tatsuhiko Ozawa, Kiyohiko Angata, Takashi Sato, Akira Togayachi, Izuru Nagashima, Hiroki Shimizu, Aiko Murayama, Nozomu Muraishi, and et al. 2024. "Impact of preS1 Evaluation in the Management of Chronic Hepatitis B Virus Infection" Medicina 60, no. 8: 1334. https://doi.org/10.3390/medicina60081334







