Myostatin Changes in Females with UI after Magnetic Stimulation: A Quasi-Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Study Population
2.1.2. Study Device
2.1.3. Study Device Protocols and Quality Assessments
2.1.4. Myostatin Concentration
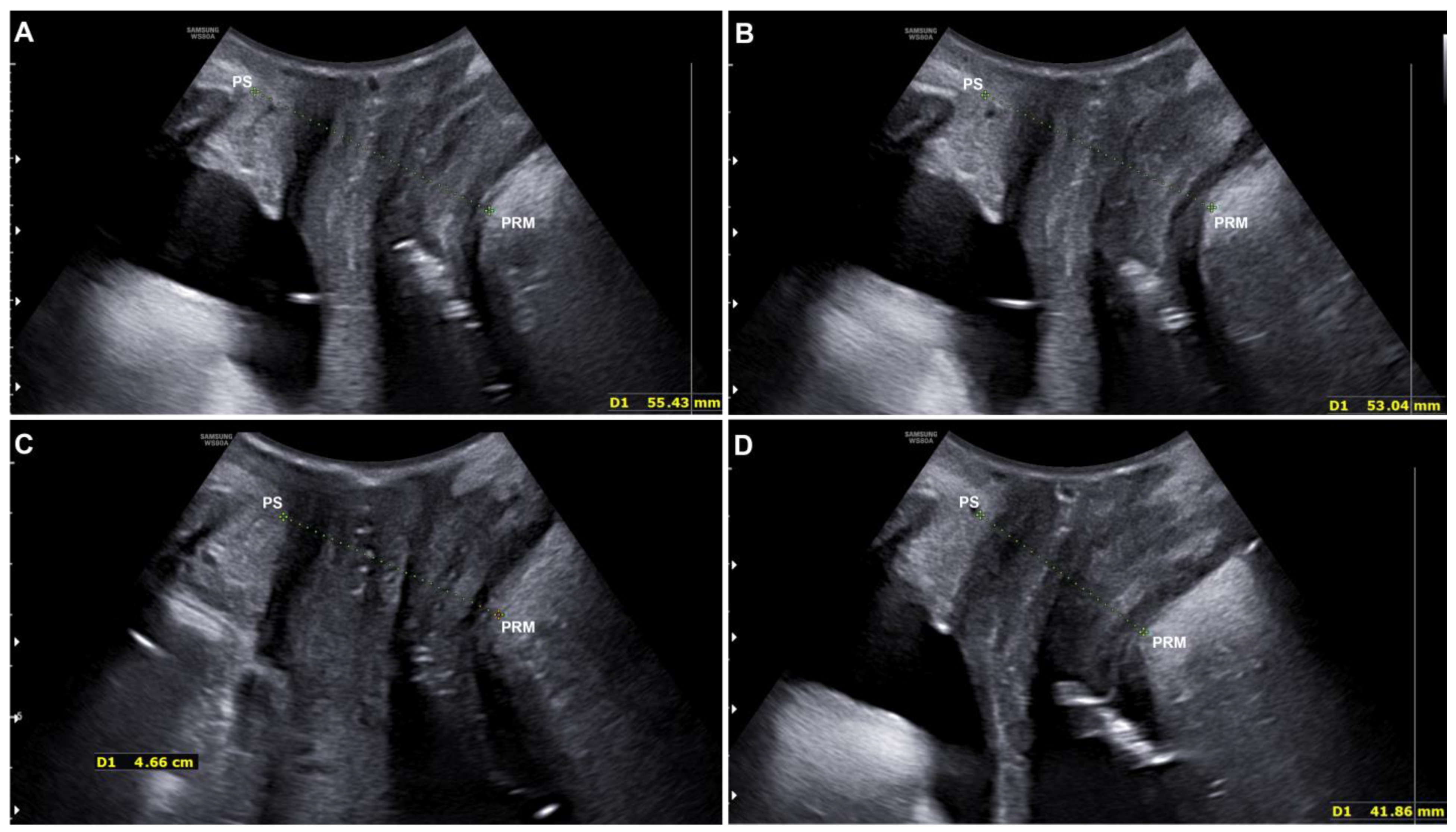
2.1.5. Quantitative Evaluation with Ultrasound
2.1.6. Statistical Analysis

3. Results
3.1. Myostatin Concentration
3.2. Quantitative Evaluation with Ultrasound
3.3. Qualitative Assessment: PFBQ
3.4. Side Effects
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shamliyan, T.; Wyman, J.; Kane, R.L. Nonsurgical Treatments for Urinary Incontinence in Adult Women: Diagnosis and Comparative Effectiveness; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012. [PubMed]
- Lukanovi’c, D.; Blaganje, M.; Barbic, M. Urinary incontinence treatment algorithm. Zdrav. Vestn. 2021, 90, 275–287. [Google Scholar]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The Standardisation of Terminology in Lower Urinary Tract Function: Report from the Standardisation Sub-Committee of the International Continence Society. Urology 2003, 61, 37–49. [Google Scholar] [CrossRef]
- Harris, S.; Stephen, W. Mixed Urinary Incontinence. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024; Bookshelf ID: NBK534234. [Google Scholar] [PubMed]
- Padmanabhan, P.; Dmochowski, R. Urinary incontinence in women: A comprehensive review of the pathophysiology, diagnosis and treatment. Minerva Ginecol. 2014, 66, 469–478. [Google Scholar]
- Lienemann, A.; Fischer, T. Functional imaging of the pelvic floor. Eur. J. Radiol. 2003, 47, 117–122. [Google Scholar] [CrossRef]
- Ayeleke, R.O.; Hay-Smith, E.J.; Omar, M.I. Pelvic floor muscle training added to another active treatment versus the same active treatment alone for urinary incontinence in women. Cochrane Database Syst. Rev. 2013, 11, CD010551. [Google Scholar]
- Perucchini, D.; DeLancey, J.O.L. Pelvic Floor Re-Education: Principles and Practice; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Greer, J.A.; Arya, L.A.; Smith, A.L. Urinary Incontinence: Diagnosis and Treatment in the Elderly. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2013, 2, 66–75. [Google Scholar] [CrossRef][Green Version]
- Yount, S.M.; Fay, R.A.; Kissler, K.J. Prenatal and Postpartum Experience, Knowledge and Engagement with Kegels: A Longitudinal, Prospective, Multisite Study. J. Women’s Health 2021, 30, 891–901. [Google Scholar] [CrossRef]
- Lopopolo, G.; Salsi, B.; Banfi, A.; Isaza, P.G.; Fusco, I. Is It Possible to Improve Urinary Incontinence and Quality of Life in Female Patients? A Clinical Evaluation of the Efficacy of Top Flat Magnetic Stimulation Technology. Bioengineering 2022, 9, 140. [Google Scholar] [CrossRef]
- Biondo, A.; González-Isaza, P.; Fusco, I. Efficacy of Top Flat Magnetic Stimulation Technology for Female Stress and Urge Urinary Incontinence: A Clinical Evaluation. World J. Nephrol. Urol. 2022, 11, 18–23. [Google Scholar] [CrossRef]
- Vodusek, D.B. Anatomy and neurocontrol of the pelvic floor. Digestion 2004, 69, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.N.; Bordoni, B. Anatomy, Abdomen and Pelvis: Levator Ani Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; Bookshelf ID: NBK556078. [Google Scholar]
- Dietz, H.P.; Simpson, J.M. Levator trauma is associated with pelvic organ prolapse. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 979–984. [Google Scholar] [CrossRef]
- Joulia-Ekaza, D.; Cabello, G. Myostatin regulation of muscle development: Molecular basis, natural mutations, physiopathological aspects. Exp. Cell Res. 2006, 312, 2401–2414. [Google Scholar]
- Min, J.; Li, B.; Liu, C.; Hong, S.; Tang, J.; Hu, M.; Liu, Y.; Li, S.; Hong, L. Therapeutic Effect and Mechanism of Electrical Stimulation in Female Stress Urinary Incontinence. Urology 2017, 104, 45–51. [Google Scholar] [CrossRef]
- Harland, N.; Walz, S.; Eberli, D.; Schmid, F.A.; Aicher, W.K.; Stenzl, A.; Amend, B. Stress Urinary Incontinence: An Unsolved Clinical Challenge. Biomedicines 2023, 11, 2486. [Google Scholar] [CrossRef]
- Glass, D.J. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr. Top. Microbiol. Immunol. 2010, 346, 267–278. [Google Scholar]
- Esposito, P.; Picciotto, D.; Battaglia, Y.; Costigliolo, F.; Viazzi, F.; Verzola, D. Myostatin: Basic biology to clinical application. Adv. Clin. Chem. 2022, 106, 181–234. [Google Scholar]
- Whittemore, L.A.; Song, K.; Li, X.; Aghajanian, J.; Davies, M.; Girgenrath, S.; Hill, J.J.; Jalenak, M.; Kelley, P.; Knight, A.; et al. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem. Biophys. Res. Commun. 2003, 300, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Akita, Y.; Sumino, Y.; Mori, K.; Nomura, T.; Sato, F.; Mimata, H. Myostatin inhibits proliferation of human urethral rhabdosphincter satellite cells. Int. J. Urol. 2013, 20, 522–529. [Google Scholar] [CrossRef]
- Frigerio, M.; Barba, M.; Cola, A.; Marino, G.; Volontè, S.; Melocchi, T.; De Vicari, D.; Maruccia, S. Flat Magnetic Stimulation for Stress Urinary Incontinence: A Prospective Comparison Study. Bioengineering 2023, 10, 295. [Google Scholar] [CrossRef]
- Haylen, B.T.; De Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int. Urogynecol. J. 2010, 21, 5–26. [Google Scholar] [CrossRef]
- Dominguez, A.P.; Isaza, P.G.; Pantoja, S.N.; Fusco, I. Role of top flat magnetic stimulation for urinary incontinence as a debilitating condition of pelvic floor dysfunction: An observational evaluation of Latin American population. World J. Urol. 2023, 41, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.V.; Karp, D.R.; Aguilar, V.C.; Davila, G.W. Validation of a global pelvic floor symptom bother questionnaire. Int. Urogynecol. J. 2010, 21, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.P.; Haylen, B.T.; Broome, J. Ultrasound in the quantification of female pelvic organ prolapse. Ultrasound Obstet. Gynecol. 2001, 18, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciencies; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Persu, C.; Chapple, C.R.; Cauni, V.; Gutue, S.; Geavlete, P. Pelvic Organ Prolapse Quantification System (POP-Q)—A new era in pelvic prolapse staging. J. Med. Life 2011, 4, 75–81. [Google Scholar]
- Filippini, M.; Biordi, N.; Curcio, A.; Comito, A.; Pennati, B.M.; Farinelli, M. A Qualitative and Quantitative Study to Evaluate the Effectiveness and Safety of Magnetic Stimulation in Women with Urinary Incontinence Symptoms and Pelvic Floor Disorders. Medicina 2023, 59, 879. [Google Scholar] [CrossRef]
- González-Isaza, P.; Sánchez-Borrego, R.; Lugo Salcedo, F.; Rodríguez, N.; Vélez Rizo, D.; Fusco, I.; Callarelli, S. Pulsed Magnetic Stimulation for Stress Urinary Incontinence and Its Impact on Sexuality and Health. Medicina 2022, 58, 1721. [Google Scholar] [CrossRef]
- Frigerio, M.; Barba, M.; Cola, A.; Braga, A.; Celardo, A.; Munno, G.M.; Schettino, M.T.; Vagnetti, P.; De Simone, F.; Di Lucia, A.; et al. Quality of Life, Psychological Wellbeing, and Sexuality in Women with Urinary Incontinence-Where Are We Now: A Narrative Review. Medicina 2022, 58, 525. [Google Scholar] [CrossRef]
- Strasser, H.; Tiefenthaler, M.; Steinlechner, M.; Eder, I.; Bartsch, G.; Konwalinka, G. Age dependent apoptosis and loss of rhabdosphincter cells. J. Urol. 2000, 164, 1781–1785. [Google Scholar] [CrossRef]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.; van Kranenburg, J.; Verdijk, L.B.; van Loon, L.J.C. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef]
- Sammarco, A.G.; Nandikanti, L.; Kobernik, E.K.; Xie, B.; Jankowski, A.; Swenson, C.W.; DeLancey, J.O.L. Interactions among pelvic organ protrusion, levator ani descent, and hiatal enlargement in women with andwithout prolapse. Am. J. Obstet. Gynecol. 2017, 217, 614.e1–614.e7. [Google Scholar] [CrossRef]
- Delancey, J.O.; Hurd, W.W. Size of the urogenital hiatus in the levator ani muscles in normal women and women with pelvic organ prolapse. Obstet. Gynecol. 1998, 91, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Vakili, B.; Zheng, Y.T.; Loesch, H.; Echols, K.T.; Franco, N.; Chesson, R.R. Levator contraction strength and genital hiatus as risk factors for recurrent pelvic organ prolapse. Am. J. Obstet. Gynecol. 2005, 192, 1592–1598. [Google Scholar] [CrossRef]
- Binns, A.; Gray, M.; Henson, A.C.; Fort, I.L. Changes in Lean Mass and Serum Myostatin with Habitual Protein Intake and High-Velocity Resistance Training. J. Nutr. Health Aging 2017, 21, 1111–1117. [Google Scholar] [CrossRef]
- de Sordi, C.M.; Dos Reis-Neto, E.T.; Keppeke, G.D.; Shinjo, S.K.; Inoue Sato, E. Serum Myostatin and Follistatin Levels in Patients with Dermatomyositis and Polymyositis. Multicent. Study J. Clin. Rheumatol. 2022, 28, 33–37. [Google Scholar] [CrossRef]
- Baczek, J.; Silkiewicz, M.; Wojszel, Z.B. Myostatin as a Biomarker of Muscle Wasting and other Pathologies-State of the Art and Knowledge Gaps. Nutrients 2020, 12, 2401. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, X.; Chen, D. Myostatin: A novel insight into its role in metabolism, signal pathways, and expression regulation. Cell. Signal. 2011, 23, 1441–1446. [Google Scholar] [CrossRef]
- Yuan, H.; Ruan, Y.; Tan, Y.; Reed-Maldonado, A.B.; Chen, Y.; Zhao, D.; Wang, Z.; Zhou, F.; Peng, D.; Banie, L.; et al. Regenerating Urethral Striated Muscle by CRISPRi/dCas9-KRAB-Mediated Myostatin Silencing for Obesity-Associated Stress Urinary Incontinence. CRISPR J. 2020, 3, 562–572. [Google Scholar] [CrossRef]
- Reardon, K.A.; Davis, J.; Kapsa, R.M.; Choong, P.; Byrne, E. Myostatin, insulin-like growth factor-1, and leukemia inhibitory factor mRNAs are upregulated in chronic human disuse muscle atrophy. Muscle Nerve 2001, 24, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Weber-Rajek, M.; Radzimińska, A.; Strączyńska, A.; Podhorecka, M.; Kozakiewicz, M.; Perkowski, R.; Jarzemski, P.; Kędziora-Kornatowska, K.; Goch, A. A randomized-controlled trial pilot study examining the effect of extracorporeal magnetic innervation in the treatment of stress urinary incontinence in women. Clin. Interv. Aging 2018, 13, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-M.; Xiao, H.; Ji, Z.-G.; Yan, W.-G.; Zhang, Y.-S. TVT versus TOT in the Treatment of Female Stress Urinary Incontinence: A Systematic Review and Meta-Analysis. Ther. Clin. Risk Manag. 2018, 14, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Hașegan, A.; Mihai, I.; Teodoru, C.A.; Matacuta, I.B.; Dura, H.; Todor, S.B.; Ichim, C.; Tanasescu, D.; Grigore, N.; Bolca, C.N.; et al. Exploring the Challenges of Using Minimal Invasive Surgery to Treat Stress Urinary Incontinence: Insights from a Retrospective Case-Control Study. Diagnostics 2024, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- Rosen, N.O.; Dawson, S.J.; Brooks, M.; Kellogg-Spadt, S. Treatment of Vulvodynia: Pharmacological and Non-Pharmacological Approaches. Drugs 2019, 79, 483–493. [Google Scholar] [CrossRef] [PubMed]

| Number of Patients | 19 |
| UI Type(%) | SUI (75.00%) UUI (25.00%) |
| Prolapse(%) | 63.15% |
| Uterine (Grade II: 13.33%) Rectocele (Grade I: 6.66%, Grade II: 13.33%) Cystocele (Grade I: 33.33%, Grade II: 33.33%) | |
| Menopausal Patients(%) | 31.57% |
| Average Age (Mean ± SD) | 49.42 ± 9.72 (range 27–72) |
| Number of Pregnancies (Mean ± SD) | 1.31 ± 0.88 (range 0–3) |
| Myostatin Concentration | p Value | ||
|---|---|---|---|
| T0 (ng/mL) | Tend (ng/mL) | ||
| Mean | 14.43 | 7.18 | p < 0.01 |
| STDev | 9.53 | 3.96 | p < 0.01 |
| At Rest (mm) | Interval Confidence Values | Contraction (mm) | Interval Confidence Values | |
|---|---|---|---|---|
| Before treatment | 56.57 ± 6.52 | 52.72; 60.42 | 47.49 ± 4.86 | 44.62; 50.36 |
| End of the treatment | 54.32 ± 6.38 | 50.55; 58.09 | 44.80 ± 5.07 | 41.81; 47.80 |
| Significance (T0 vs. Tend) | p < 0.001 | p < 0.001 |
| Baseline | End of the Treatment | Significance | |
|---|---|---|---|
| Questionnaire PFBQ | 38.51 ± 21.93 | 28.51 ± 13.87 | p < 0.01 |
| Interval confidence values | 25.20; 51.83 | 20.09; 36.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippini, M.; Bugli, S.; Biordi, N.; Muccioli, F.; Reggini, V.; Benedettini, M.; Migliore, S.; Pieri, L.; Comito, A.; Pennati, B.M.; et al. Myostatin Changes in Females with UI after Magnetic Stimulation: A Quasi-Experimental Study. Medicina 2024, 60, 1399. https://doi.org/10.3390/medicina60091399
Filippini M, Bugli S, Biordi N, Muccioli F, Reggini V, Benedettini M, Migliore S, Pieri L, Comito A, Pennati BM, et al. Myostatin Changes in Females with UI after Magnetic Stimulation: A Quasi-Experimental Study. Medicina. 2024; 60(9):1399. https://doi.org/10.3390/medicina60091399
Chicago/Turabian StyleFilippini, Maurizio, Simona Bugli, Nicoletta Biordi, Fausto Muccioli, Valentina Reggini, Milena Benedettini, Serena Migliore, Laura Pieri, Alessandra Comito, Beatrice Marina Pennati, and et al. 2024. "Myostatin Changes in Females with UI after Magnetic Stimulation: A Quasi-Experimental Study" Medicina 60, no. 9: 1399. https://doi.org/10.3390/medicina60091399
APA StyleFilippini, M., Bugli, S., Biordi, N., Muccioli, F., Reggini, V., Benedettini, M., Migliore, S., Pieri, L., Comito, A., Pennati, B. M., Fusco, I., Isaza, P. G., Dominguez, A. P., Zingoni, T., & Farinelli, M. (2024). Myostatin Changes in Females with UI after Magnetic Stimulation: A Quasi-Experimental Study. Medicina, 60(9), 1399. https://doi.org/10.3390/medicina60091399








