Do Stainless-Steel Pins Coated with Fibroblast Growth Factor–Calcium Phosphatase Composite Layers Have Anti-Infective Effects?
Abstract
1. Introduction
2. Materials and Methods
2.1. Screws Coated with Fibroblast Growth Factor–Calcium Phosphate Composite Layers
2.2. Scanning Electron Microscope of the Coating Layer
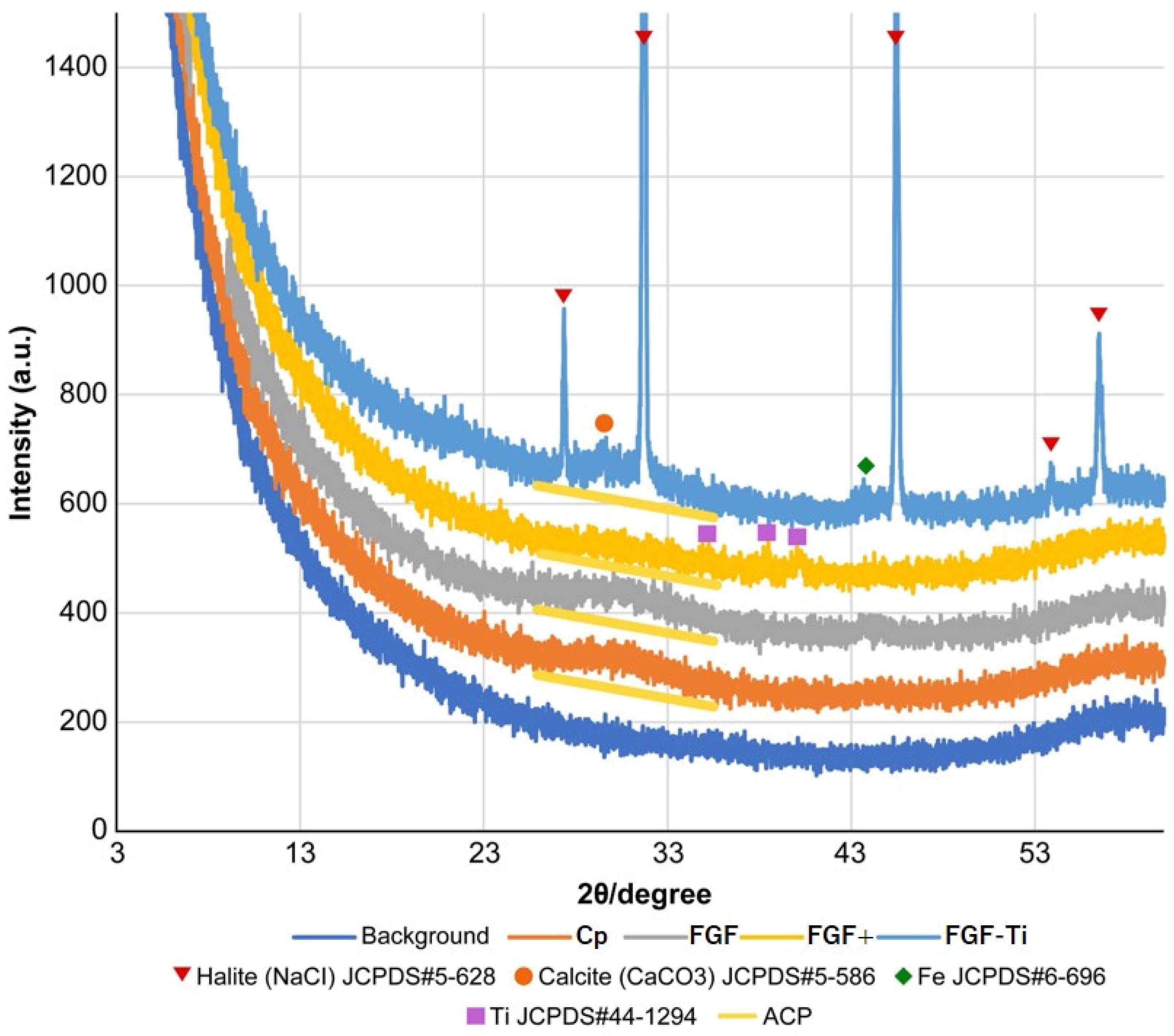
2.3. X-ray Diffraction of the Coating Layer
2.4. Coating Detaching Test
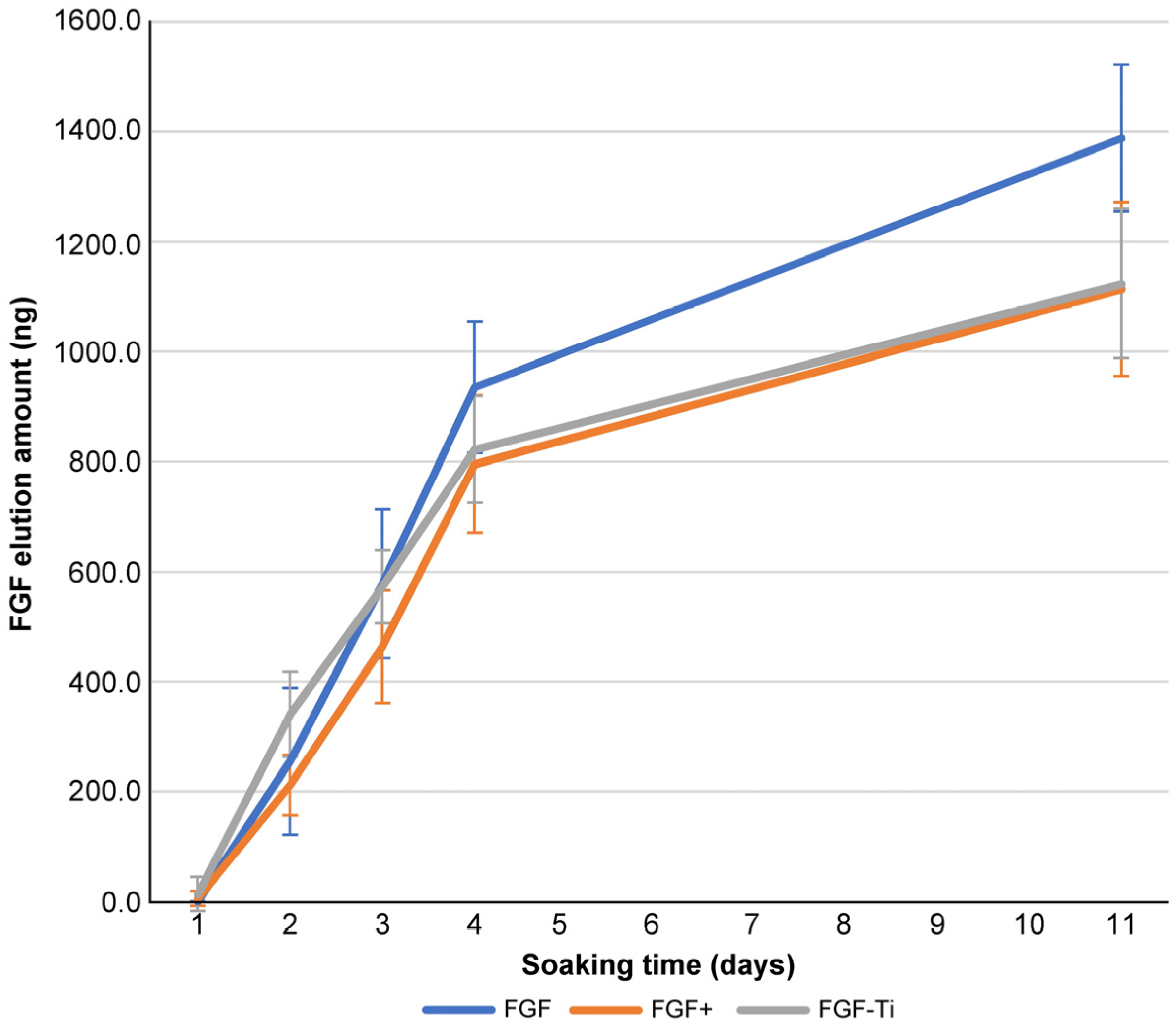
2.5. Sustained Release Test
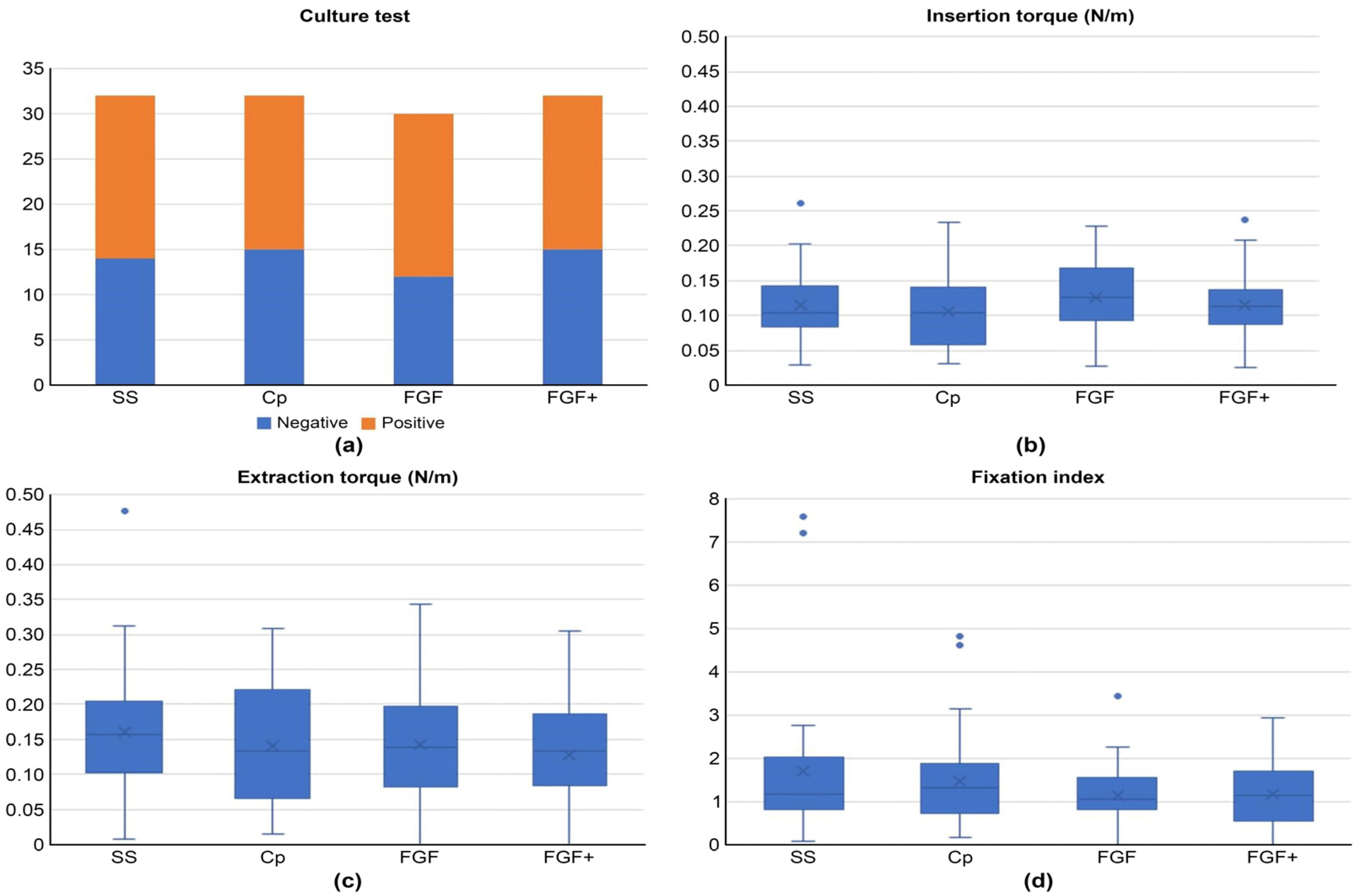
2.6. Bioassay
2.7. Animal Experiments
2.8. Statistical Analysis
3. Results
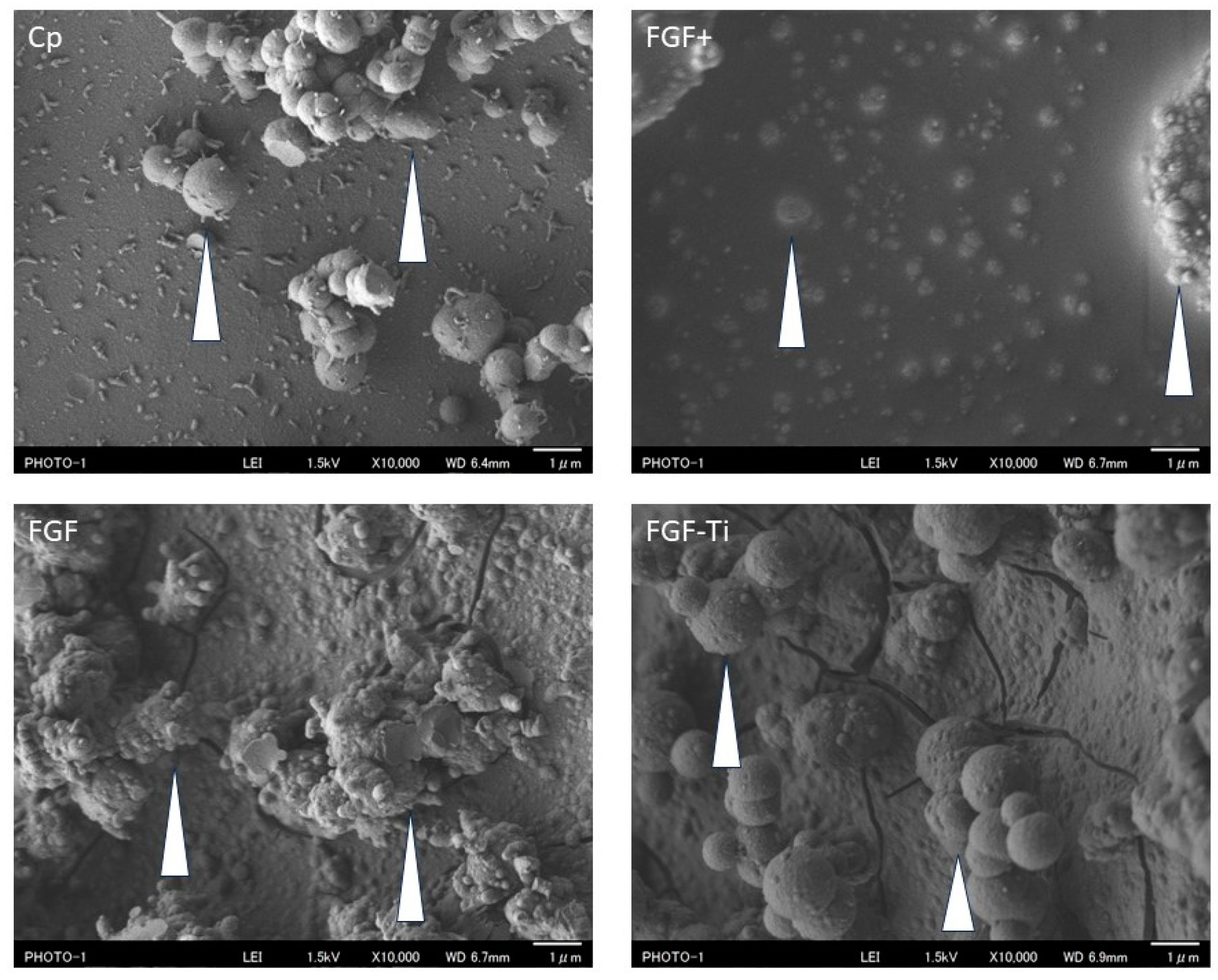
3.1. Scanning Electron Microscope of the Coated Layer
3.2. X-ray Diffraction of the Coated Layer
3.3. Coating Detachment Test
3.4. Sustained Release Test
3.5. Bioassay
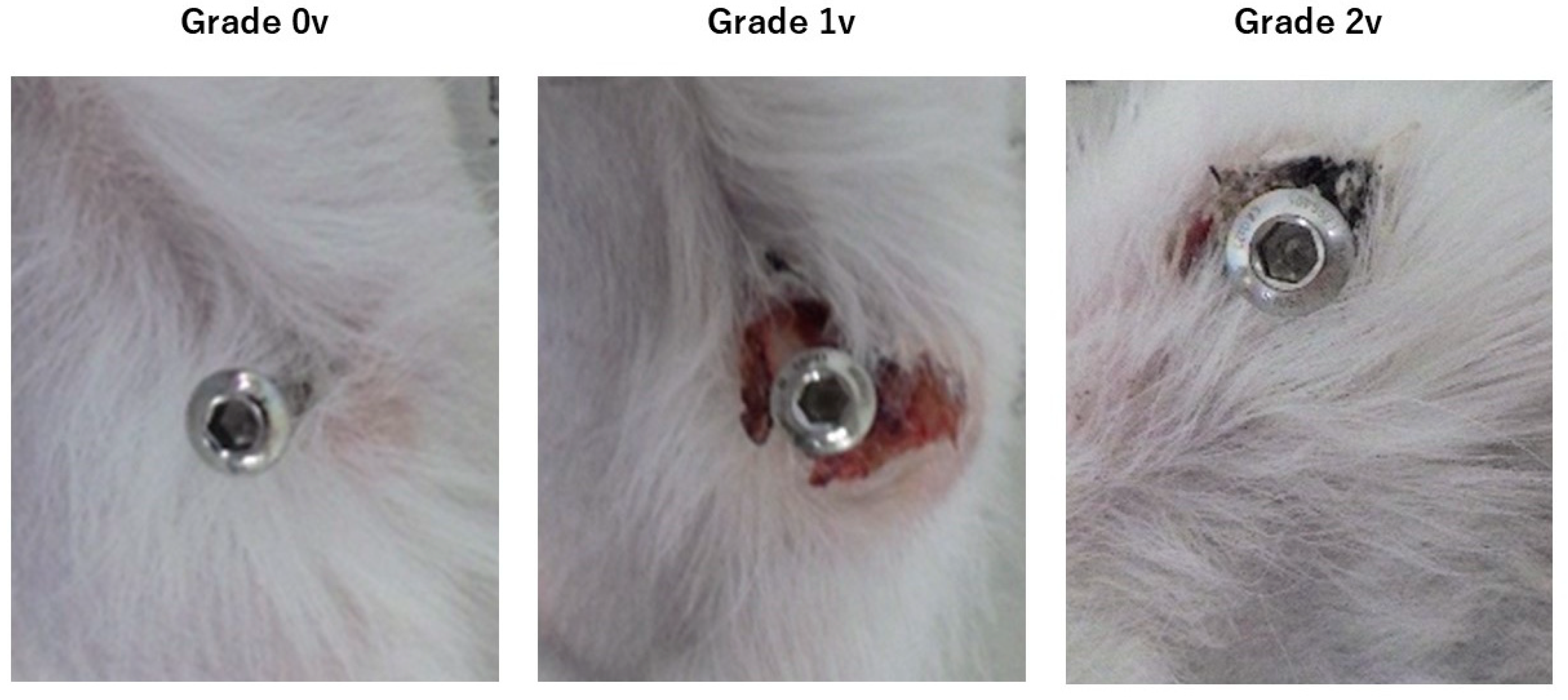
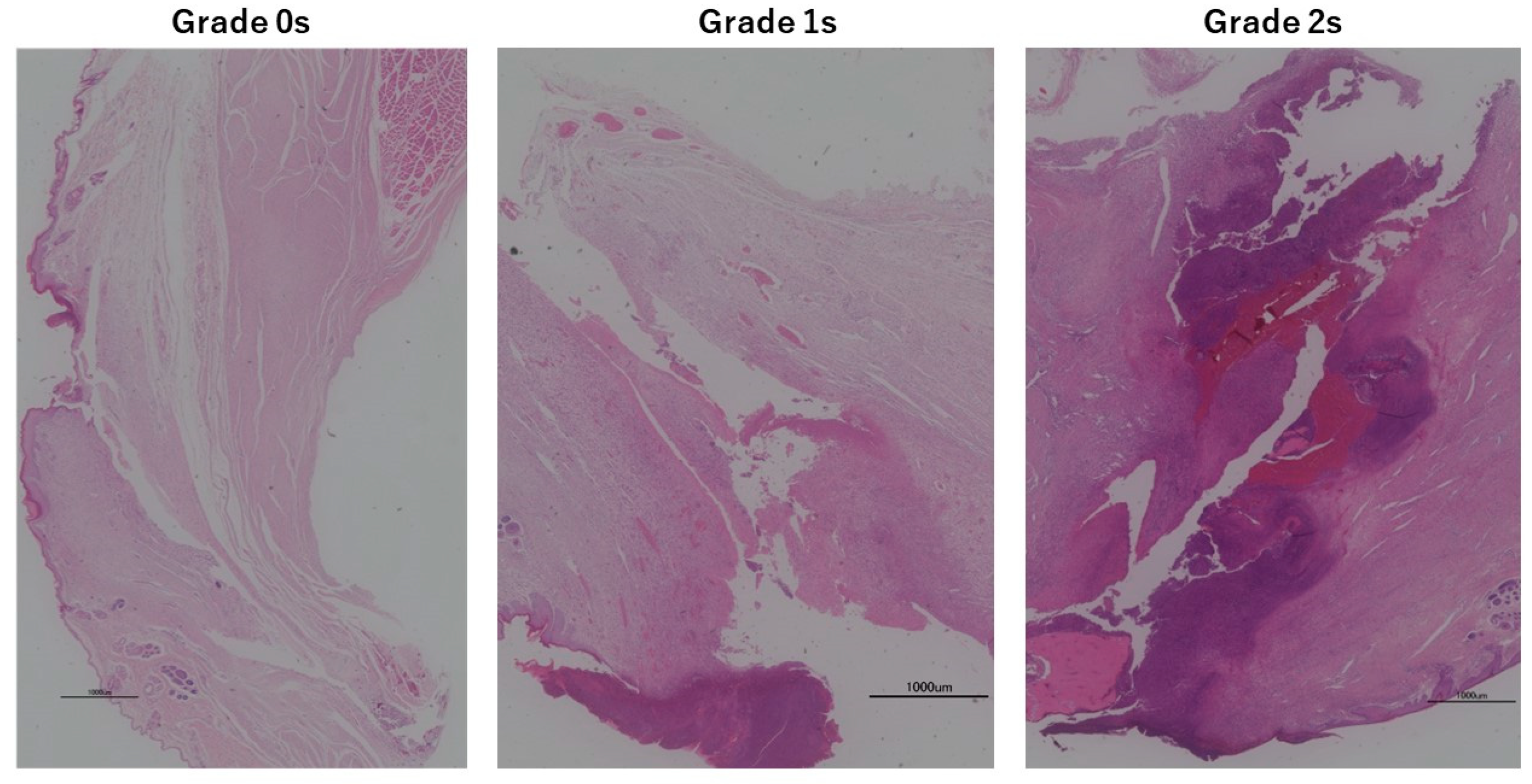
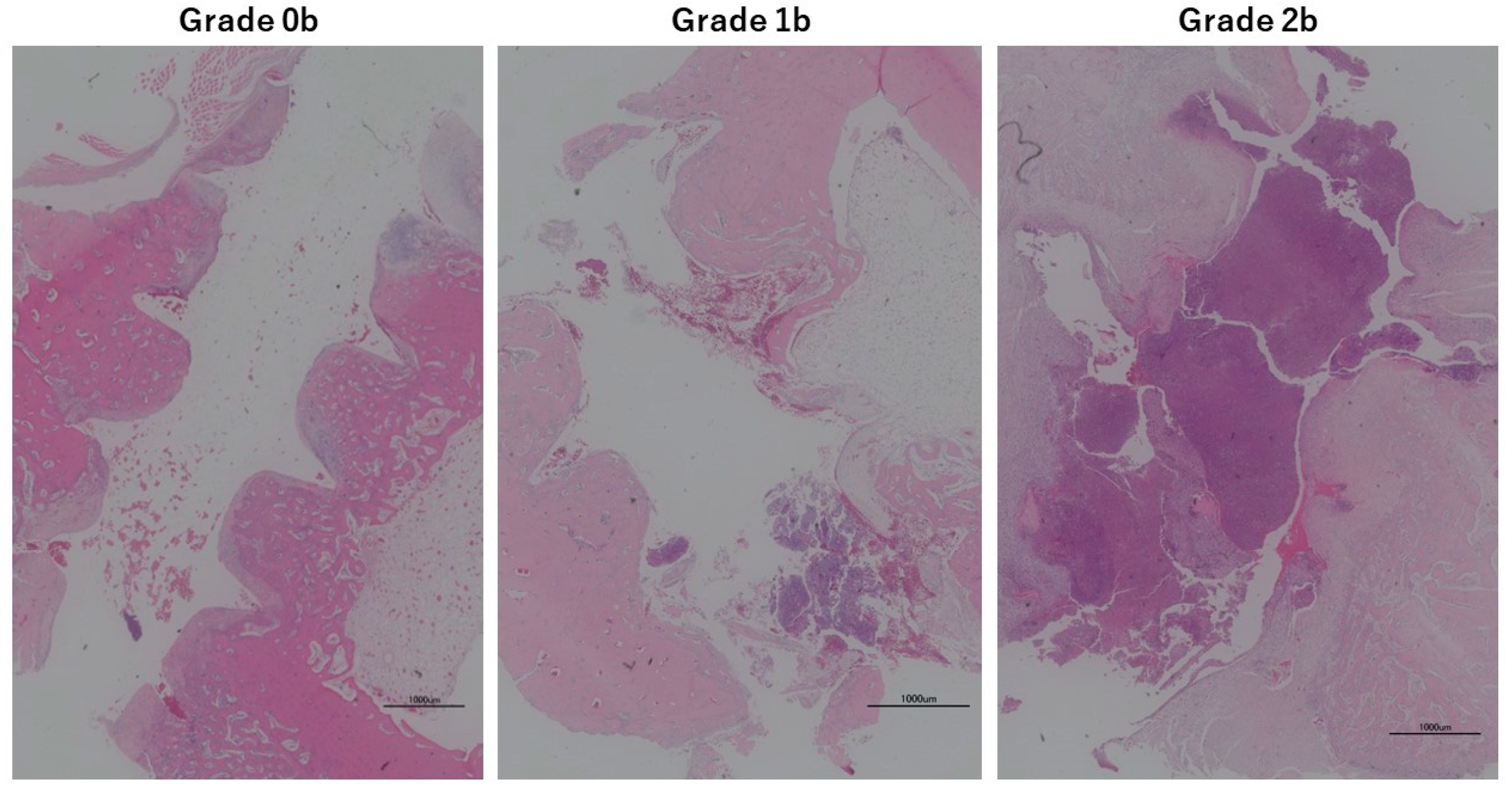
3.6. Animal Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahan, J.; Seligson, D.; Henry, S.L.; Hynes, P.; Dobbins, J. Factors in pin tract infections. Orthopedics 1991, 14, 305–308. [Google Scholar] [PubMed]
- Kazmers, N.H.; Fragomen, A.T.; Rozbruch, S.R. Prevention of pin site infection in external fixation: A review of the literature. Strategies Trauma. Limb Reconstr. 2016, 11, 75–85. [Google Scholar] [PubMed]
- DeJong, E.S.; DeBerardino, T.M.; Brooks, D.E.; Nelson, B.J.; Campbell, A.A.; Bottoni, C.R.; Pusateri, A.E.; Walton, R.S.; Guymon, C.H.; McManus, A.T. Antimicrobial efficacy of external fixator pins coated with a lipid stabilized hydroxyapatite/chlorhexidine complex to prevent pin tract infection in a goat model. J. Trauma. 2001, 50, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Jennison, T.; McNally, M.; Pandit, H. Prevention of infection in external fixator pin sites. Acta Biomater. 2014, 10, 595–603. [Google Scholar] [CrossRef]
- Ferreira, N.; Marais, L.C. Prevention and management of external fixator pin track sepsis. Strateg. Trauma. Limb Reconstr. 2012, 7, 67–72. [Google Scholar]
- Schalamon, J.; Petnehazy, T.; Ainoedhofer, H.; Zwick, E.B.; Singer, G.; Hoellwarth, M.E. Pin tract infection with external fixation of pediatric fractures. J. Pediatr. Surg. 2007, 42, 1584–1587. [Google Scholar] [CrossRef]
- Lee, C.K.; Chua, Y.P.; Saw, A. Antimicrobial gauze as a dressing reduces pin site infection: A randomized controlled trial. Clin. Orthop. Relat. Res. 2012, 470, 610–615. [Google Scholar] [CrossRef]
- Parameswaran, A.D.; Roberts, C.S.; Seligson, D.; Voor, M. Pin tract infection with contemporary external fixation: How much of a problem? J. Orthop. Trauma. 2003, 17, 503–507. [Google Scholar] [CrossRef]
- W-Dahl, A.; Toksvig-Larsen, S.; Lindstrand, A.A. No difference between daily and weekly pin site care: A randomized study of 50 patients with external fixation. Acta Orthop. Scand. 2003, 74, 704–708. [Google Scholar] [CrossRef]
- Cam, R.; Demir Korkmaz, F.; Oner Şavk, S. Effects of two different solutions used in pin site care on the development of infection. Acta Orthop. Traumatol. Turc. 2014, 48, 80–85. [Google Scholar] [CrossRef]
- Ktistakis, I.; Guerado, E.; Giannoudis, P.V. Pin-site care: Can we reduce the incidence of infections? Injury 2015, 46 (Suppl. S3), S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Wassall, M.A.; Santin, M.; Isalberti, C.; Cannas, M.; Denyer, S.P. Adhesion of bacteria to stainless steel and silver-coated orthopedic external fixation pins. J. Biomed. Mater. Res. 1997, 36, 325–330. [Google Scholar] [CrossRef]
- Coester, L.M.; Nepola, J.V.; Allen, J.; Marsh, J.L. The effects of silver coated external fixation pins. Iowa Orthop. J. 2006, 26, 48–53. [Google Scholar] [PubMed]
- Nakamura, H.; Matsuno, T.; Hashimoto, Y.; Nakamura, T.; Mataga, I. Comparison of a hydroxyapatite-coated and an anodic oxidized titanium implant for experimentally induced peri-implantitis: Macroscopic and novel radiographic evaluations in a canine model. J. Hard Tissue Biol. 2015, 24, 347–356. [Google Scholar] [CrossRef][Green Version]
- Saithna, A. The influence of hydroxyapatite coating of external fixator pins on pin loosening and pin track infection: A systematic review. Injury 2010, 41, 128–132. [Google Scholar] [CrossRef]
- Stoffel, C.; Eltz, B.; Salles, M.J. Role of coatings and materials of external fixation pins on the rates of pin tract infection: A systematic review and meta-analysis. World J. Orthop. 2021, 12, 920–930. [Google Scholar] [CrossRef]
- Shirai, T.; Watanabe, K.; Matsubara, H.; Nomura, I.; Fujiwara, H.; Arai, Y.; Ikoma, K.; Terauchi, R.; Kubo, T.; Tsuchiya, H. Prevention of pin tract infection with iodine-supported titanium pins. J. Orthop. Sci. 2014, 19, 598–602. [Google Scholar] [CrossRef]
- Mutsuzaki, H.; Ito, A.; Sogo, Y.; Sakane, M.; Oyane, A.; Ochiai, N. Enhanced wound healing associated with Sharpey’s fiber-like tissue formation around FGF-2-apatite composite layers on percutaneous titanium screws in rabbits. Arch. Orthop. Trauma. Surg. 2012, 132, 113–121. [Google Scholar] [CrossRef]
- Davies, R.; Holt, N.; Nayagam, S. The care of pin sites with external fixation. J. Bone Joint Surg. Br. 2005, 87, 716–719. [Google Scholar] [CrossRef]
- Hanch, L.L. Biomaterials. Science 1980, 208, 826–831. [Google Scholar] [CrossRef]
- Ganser, A.; Thompson, R.E.; Tami, I.; Neuhoff, D.; Steiner, A.; Ito, K. An in vivo experimental comparison of stainless steel and titanium Schanz screws for external fixation. Eur. J. Trauma. Emerg. Surg. 2007, 33, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Mutsuzaki, H.; Ito, A.; Sakane, M.; Sogo, Y.; Oyane, A.; Ochiai, N. Fibroblast growth factor-2-apatite composite layers on titanium screw to reduce pin tract infection rate. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Oyane, A.; Ito, A. Chapter 6. Signal Molecule-Calcium Phosphate Composites: Novel Approaches to Controlling Cellular and/or Biological Reactions and Functions. In Advances in Calsium Phosphate Biomaterials; Springer: Berlin/Heidelberg, Germany, 2014; pp. 171–197. [Google Scholar]
- Wu, M.; Chen, G.; Li, Y.P. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Fromigué, O.; Modrowski, D.; Marie, P.J. Growth factors and bone formation in osteoporosis: Roles for fibroblast growth factor and transforming growth factor beta. Curr. Pharm. Des. 2004, 10, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Zeng, H.C.; Bae, Y.; Dawson, B.C.; Chen, Y.; Bertin, T.; Munivez, E.; Campeau, P.M.; Tao, J.; Chen, R.; Lee, B.H. MicroRNA miR-23a cluster promotes osteocyte differentiation by regulating TGF-beta signalling in osteoblasts. Nat. Commun. 2017, 8, 15000. [Google Scholar] [CrossRef]
- Behr, B.; Sorkin, M.; Lehnhardt, M.; Renda, A.; Longaker, M.T.; Quarto, N. A comparative analysis of the osteogenic effects of BMP-2, FGF-2, and VEGFA in a calvarial defect model. Tissue Eng. Part. A 2012, 18, 1079–1086. [Google Scholar] [CrossRef]
- Nimori, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar]
- Disegi, J.A.; Eschbach, L. Stainless steel in bone surgery. J. Care Inj. 2000, 31, D2–D6. [Google Scholar] [CrossRef]
- Disegi, J.A. Titanium alloys for fracture fixation implants. J. Care Inj. 2000, 31, D14–D17. [Google Scholar] [CrossRef]
- Sogo, Y.; Sogo, Y.; Fujii, K.; Yanagisawa, Y.; Kobayashi, F.; Murai, S.; Mutsuzaki, H.; Hara, Y.; Yamazaki, M.; Ito, A. Impacts of chemically different surfaces of implants on a biologicalactivity of fibroblast growth factor-2-apatite composite layers formedon the implants. Orthop. Traumatol. Surg. Res. 2021, 107, 102748. [Google Scholar] [CrossRef]
- Placzek, R.; Ruffer, M.; Deuretzbacher, G.; Heijens, E.; Meiss, A.L. The fixation strength of hydroxyapatite-coated Schanz screws and standard stainless steel Schanz screws in lower extremity lengthening: A comparison based on a new torque value index: The fixation index. Arch. Orthop. Trauma. Surg. 2006, 126, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Indurkar, A.; Choudhary, R.; Rubenis, K.; Nimbaklar, M.; Sarakovskis, A.; Boccaccini, A.R.; Locs, J. Amorphous calcium phosphate and Amorphous calcium phosphate Carboxylate: Synthesis and Characterization. ACS Omega 2023, 8, 26782–26792. [Google Scholar] [CrossRef] [PubMed]
- Mutsuzaki, H.; Ito, A.; Sogo, Y.; Sakane, M.; Oyane, A.; Yamazaki, M. The calcium phosphate matrix of FGF-2-Apatite composite layers contribute to their biological effects. Int. J. Mol. Sci. 2014, 15, 10252–10270. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, Y.; Ito, A.; Hara, Y.; Mutsuzaki, H.; Murai, S.; Fujii, K.; Sogo, Y.; Hirose, M.; Oyane, A.; Kobayashi, F.; et al. Initial clinical trial of pins coated with fibroblast growth factor-2–apatite composite layer in external fixation of distal radius fractures. J. Orthop. 2019, 16, 69–73. [Google Scholar] [CrossRef]
- Mutsuzaki, H.; Ito, A.; Sakane, M.; Sogo, Y.; Oyane, A.; Ebihara, Y.; Ichinose, N.; Ochiai, N. Calcium phosphate coating formed in infusion fluid mixture to enhance fixation strength of titanium screws. J. Mater. Sci. Mater. Med. 2007, 18, 1799–1808. [Google Scholar] [CrossRef]
- Donley, T.G.; Gillette, W.B. Titanium endosseous implant-soft tissue interface: A literature review. J. Periodontol. 1991, 62, 153–160. [Google Scholar] [CrossRef]
- Gould, T.R.L.; Westbury, L.; Brunette, D.M. Ultrastructural study of the attachment of human gingiva to titanium in vivo. J. Prosthet. Dent. 1984, 52, 418–420. [Google Scholar] [CrossRef]
- Gould, T.R.L.; Brunette, D.M.; Westbury, L. The attachment mechanism of epithelial cells to titanium in vitro. J. Periodontal Res. 1981, 16, 611–616. [Google Scholar] [CrossRef]
- Arimori, T.; Miyazaki, N.; Mihara, E.; Takizawa, M.; Taniguchi, Y.; Cabañas, C.; Sekiguchi, K.; Takagi, J. Structural mechanism of laminin recognition by integrin. Nat. Commun. 2021, 12, 4012. [Google Scholar] [CrossRef]
- Kraft, C.N.; Hansis, M.; Arens, S.; Menger, M.D.; Vollmar, B. Striated muscle microvascular response to silver implants: A comparative in vivo study with titanium and stainless steel. J. Biomed. Mater. Res. 2000, 49, 192–199. [Google Scholar] [CrossRef]
- Akyol, S.; Bozkus, H.; Adin Cinar, S.; Hanci, M.M. Which is better. Stainless steel or titanium alloy? Turk. Neurosurg. 2018, 28, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Yasunaga, M.; Hirose, M.; Kakehata, M.; Yashiro, H.; Yamazaki, A.; Ito, A. Cell attachment area of rat mesenchymal stem cells correlates with their osteogenic differentiation level on substrates without osteoconductive property. Biochem. Biophys. Res. Commun. 2020, 525, 1081–1086. [Google Scholar] [CrossRef]




| Infusion Solution Containing Calcium |
|---|
| Ringer’s solution, 500 mL (Otsuka, Otsuka Pharmaceutical Factory, Tokushima, Japan) |
| Calcium chloride correction solution, 1 mEq/mL, 20 mL (Otsuka Pharmaceutical Factory, Tokushima, Japan) |
| Intravenous solution containing phosphoric acid |
| Klinisalz infusion solution, 500 mL (Kyowa CritiCare, Tokyo, Japan) |
| Dipotassium phosphate injection, 20 mEq Kit, 20 mL (Terumo, Tokyo, Japan) |
| Alkalizing agent (sodium bicarbonate solution) |
| Meylon Intravenous Injection 7%, 20 mL (Otsuka Pharmaceutical Factory, Tokushima, Japan) |
| Dissolving and diluting solution |
| Otsuka Physiological Saline Injection, 20 mL (Otsuka Pharmaceutical Factory, Tokushima, Japan) |
| Water for injection PL, 20 mL (Fuso, Fuso Pharmaceutical Industries, Osaka, Japan) |
| FGF-2 (trafermin) |
| Fiblast Spray 500 (Kaken Pharmaceuticals, Tokyo, Japan) |
| Name of Soaking Solution | F0 | F4 |
|---|---|---|
| FGF-2 (μg/mL) | 0 | 4 |
| Na+ (mM) | 138–139 | 138–139 |
| K+ (mM) | 6.1–7.4 | 6.1–7.4 |
| Ca2+ (mM) | 3.7–4.9 | 3.7–4.9 |
| Mg2+ (mM) | 0.2 | 0.2 |
| Cl− (mM) | 134–137 | 134–137 |
| PO43− (mM) | 1.3–1.8 | 1.3–1.8 |
| HCO3− (mM) | 15.1 | 15.1 |
| Temperature (°C) | 37 | 37 |
| Name | Substrate | Technique | Components of Coating Layer |
|---|---|---|---|
| Cp-FGF titanium method | Titanium | Immersed in F4 solution for 48 h | Calcium phosphate + FGF-2 |
| Cp-FGF stainless-steel method | Stainless steel | Immersed in liquid F0 for 4 h, then immersed in liquid F4 for 44 h | Calcium phosphate + FGF-2 |
| CP method | Stainless steel, titanium | Immersed in F0 solution for 48 h | calcium phosphate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Totoki, Y.; Mutsuzaki, H.; Yanagisawa, Y.; Sogo, Y.; Yasunaga, M.; Noguchi, H.; Matsumoto, Y.; Koda, M.; Ito, A.; Yamazaki, M. Do Stainless-Steel Pins Coated with Fibroblast Growth Factor–Calcium Phosphatase Composite Layers Have Anti-Infective Effects? Medicina 2024, 60, 1419. https://doi.org/10.3390/medicina60091419
Totoki Y, Mutsuzaki H, Yanagisawa Y, Sogo Y, Yasunaga M, Noguchi H, Matsumoto Y, Koda M, Ito A, Yamazaki M. Do Stainless-Steel Pins Coated with Fibroblast Growth Factor–Calcium Phosphatase Composite Layers Have Anti-Infective Effects? Medicina. 2024; 60(9):1419. https://doi.org/10.3390/medicina60091419
Chicago/Turabian StyleTotoki, Yasukazu, Hirotaka Mutsuzaki, Yohei Yanagisawa, Yu Sogo, Mayu Yasunaga, Hiroshi Noguchi, Yukei Matsumoto, Masao Koda, Atsuo Ito, and Masashi Yamazaki. 2024. "Do Stainless-Steel Pins Coated with Fibroblast Growth Factor–Calcium Phosphatase Composite Layers Have Anti-Infective Effects?" Medicina 60, no. 9: 1419. https://doi.org/10.3390/medicina60091419
APA StyleTotoki, Y., Mutsuzaki, H., Yanagisawa, Y., Sogo, Y., Yasunaga, M., Noguchi, H., Matsumoto, Y., Koda, M., Ito, A., & Yamazaki, M. (2024). Do Stainless-Steel Pins Coated with Fibroblast Growth Factor–Calcium Phosphatase Composite Layers Have Anti-Infective Effects? Medicina, 60(9), 1419. https://doi.org/10.3390/medicina60091419







