External Validation of the New 2023 International Federation of Gynecology and Obstetrics Staging System in Endometrial Cancer Patients: 12-Year Experience from an European Society of Gynecological Oncology-Accredited Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Characteristics
2.2. Patients
- Histological confirmation of endometrial cancer.
- Complete treatment at our hospital.
- Synchronous neoplasm.
- Recurrent endometrial cancer.
- Missing important registry data.
2.3. Data Collection
- Patient’s identifiers:
- ○
- Name.
- ○
- Hospital identification number.
- Patient’s age.
- Body Mass Index (BMI).
- Charlson Comorbidity Index (CCI).
- Histological type.
- Lymphovascular space invasion (LVSI).
- Tumor grade.
- FIGO staging (2009).
- FIGO staging (2023).
- Molecular classification:
- ○
- DNA polymerase epsilon (POLE) mutation.
- ○
- Mismatch repair-deficient subtype or microsatellite instability (MMRd or MSI).
- ○
- p53 abnormal (mutation type).
- Time-related data:
- ○
- Date of diagnosis.
- ○
- Date of recurrence.
- ○
- Date of last follow-up or death.
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Masood, M.; Singh, N. Endometrial carcinoma: Changes to classification (WHO 2020). Diagn. Histopathol 2021, 27, 493–499. [Google Scholar] [CrossRef]
- Guan, H.; Semaan, A.; Bandyopadhyay, S.; Arabi, H.; Feng, J.; Fathallah, L.; Pansare, V.; Qazi, A.; Abdul-Karim, F.; Morris, R.T.; et al. Prognosis and reproducibility of new and existing binary grading systems for endometrial carcinoma compared to FIGO grading in hysterectomy specimens. Int. J. Gynecol. Cancer 2011, 21, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Sidhu, D.; Duggan, M.A.; Arseneau, J.; Cesari, M.; Clement, P.B.; Ewanowich, C.A.; Kalloger, S.E.; Köbel, M. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod. Pathol. 2013, 26, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Gilks, C.B.; Oliva, E.; Soslow, R.A. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am. J. Surg. Pathol. 2013, 37, 874–881. [Google Scholar] [CrossRef]
- Levine, D.A.; The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; FIGO Women’s Cancer Committee Endometrial Cancer Staging Subcommittee. FIGO staging of endometrial cancer: 2023. J. Gynecol. Oncol. 2023, 34, e85. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Höhn, A.K.; Brambs, C.E.; Hiller, G.G.R.; May, D.; Schmoeckel, E.; Horn, L.-C. 2020 WHO Classification of Female Genital Tumors. Geburtshilfe Frauenheilkd 2021, 81, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Sagae, S.; Saito, T.; Satoh, M.; Ikeda, T.; Kimura, S.; Mori, M.; Sato, N.; Kudo, R. The reproducibility of a binary tumor grading system for uterine endometrial endometrioid carcinoma, compared with FIGO system and nuclear grading. Oncology 2004, 67, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N. FIGO staging of endometrial cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef] [PubMed]
- León-Castillo, A.; Gilvazquez, E.; Nout, R.; Smit, V.T.; McAlpine, J.N.; McConechy, M.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; et al. Clinicopathological and molecular characterisation of “multiple-classifier” endometrial carcinomas. J. Pathol. 2020, 250, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Schwameis, R.; Fanfani, F.; Ebner, C.; Zimmermann, N.; Peters, I.; Nero, C.; Marth, C.; Ristl, R.; Leitner, K.; Grimm, C.; et al. Verification of the prognostic precision of the new 2023 FIGO staging system in endometrial cancer patients—An international pooled analysis of three ESGO accredited centres. Eur. J. Cancer 2023, 193, 113317. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Park, N.; Lee, M.; Lee, C.; Kim, H. The new 2023 FIGO staging system for endometrial cancer: What is different from the previous 2009 FIGO staging system? J. Gynecol. Oncol. 2024, 35, e59. [Google Scholar] [CrossRef] [PubMed]
- Gravbrot, N.; Weil, C.R.; DeCesaris, C.M.; Gaffney, D.K.; Suneja, G.; Burt, L.M. Differentiation of survival outcomes by anatomic involvement and histology with the revised 2023 International Federation of Gynecology and Obstetrics staging system for endometrial cancer. Eur. J. Cancer 2024, 201, 113913. [Google Scholar] [CrossRef] [PubMed]
- Leitao, M.M. 2023 changes to FIGO endometrial cancer staging: Counterpoint. Gynecol. Oncol. 2024, 184, 146–149. [Google Scholar] [CrossRef] [PubMed]


| Stage | Description |
|---|---|
| Stage I | Tumor confined to the corpus uteri |
| IA | No or less than half myometrial invasion |
| IB | Invasion equal to or more than half of the myometrium |
| Stage II | Tumor invades cervical stroma but does not extend beyond the uterus |
| Stage III | Local and/or regional spread of the tumor |
| IIIA | Tumor invades the serosa of the corpus uteri and/or adnexa |
| IIIB | Vaginal and/or parametrial involvement |
| IIIC | Metastases to pelvic and/or para-aortic lymph nodes |
| IIIC1 | Positive pelvic nodes |
| IIIC2 | Positive para-aortic lymph nodes with or without positive pelvic lymph nodes |
| Stage IV | Tumor invades bladder and/or bowel mucosa, and/or distant metastases |
| IVA | Tumor invasion of bladder and/or bowel mucosa |
| IVB | Distant metastases, including intra-abdominal metastases and/or inguinal lymph nodes |
| Stage | Description |
|---|---|
| Stage I | Confined to the uterine corpus and ovary |
| IA | Disease limited to the endometrium or non-aggressive histological type |
| IAmPOLEmut | POLEmut endometrial carcinoma, confined to the uterine corpus or with cervical extension, regardless of LVSI or histological type |
| IA1 | Non-aggressive histological type limited to an endometrial polyp or confined to the endometrium |
| IA2 | Non-aggressive histological types involving less than half of the myometrium with no or focal LVSI |
| IA3 | Low-grade endometrioid carcinomas limited to the uterus and ovary |
| IB | Non-aggressive histological types with invasion of half or more of the myometrium and with no or focal LVSI |
| IC | Aggressive histological types limited to a polyp or confined to the endometrium |
| Stage II | Invasion of cervical stroma without extrauterine extension or with substantial LVSI or aggressive histological types with myometrial invasion |
| IIA | Invasion of the cervical stroma of non-aggressive histological types |
| IIB | Substantial LVSI of non-aggressive histological types |
| IIC | Aggressive histological types with any myometrial involvement |
| IICmp53abn | p53abn endometrial carcinoma confined to the uterine corpus with any myometrial invasion, with or without cervical invasion, and regardless of the degree of LVSI or histological type |
| Stage III | Local and/or regional spread of the tumor of any histological subtype |
| IIIA | Invasion of uterine serosa, adnexa, or both by direct extension or metastasis |
| IIIA1 | Spread to ovary or fallopian tube (except when meeting stage IA3 criteria) |
| IIIA2 | Involvement of uterine subserosa or spread through the uterine serosa |
| IIIB | Metastasis or direct spread to the vagina and/or to the parametria or pelvic peritoneum |
| IIIB1 | Metastasis or direct spread to the vagina and/or the parametria |
| IIIB2 | Metastasis to the pelvic peritoneum |
| IIIC | Metastasis to the pelvic or para-aortic lymph nodes or both |
| IIIC1 | Metastasis to the pelvic lymph nodes |
| IIIC1i | Micrometastasis |
| IIIC1ii | Macrometastasis |
| IIIC2 | Metastasis to para-aortic lymph nodes up to the renal vessels, with or without metastasis to the pelvic lymph nodes |
| IIIC2i | Micrometastasis |
| IIIC2ii | Macrometastasis |
| Stage IV | Spread to the bladder mucosa and/or intestinal mucosa and/or distance metastasis |
| IVA | Invasion of the bladder mucosa and/or the intestinal/bowel mucosa |
| IVB | Abdominal peritoneal metastasis beyond the pelvis |
| IVC | Distant metastasis, including metastasis to any extra- or intra-abdominal lymph nodes above the renal vessels, lungs, liver, brain, or bone |
| Number of Patients (N) | Percentage (%) | ||
|---|---|---|---|
| Age (years) | mean: 63.5 | SD: 11.6 | |
| BMI (kg/m2) | median: 32.8 | IQR: 28–38.2 | |
| BMI categories | median: 2 | IQR: 1–4 | |
| <18.5 | 0 | 53 | |
| ≥18.5–24.9 | 34 | 12.9 | |
| ≥25–29.9 | 59 | 13.7 | |
| ≥30–34.4 | 155 | 36.6 | |
| ≥35 | 55 | 12.8 | |
| Missing | 168 | 39 | |
| CCI | median: 3 | IQR: 2–4 | |
| 0–2 | 115 | 26.7 | |
| 3–4 | 179 | 41.5 | |
| ≥5 | 40 | 9.3 | |
| Missing | 97 | 22.5 | |
| Histology | |||
| Carcinosarcoma | 14 | 3.2 | |
| Clear cell | 6 | 1.4 | |
| Endometrioid | 355 | 82.4 | |
| Mixed | 18 | 4.2 | |
| Mucinous | 2 | 0.5 | |
| Serous | 31 | 7.2 | |
| Undifferentiated | 5 | 1.2 | |
| Grade | |||
| Low | 306 | 71 | |
| High | 125 | 29 | |
| LVSI | |||
| No | 343 | 79.6 | |
| Focal | 43 | 10 | |
| Substantial | 45 | 10.4 | |
| Molecular subtypes | |||
| MMRd | 7 | 1.6 | |
| POLEmut | 11 | 2.6 | |
| p53abn | 13 | 3 | |
| NSMP | 2 | 0.5 | |
| Unknown | 398 | 92.3 | |
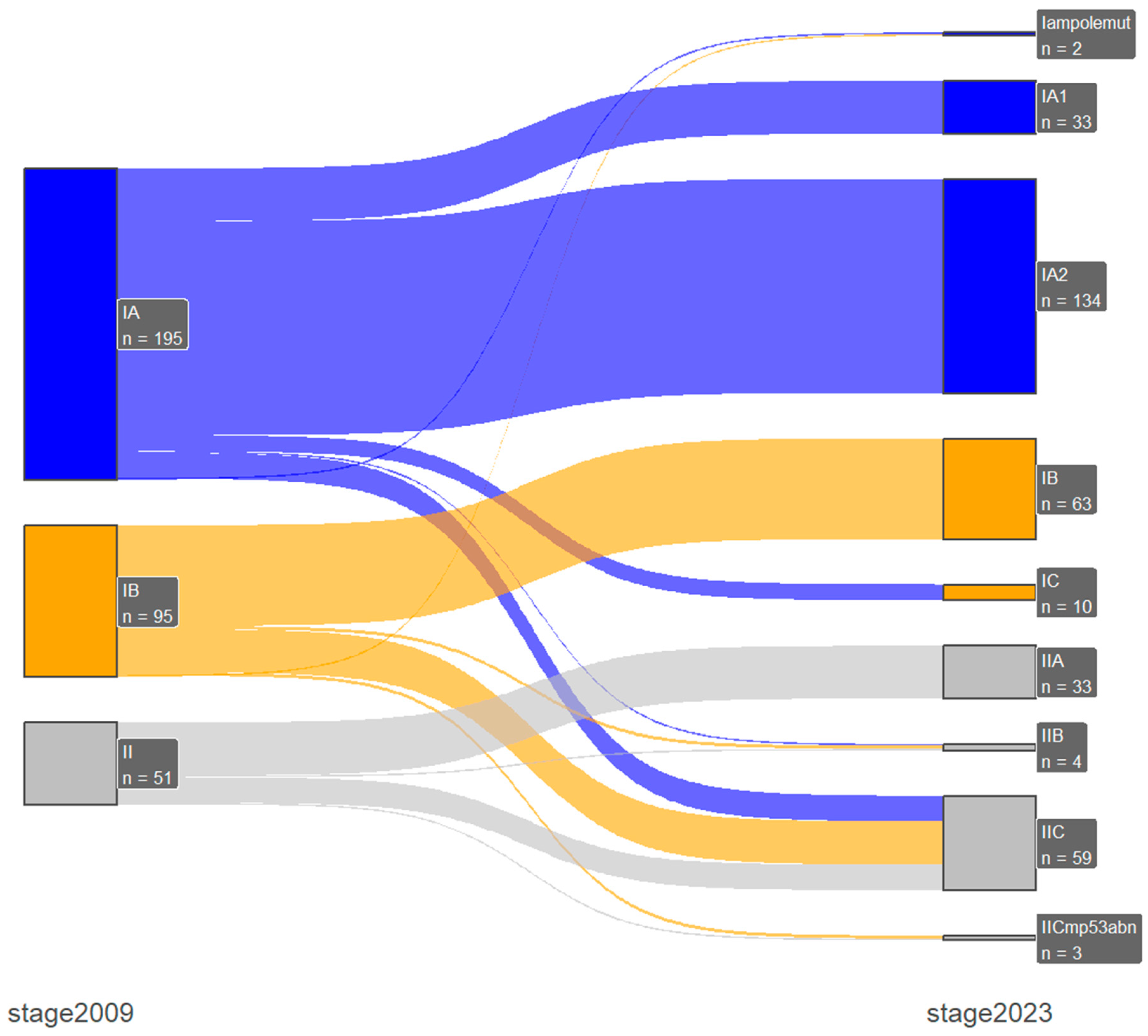
| 2009 FIGO | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IA | IB | II | IIIA | IIIB | IIIC1 | IIIC2 | IVA | IVB | ||
| 2023 FIGO | n = 195 | n = 95 | n = 51 | n = 10 | n = 14 | n = 24 | n = 20 | n = 5 | n = 17 | |
| IAmpolemut | n = 2 | 1 | 1 (↓) | - | - | - | - | - | - | - |
| IA1 | n = 33 | 33 | - | - | - | - | - | - | - | - |
| IA2 | n = 134 | 134 | - | - | - | - | - | - | - | - |
| IA3 | n = 1 | - | - | - | 1 (↓) | - | - | - | - | - |
| IB | n = 63 | - | 63 | - | - | - | - | - | - | - |
| IC | n = 10 | 10 (↑) | - | - | - | - | - | - | - | - |
| IIA | n = 33 | - | - | 33 | - | - | - | - | - | - |
| IIB | n = 4 | 1 (↑) | 2 (↑) | 1 | - | - | - | - | - | - |
| IIC | n = 59 | 16 (↑) | 27 (↑) | 16 | - | - | - | - | - | - |
| IICmp53abn | n = 3 | - | 2 (↑) | 1 | - | - | - | - | - | - |
| IIIA1 | n = 4 | - | - | - | 4 | - | - | - | - | - |
| IIIA2 | n = 4 | - | - | - | 4 | - | - | - | - | - |
| IIIB1 | n = 12 | - | - | - | - | 12 | - | - | - | - |
| IIIB2 | n = 3 | - | - | - | 1 (↑) | 2 | - | - | - | - |
| IIIC1i | n = 11 | - | - | - | - | - | 11 | - | - | - |
| IIIC1ii | n = 13 | - | - | - | - | - | 13 | - | - | - |
| IIIC2i | n = 3 | - | - | - | - | - | - | 3 | - | - |
| IIIC2ii | n = 17 | - | - | - | - | - | - | 17 | - | - |
| IVA | n = 5 | - | - | - | - | - | - | - | 5 | - |
| IVB | n = 11 | - | - | - | - | - | - | - | - | 11 |
| IVC | n = 6 | - | - | - | - | - | - | - | - | 6 (↑) |
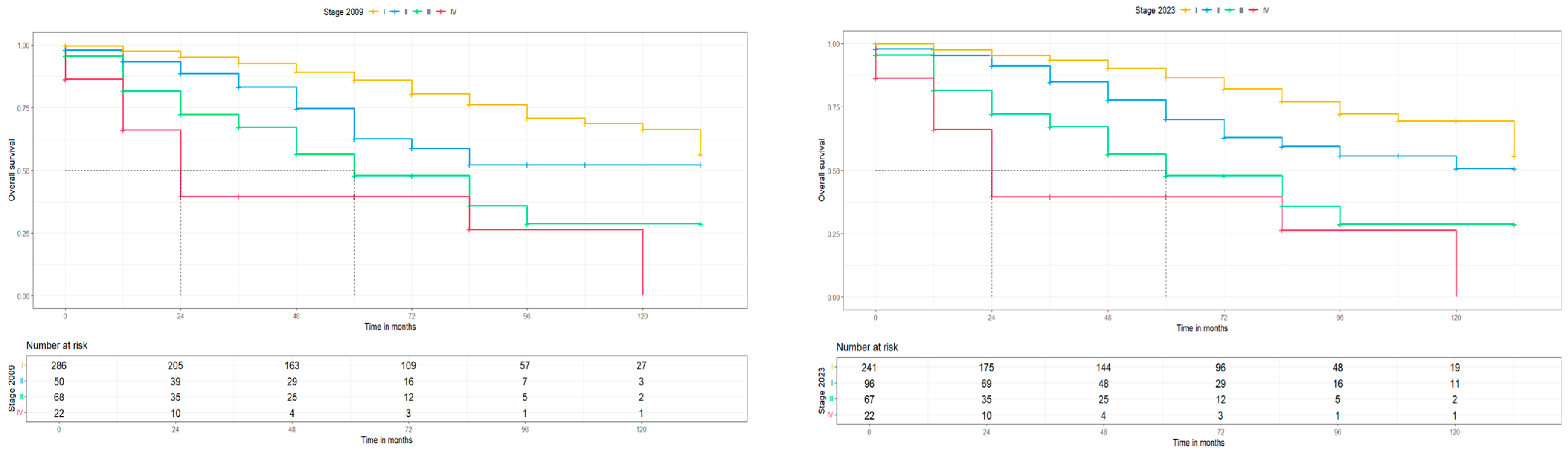
| 2009 Stage | 2023 Stage | |||||
|---|---|---|---|---|---|---|
| Patients n (%) | 5-Year PFS in % (95% CI) | 5-Year OS in % (95% CI) | Patients n (%) | 5-Year PFS in % (95% CI) | 5-Year OS in % (95% CI) | |
| I | 290 (67.3) | 83.3 (78.4, 88.4) | 86 (81.2, 91.2) | 243 (56.4) | 84 (78.9, 89.5) | 86.6 (81.5, 92.1) |
| IA | 195 (45.2) | 87 (81.8, 92.5) | 88.7 (83.4, 94.3) | 150 (34.8) | 86.7 (81.2, 92.7) | 88.9 (83.3, 94.9) |
| IAmpolemut | 2 (0.5) | n.e. | n.e. | |||
| IA1 | 23 (5.3) | n.e. | n.e. | |||
| IA2 | 124 (28.8) | 85.4 (79., 92.4) | 88.7 (82.5, 95.3) | |||
| IA3 | 1 (0.3) | n.e. | n.e. | |||
| IB | 95 (22.) | 74.7 (64.8, 86.2) | 80.2 (70.5, 91.3) | 63 (14.6) | 76.2 (64.7, 89.8) | 81 (69.7, 94.1) |
| IC | 10 (2.3) | n.e. | n.e. | |||
| II | 51 (11.8) | 57.2 (43.8, 74.6) | 62.8 (48.9, 80.7) | 99 (23.0) | 66.9 (56.7, 79.0) | 70.2 (59.6, 82.8) |
| IIA | 33 (7.7) | 61 (45.3, 82.2) | 62.5 (46.5, 84.0) | |||
| IIB | 4 (0.9) | n.e. | n.e. | |||
| IIC | 59 (13.7) | 60.2 (45.4, 79.8) | 75.4 (61.9, 91.8) | |||
| IICmp53abn | 3 (0.7) | n.e. | n.e. | |||
| III | 68 (15.8) | 49.4 (36.4, 67.1) | 48.0 (34.8, 66.1) | 67 (15.5) | 49.3 (36.3, 67.0) | 47.9 (34.8, 66.0) |
| IIIA | 10 (2.3) | n.e. | n.e. | 8 (1.9) | n.e. | n.e. |
| IIIA1 | 4 (0.9) | n.e. | n.e. | |||
| IIIA2 | 4 (0.9) | n.e. | n.e. | |||
| IIIB | 14 (3.2) | 46.3 (21.3, 100) | 46.3 (21.3, 100) | 15 (3.5) | 46.8 (21.6, 100) | 46.8 (21.6, 100.0) |
| IIIB1 | 12 (2.8) | 19.3 (23.0, 100) | 49.5 (19.3, 23.1) | |||
| IIIB2 | 3 (0.7) | n.e. | n.e. | |||
| IIIC | 44 (10.2) | 52.4 (37.3, 73.8) | 51.9 (36.4, 73.9) | |||
| IIIC1 | 24 (5.6) | 48.3 (29.5, 79) | 51.2 (32.2, 81.3) | 24 (4.9) | 48.3 (29.5, 79) | 51.2 (32.2, 81.3) |
| IIIC1i | 11 (2.6) | 60.6 (18.4, 33.4) | 37.9 (8.9, 100) | |||
| IIIC1ii | 13 (3.0) | 55 (33.4, 90.6) | 33.2 (13.6, 81) | |||
| IIIC2 | 20 (2.6) | 57.3 (35.7, 92.1) | 52.4 (30.0, 91.5) | 20 (4.6) | 52.4 (30.0, 91.5) | 52.4 (30.0, 96.5) |
| IIIC2i | 3 (0.7) | n.e. | ||||
| IIIC2ii | 17 (3.9) | 55 (33.4, 90.6) | 51.1 (28.9, 90.3) | |||
| IV | 22 (5.1) | n.e. | n.e. | 22 (5.1) | n.e. | n.e |
| IVA | 5 (1.2) | n.e. | n.e. | 5 (1.2) | n.e. | n.e. |
| IVB | 17 (3.9) | n.e. | n.e. | 11 (2.6) | n.e. | n.e. |
| IVC | 6 (1.4) | n.e. | n.e. |
| Number of Patients (N) | Percentage (%) | ||
|---|---|---|---|
| Histology | |||
| Carcinosarcoma | 3 | 6.2 | |
| Clear cell | 2 | 4.2 | |
| Endometrioid | 24 | 50 | |
| Mixed | 10 | 20.8 | |
| Mucinous | - | - | |
| Serous | 8 | 16.7 | |
| Undifferentiated | 1 | 2.1 | |
| Grade | |||
| Low | 7 | 14.6 | |
| High | 41 | 85.4 | |
| LVSI | |||
| No | 30 | 62.5 | |
| Focal | 13 | 27.1 | |
| Substantial | 5 | 10.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsolakidis, D.; Zouzoulas, D.; Sofianou, I.; Karalis, T.; Chatzistamatiou, K.; Theodoulidis, V.; Topalidou, M.; Timotheadou, E.; Grimbizis, G. External Validation of the New 2023 International Federation of Gynecology and Obstetrics Staging System in Endometrial Cancer Patients: 12-Year Experience from an European Society of Gynecological Oncology-Accredited Center. Medicina 2024, 60, 1421. https://doi.org/10.3390/medicina60091421
Tsolakidis D, Zouzoulas D, Sofianou I, Karalis T, Chatzistamatiou K, Theodoulidis V, Topalidou M, Timotheadou E, Grimbizis G. External Validation of the New 2023 International Federation of Gynecology and Obstetrics Staging System in Endometrial Cancer Patients: 12-Year Experience from an European Society of Gynecological Oncology-Accredited Center. Medicina. 2024; 60(9):1421. https://doi.org/10.3390/medicina60091421
Chicago/Turabian StyleTsolakidis, Dimitrios, Dimitrios Zouzoulas, Iliana Sofianou, Tilemaxos Karalis, Kimon Chatzistamatiou, Vasilis Theodoulidis, Maria Topalidou, Eleni Timotheadou, and Grigoris Grimbizis. 2024. "External Validation of the New 2023 International Federation of Gynecology and Obstetrics Staging System in Endometrial Cancer Patients: 12-Year Experience from an European Society of Gynecological Oncology-Accredited Center" Medicina 60, no. 9: 1421. https://doi.org/10.3390/medicina60091421
APA StyleTsolakidis, D., Zouzoulas, D., Sofianou, I., Karalis, T., Chatzistamatiou, K., Theodoulidis, V., Topalidou, M., Timotheadou, E., & Grimbizis, G. (2024). External Validation of the New 2023 International Federation of Gynecology and Obstetrics Staging System in Endometrial Cancer Patients: 12-Year Experience from an European Society of Gynecological Oncology-Accredited Center. Medicina, 60(9), 1421. https://doi.org/10.3390/medicina60091421





