Evaluation of Treatment Protocols in Surgically Assisted Rapid Maxillary Expansion by Finite Element Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Modeling of the Maxilla and Teeth Models
2.3. Finite Element Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, W.C.; Shieh, Y.; Liao, Y.F.; Lee, C.H.; Huang, C.S. Long-term maxillary three-dimensional changes following maxillary protraction with or without expansion: A systematic review and meta-analysis. J. Dent. Sci. 2021, 16, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Goncales, E.S.; Polido, W. Surgical and orthodontic treatment of maxillary transverse deficiency. Concepts for the oral and maxillofacial surgeon and case report. Rev. Inst. Cien. Saude 1998, 16, 55. [Google Scholar]
- Betts, N.J.; Vanarsdall, R.L.; Barber, H.D.; Higgins-Barber, K.; Fonseca, R. Diagnosis and treatment of transverse maxillary deficiency. Int. J. Adult Orthodon. Orthognath. Surg. 1995, 10, 75–96. [Google Scholar] [PubMed]
- Koudstaal, M.J.; Wolvius, E.B.; Schulten, A.J.M.; Hop, W.C.J.; Van der Wal, K.G.H. Stability, tipping and relapse of bone-borne versus tooth-borne surgically assisted rapid maxillary expansion; A prospective randomized patient trial. Int. J. Oral Maxillofac. Surg. 2009, 38, 308–315. [Google Scholar] [CrossRef]
- Sant’Ana, L.F.M.; Pinzan-Vercelino, C.R.M.; Gurgel, J.A.; Carvalho, P.S.P.D. Evaluation of surgically assisted rapid maxillary expansion with and without midpalatal split. Int. J. Oral Maxillofac. Surg. 2016, 45, 997–1001. [Google Scholar] [CrossRef][Green Version]
- Suri, L.; Taneja, P. Surgically assisted rapid palatal expansion: A literature review. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 290–302. [Google Scholar] [CrossRef]
- Coloccia, G.; Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Montenegro, V.; Patano, A.; Marinelli, G.; Laudadio, C.; Limongelli, L.; Di Venere, D.; et al. Effectiveness of dental and maxillary transverse changes in tooth-borne, bone-borne, and hybrid palatal expansion through cone-beam tomography: A systematic review of the literature. Medicina 2021, 57, 288. [Google Scholar] [CrossRef]
- Sygouros, A.; Motro, M.; Ugurlu, F.; Acar, A. Surgically assisted rapid maxillary expansion: Cone-beam computed tomography evaluation of different surgical techniques and their effects on the maxillary dentoskeletal complex. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 748–757. [Google Scholar] [CrossRef]
- Seitz, O.; Landes, C.A.; Philipp, D.J.; Sader, R.; Klein, C.M. Reliable surgically assisted rapid palatal expansion by maxillary widening device. J. Craniofac. Surg. 2008, 19, 846–849. [Google Scholar] [CrossRef]
- Esen, A.; Soganci, E.; Dolanmaz, E.; Dolanmaz, D. Evaluation of stress by finite element analysis of the midface and skull base at the time of midpalatal osteotomy in models with or without pterygomaxillary dysjunction. Br. J. Oral Maxillofac. Surg. 2018, 56, 177–181. [Google Scholar] [CrossRef]
- Lanigan, D.; Mintz, S. Complications of surgically assisted rapid palatal expansion: Review of the literature and report of a case. J. Oral Maxillofac. Surg. 2002, 60, 104–110. [Google Scholar] [CrossRef]
- Jafari, A.; Shetty, K.S.; Kumar, M. Study of stress distribution and displacement of various craniofacial structures following application of transverse orthopedic forces—A three-dimensional FEM study. Angle Orthod. 2003, 73, 12–20. [Google Scholar] [PubMed]
- de Assis, D.S.F.R.; Xavier, T.A.; Noritomi, P.Y.; Gonçales, E.S. Finite element analysis of bone stress after SARPE. J. Oral Maxillofac. Surg. 2014, 72, 167.e1–167.e9. [Google Scholar] [CrossRef] [PubMed]
- Zemann, W.; Schanbacher, M.; Feichtinger, M.; Linecker, A.; Kärcher, H. Dentoalveolar changes after surgically assisted maxillary expansion: A three-dimensional evaluation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 36–42. [Google Scholar] [CrossRef]
- Ulusoy, Ç.; Dogan, M. A new method for the treatment of unilateral posterior cross-bite: A three-dimensional finite element stress analysis study. Prog. Orthod. 2018, 19, 31. [Google Scholar] [CrossRef]
- Cansiz, E.; Dogru, S.; Arslan, Y. Comparative evaluation of the mechanical properties of resorbable and titanium miniplates used for fixation of mandibular condyle fractures. J. Mech. Med. Biol. 2015, 15, 1540032. [Google Scholar] [CrossRef]
- Cattaneo, P.M.; Dalstra, M.; Melsen, B. Strains in periodontal ligament and alveolar bone associated with orthodontic tooth movement analyzed by finite element. Orthod. Craniofac. Res. 2009, 12, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Dogru, S.C.; Cansiz, E.; Arslan, Y.Z. A review of finite element applications in oral and maxillofacial biomechanics. J. Mech. Med. Biol. 2018, 18, 1830002. [Google Scholar] [CrossRef]
- Jensen, T.; Johannesen, L.; Rodrigo-Domingo, M. Periodontal changes after surgically assisted rapid maxillary expansion (SARME). Oral Maxillofac. Surg. 2015, 19, 381–386. [Google Scholar] [CrossRef][Green Version]
- Chamberland, S.; Proffit, W.R. Closer look at the stability of surgically assisted rapid palatal expansion. J. Oral Maxillofac. Surg. 2008, 66, 1895–1900. [Google Scholar] [CrossRef]
- Barone, T.R.; Cahali, M.B.; Vasconcelos, C.; Barone, J.R. A comparison of tooth-borne and bone-anchored expansion devices in SARME. Oral Maxillofac. Surg. 2020, 24, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, F.; Solidoro, L.; Bartolucci, M.L.; Parenti, S.I.; Paganelli, C.; Alessandri-Bonetti, G. Skeletal and dental effects of surgically assisted rapid palatal expansion: A systematic review of randomized controlled trials. Eur. J. Orthod. 2019, 42, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Hamedi Sangsari, A.; Sadr-Eshkevari, P.; Al-Dam, A.; Friedrich, R.E.; Freymiller, E.; Rashad, A. Surgically assisted rapid palatomaxillary expansion with or without pterygomaxillary disjunction: A systematic review and meta-analysis. J. Oral Maxillofac. Surg. 2016, 74, 338–348. [Google Scholar] [CrossRef]
- Zandi, M.; Miresmaeili, A.; Heidari, A.; Lamei, A. The necessity of pterygomaxillary disjunction in surgically assisted rapid maxillary expansion: A short-term, double-blind, historical controlled clinical trial. J. Craniomaxillofac. Surg. 2016, 44, 1181–1186. [Google Scholar] [CrossRef]
- De Gijt, J.P.; Gül, A.; Tjoa, S.T.H.; Wolvius, E.B.; van der Wal, K.G.H.; Koudstaal, M.J. Follow-up of surgically-assisted rapid maxillary expansion after 6.5 years: Skeletal and dental effects. Br. J. Oral Maxillofac. Surg. 2017, 55, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Olejnik, A.; Gerber, H.; Frątczak, R.; Zawiślak, E. Comparison of tooth- and bone-borne appliances on the stress distributions and displacement patterns in the facial skeleton in surgically assisted rapid maxillary expansion—A finite element analysis (FEA) study. Materials 2021, 14, 1152. [Google Scholar] [CrossRef]
- Tehranchi, A.; Ameli, N.; Najirad, Z.; Mirhashemi, F.S. Comparison of the skeletal and dental changes of tooth-borne vs. bone-borne expansion devices in surgically assisted rapid palatal expansion: A finite element study. Dent. Res. J. 2013, 10, 777. [Google Scholar]
- Trojan, L.C.; González-Torres, L.A.; Melo, A.C.M.; de Las Casas, E.B. Stresses and strains analysis using different palatal expander appliances in upper jaw and midpalatal suture. Artif. Organs 2017, 6, E41–E51. [Google Scholar]
- Möhlhenrich, S.C.; Modabber, A.; Kniha, K.; Peters, F.; Steiner, T.; Hölzle, F.; Fritz, U.; Raith, S. Simulation of three surgical techniques combined with two different bone-borne forces for surgically assisted rapid palatal expansion of the maxillofacial complex: A finite element analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 1306–1314. [Google Scholar] [CrossRef]
- Hoxha, S.; Sawafta, A.; Akçam, M.O.; Aliyev, R. Mini implant ve diş-implant destekli hızlı maksiller genişletme: Üç boyutlu FEM çalışması. AÜ Diş. Hek. Fak. Derg. 2018, 45, 33–40. [Google Scholar]
- Kilic, E.; Kilic, B.; Kurt, G.; Sakin, C.; Alkan, A. Effects of Surgically Rapid Palatal Expansion with and without Pterygomaxillary Disjunction on Dental and Skeletal Structures: A Retrospective Review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bin Dakhil, N.; Bin Salamah, F. The Diagnosis Methods and Management Modalities of Maxillary Transverse Discrepancy. Cureus 2021, 13, e20482. [Google Scholar] [CrossRef] [PubMed]
- de Costa Senior, O.; Smeets, M.; Willaert, R.; Shaheen, E.; Jacobs, R.; Politis, C. Complications Following One-Stage versus Two-Stage Surgical Treatment of Transverse Maxillary Hypoplasia. J. Oral Maxillofac. Surg. 2021, 79, 1531–1539. [Google Scholar] [CrossRef] [PubMed]

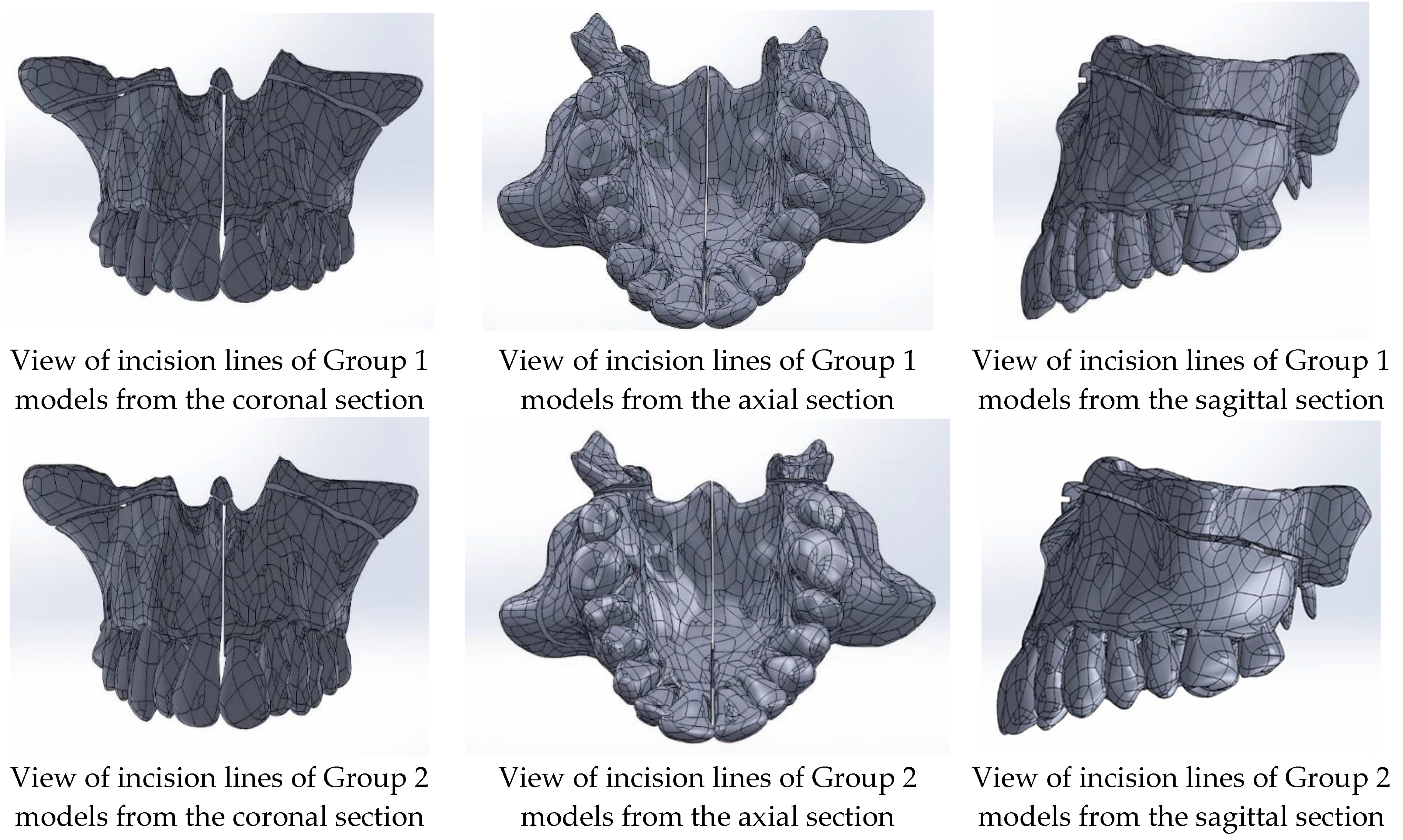
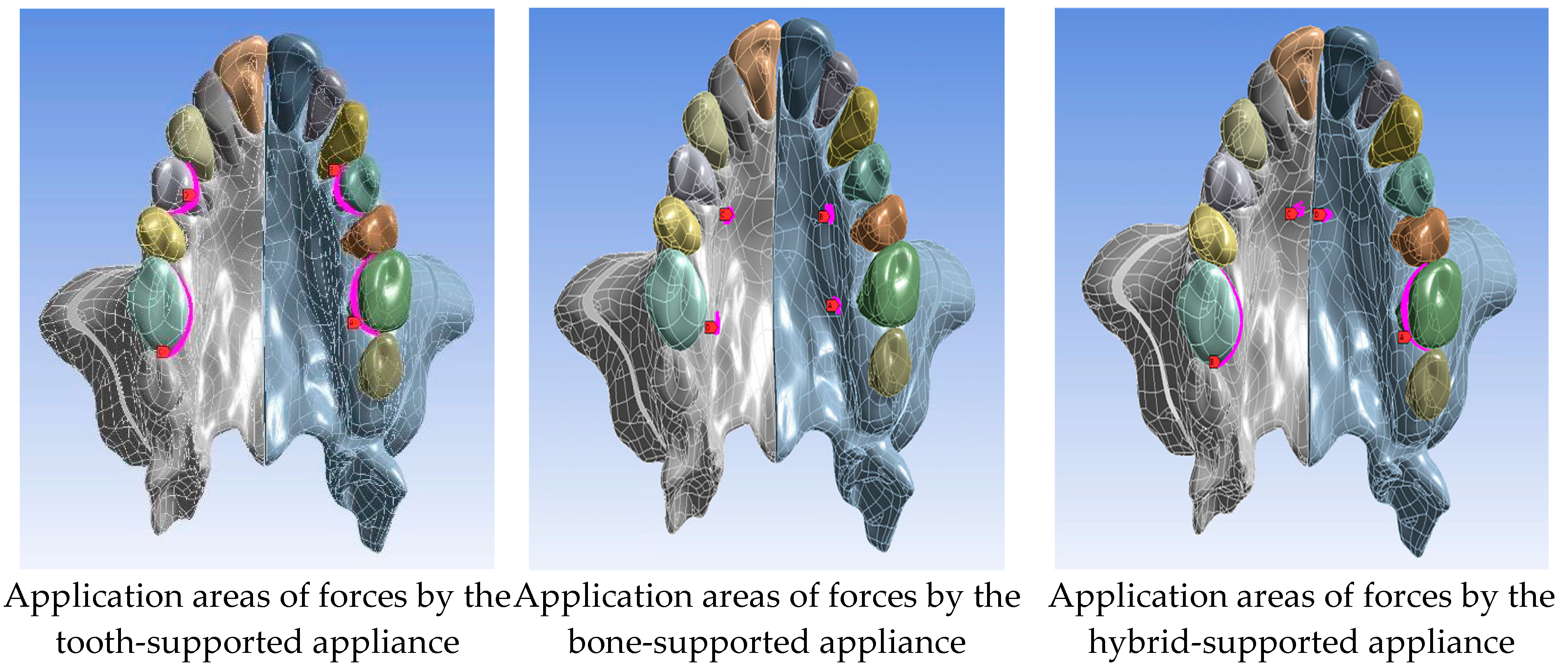
| Group 1: Midpalatal suture separation and Le Fort I corticotomy |
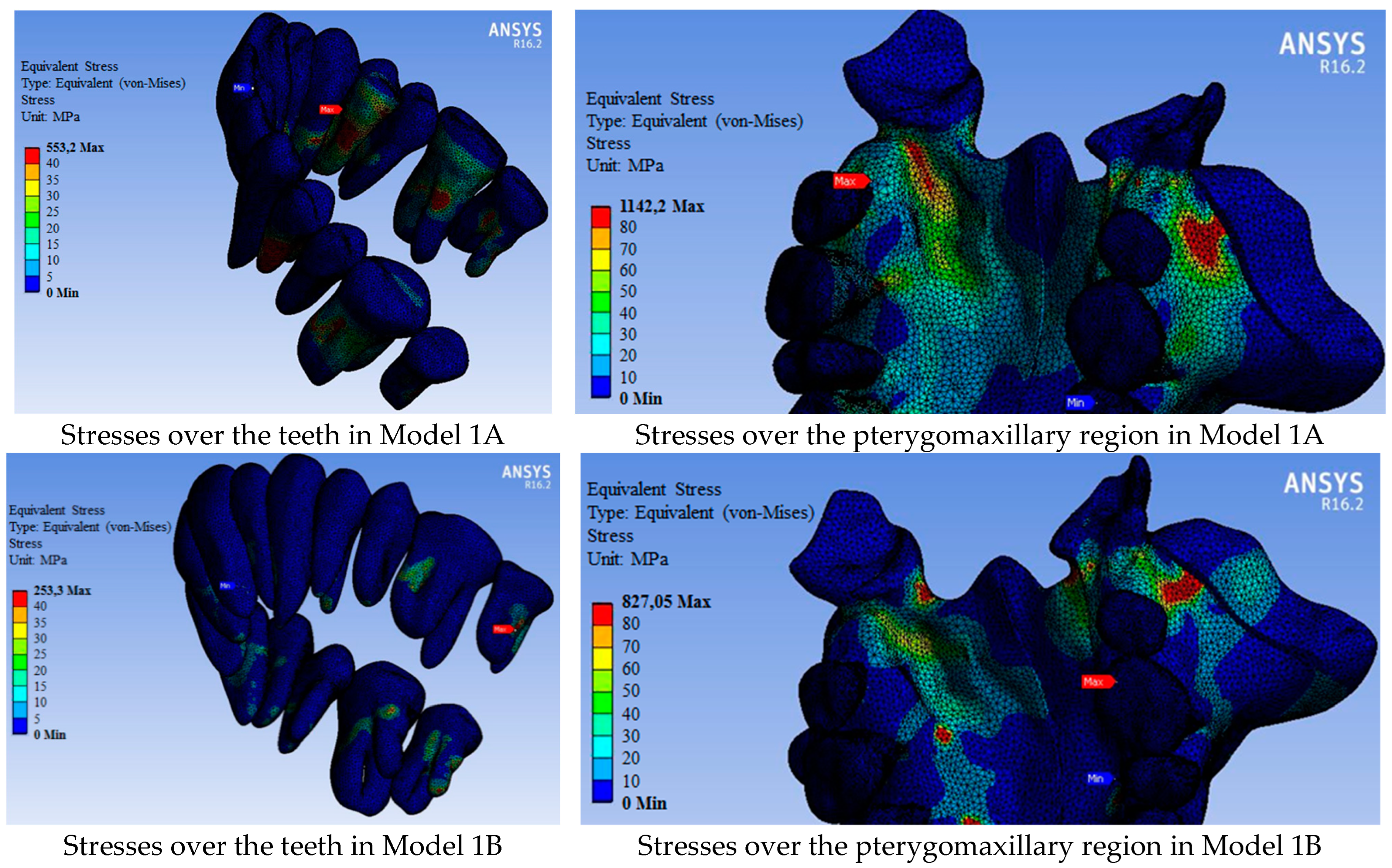
| Model 1A: tooth-supported appliance |
| Model 1B: bone-supported appliance |
| Model 1C: hybrid-supported appliance |
| Group 2: Midpalatal suture separation, Le Fort I corticotomy, and pterygomaxillary junction separation |
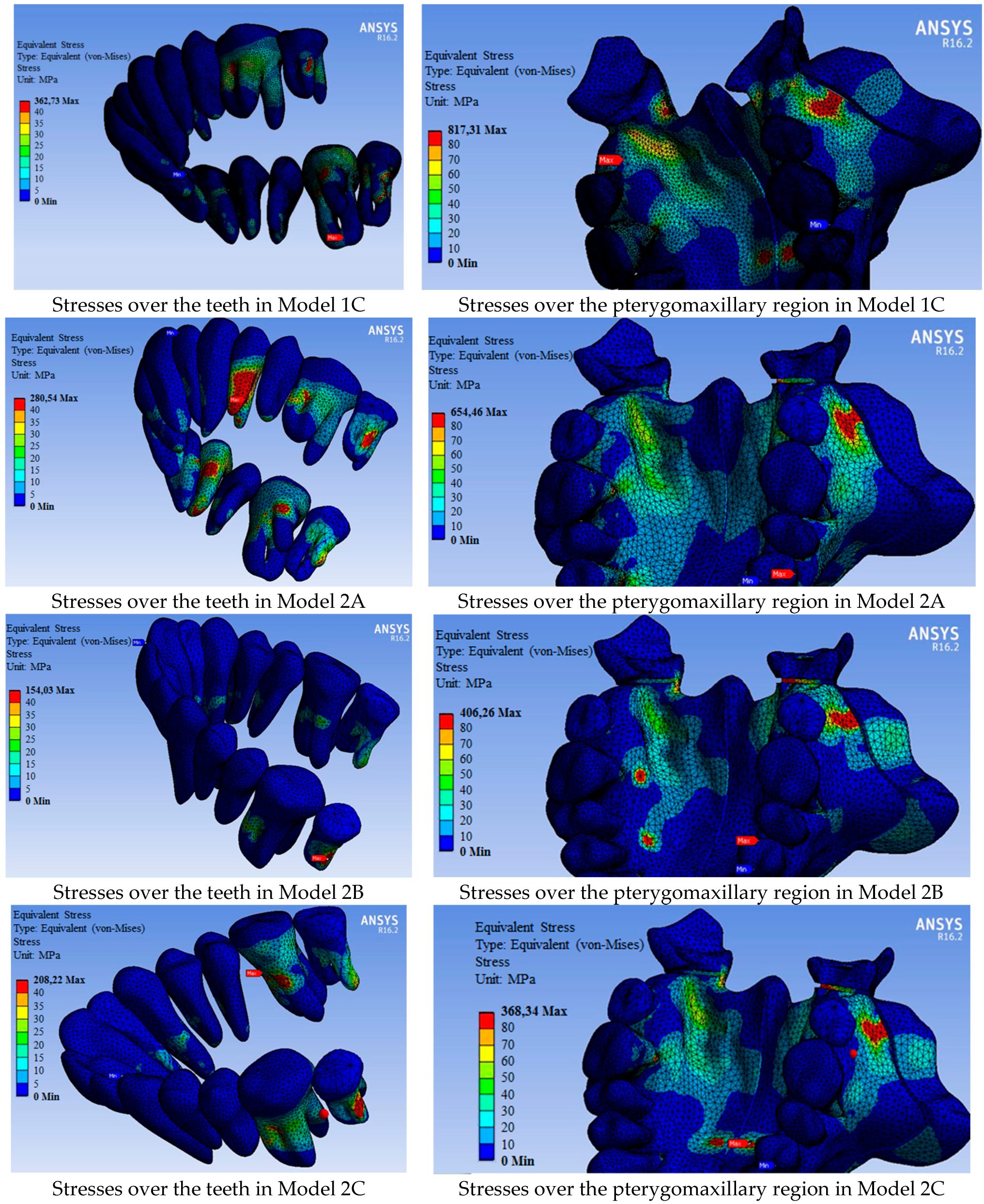
| Model 2A: tooth-supported appliance |
| Model 2B: bone-supported appliance |
| Model 2C: hybrid-supported appliance |
| Type of Appliances | Group 1 | Group 2 |
|---|---|---|
| Tooth-supported sppliance | 350 N | 325 N |
| Bone-supported appliance | 480 N | 370 N |
| Hybrid-supported appliance | 415 N | 400 N |
| Material | Poisson’s Ratio | Young’s Modulus (MPa) |
|---|---|---|
| Tooth | 0.3 | 20.000 |
| Cortical bone | 0.3 | 17.500 |
| Models | Number of Elements | Number of Nodes |
|---|---|---|
| 1A | 1140730 | 1659779 |
| 1B | 1140730 | 1659779 |
| 1C | 1140730 | 1659779 |
| 2A | 1025386 | 1341658 |
| 2B | 1025386 | 1341658 |
| 2C | 1025386 | 1341658 |
| Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|
| Tooth-Supported Appliance (Model 1A) | Bone-Supported Appliance (Model 1B) | Hybrid-Supported Appliance (Model 1C) | Tooth-Supported Appliance (Model 2A) | Bone-Supported Appliance (Model 2B) | Hybrid-Supported Appliance (Model 2C) | |
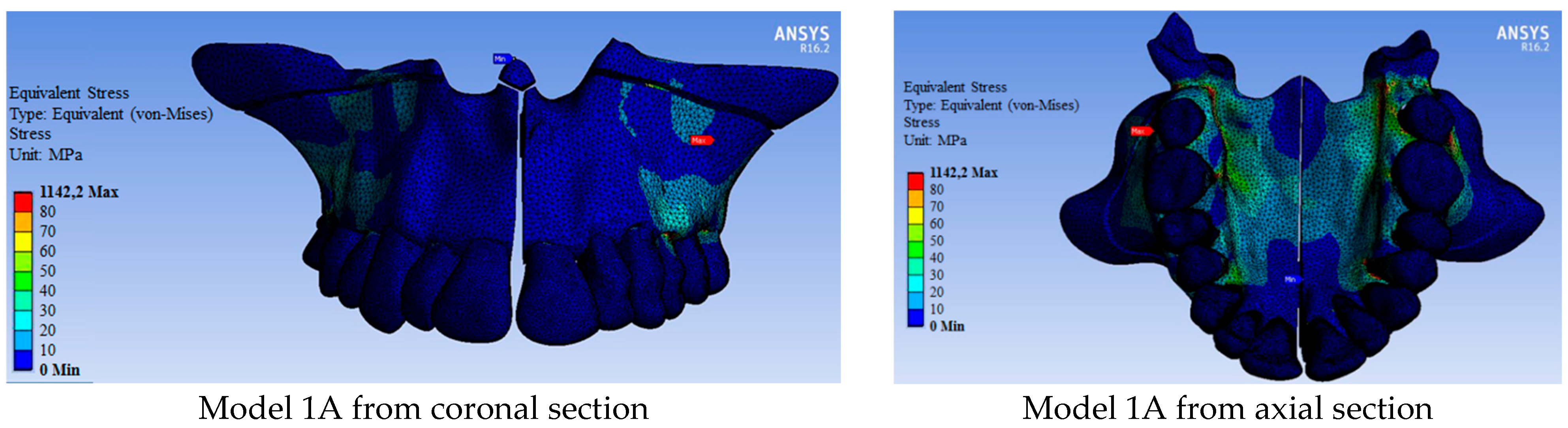
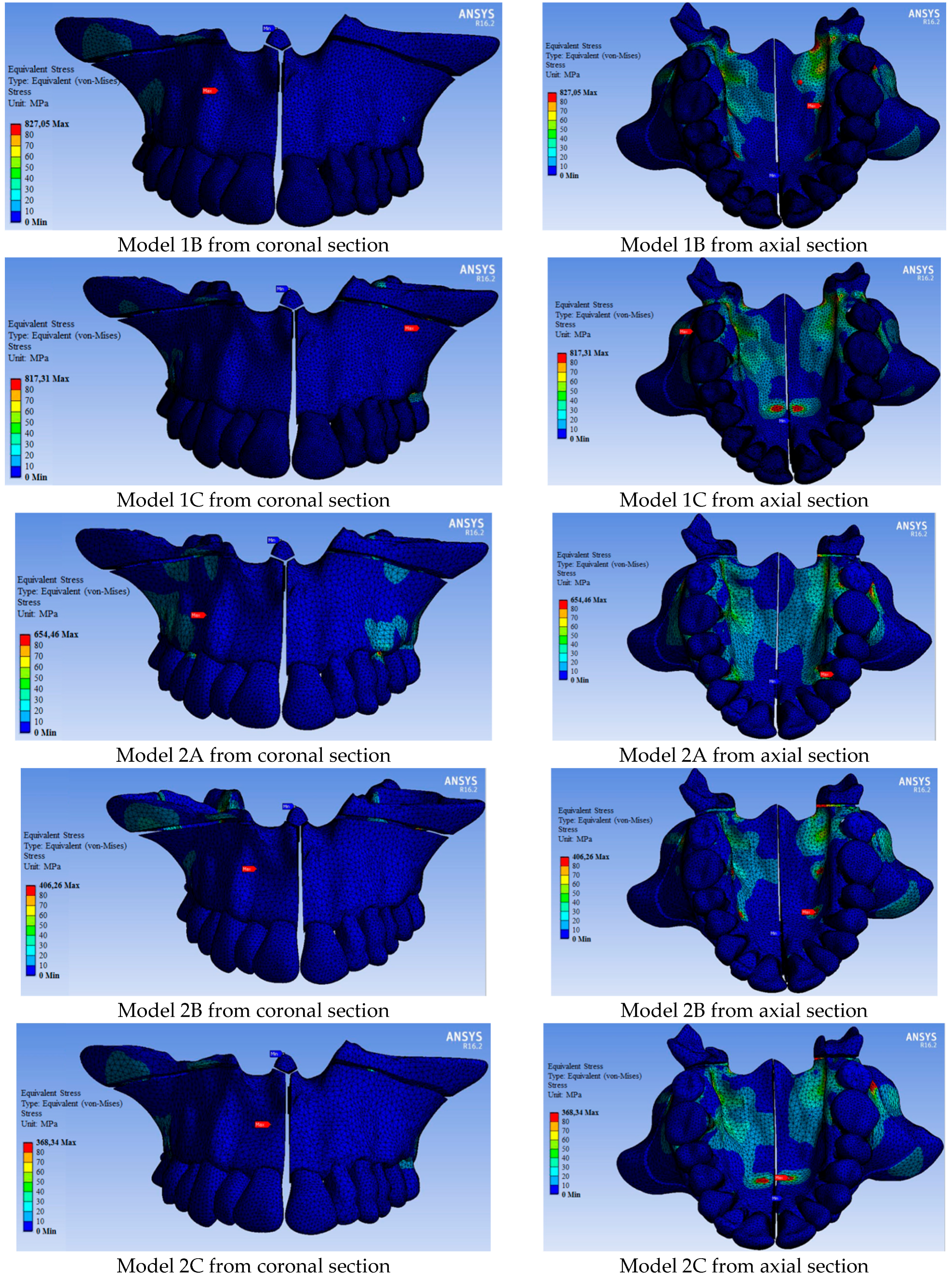
| Maximum stress | 1142.20 | 827.05 | 817.31 | 654.46 | 406.26 | 368.34 |
| Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|
| Tooth Number | Tooth-Supported Appliance (Model 1A) | Bone-Supported Appliance (Model 1B) | Hybrid-Supported Appliance (Model 1C) | Tooth-Supported Appliance (Model 2A) | Bone-Supported Appliance (Model 2B) | Hybrid-Supported Appliance (Model 2C) |
| 11 | 88.19 | 62.10 | 62.15 | 52.31 | 44.01 | 49.09 |
| 12 | 73.01 | 32.22 | 23.42 | 19.81 | 31.69 | 17.85 |
| 13 | 73.46 | 58.42 | 54.87 | 52.04 | 55.86 | 48.70 |
| 14 | 544.28 | 50.04 | 39.42 | 280.54 | 76.69 | 45.03 |
| 15 | 90.95 | 56.17 | 56.86 | 51.27 | 42.76 | 36.82 |
| 16 | 398.71 | 73.44 | 331.31 | 145.43 | 58.24 | 124.17 |
| 17 | 416.84 | 253.30 | 322.63 | 196.33 | 154.03 | 201.46 |
| 21 | 74.20 | 24.64 | 30.92 | 41.52 | 14.79 | 17.92 |
| 22 | 96.39 | 39.54 | 42.74 | 36.39 | 16.51 | 21.03 |
| 23 | 108.40 | 39.60 | 43.43 | 83.43 | 30.78 | 42.60 |
| 24 | 553.20 | 33.22 | 37.64 | 178.39 | 23.71 | 26.03 |
| 25 | 91.04 | 47.62 | 38.20 | 58.01 | 33.26 | 34.78 |
| 26 | 401.93 | 87.91 | 362.73 | 165.78 | 39.49 | 208.22 |
| 27 | 382.54 | 162.17 | 248.03 | 61.91 | 57.06 | 65.16 |
| Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|
| Tooth Number | Tooth-Supported Appliance (Model 1A) | Bone-Supported Appliance (Model 1B) | Hybrid-Supported Appliance (Model 1C) | Tooth-Supported Appliance (Model 2A) | Bone-Supported Appliance (Model 2B) | Hybrid-Supported Appliance (Model 2C) |
| 11 | 0.0041 | 0.0033 | 0.0033 | 0.0032 | 0.0028 | 0.0029 |
| 12 | 0.0039 | 0.0019 | 0.0016 | 0.0013 | 0.0017 | 0.0011 |
| 13 | 0.0040 | 0.0047 | 0.0043 | 0.0036 | 0.0041 | 0.0034 |
| 14 | 0.0302 | 0.0038 | 0.0032 | 0.0152 | 0.0053 | 0.0031 |
| 15 | 0.0049 | 0.0036 | 0.0033 | 0.0032 | 0.0026 | 0.0025 |
| 16 | 0.0367 | 0.0052 | 0.0319 | 0.0093 | 0.0041 | 0.0079 |
| 17 | 0.0241 | 0.0172 | 0.0213 | 0.0149 | 0.0119 | 0.0154 |
| 21 | 0.0039 | 0.0017 | 0.0021 | 0.0024 | 0.0009 | 0.0013 |
| 22 | 0.0051 | 0.0026 | 0.0028 | 0.0025 | 0.0013 | 0.0016 |
| 23 | 0.0056 | 0.0024 | 0.0026 | 0.0052 | 0.0016 | 0.0022 |
| 24 | 0.0284 | 0.0020 | 0.0024 | 0.0123 | 0.0014 | 0.0019 |
| 25 | 0.0049 | 0.0031 | 0.0026 | 0.0031 | 0.0018 | 0.0019 |
| 26 | 0.0217 | 0.0056 | 0.0221 | 0.0085 | 0.0026 | 0.0107 |
| 27 | 0.0247 | 0.0119 | 0.0179 | 0.0038 | 0.0035 | 0.0039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cihaner, D.; Karabulut, D.; Dogan Onur, O.; Cansiz, E.; Arslan, Y.Z. Evaluation of Treatment Protocols in Surgically Assisted Rapid Maxillary Expansion by Finite Element Analysis. Medicina 2024, 60, 1400. https://doi.org/10.3390/medicina60091400
Cihaner D, Karabulut D, Dogan Onur O, Cansiz E, Arslan YZ. Evaluation of Treatment Protocols in Surgically Assisted Rapid Maxillary Expansion by Finite Element Analysis. Medicina. 2024; 60(9):1400. https://doi.org/10.3390/medicina60091400
Chicago/Turabian StyleCihaner, Duygu, Derya Karabulut, Ozen Dogan Onur, Erol Cansiz, and Yunus Ziya Arslan. 2024. "Evaluation of Treatment Protocols in Surgically Assisted Rapid Maxillary Expansion by Finite Element Analysis" Medicina 60, no. 9: 1400. https://doi.org/10.3390/medicina60091400
APA StyleCihaner, D., Karabulut, D., Dogan Onur, O., Cansiz, E., & Arslan, Y. Z. (2024). Evaluation of Treatment Protocols in Surgically Assisted Rapid Maxillary Expansion by Finite Element Analysis. Medicina, 60(9), 1400. https://doi.org/10.3390/medicina60091400






