Epidemiology and Risk Factors of Dry Eye Disease: Considerations for Clinical Management
Abstract
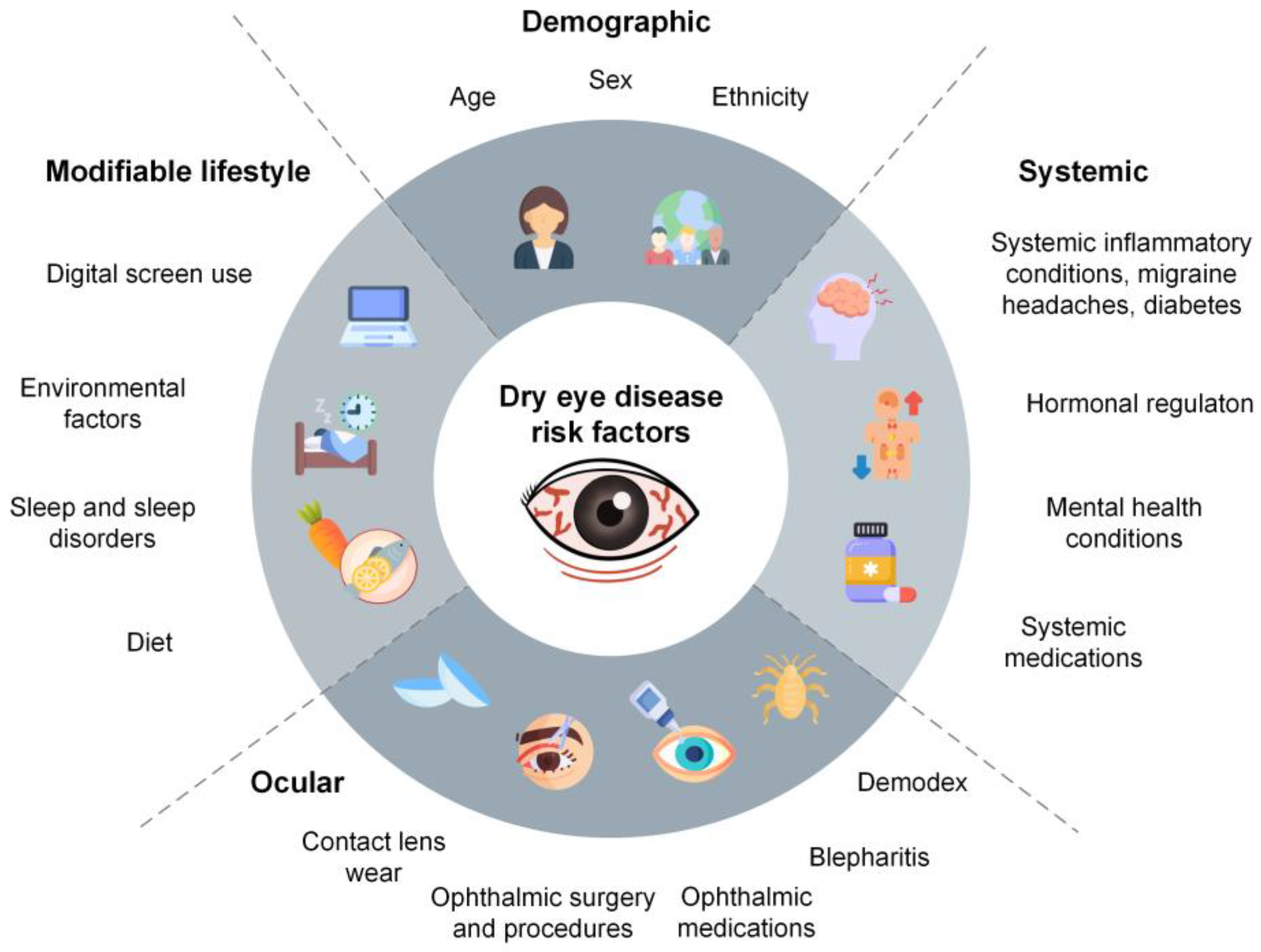
:1. Introduction
2. Prevalence
3. Demographic Risk Factors
3.1. Age
3.2. Female Sex
3.3. Genetics
3.4. Ethnicity
4. Systemic Risk Factors
4.1. Systemic Inflammation
4.2. Diabetes
4.3. Androgen Deficiency
4.4. Mental Health Conditions
4.5. Systemic Medications
5. Ocular Risk Factors
5.1. Blepharitis
5.2. Contact Lenses
5.3. Ophthalmic Procedures
5.4. Ophthalmic Medications
6. Modifiable Lifestyle Risk Factors
6.1. Environmental Factors
6.2. Digital Screen Time
6.3. Sleep and Sleep Disorders
6.4. Diet
6.5. Cosmetics and Cosmetic Procedures
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II Pathophysiology Report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benitez-del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. TFOS DEWS II Pain and Sensation report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef]
- Wang, M.T.; Muntz, A.; Wolffsohn, J.S.; Craig, J.P. Association between dry eye disease, self-perceived health status, and self-reported psychological stress burden. Clin. Exp. Optom. 2021, 104, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Mathews, P.M.; Ramulu, P.Y.; Swenor, B.S.; Utine, C.A.; Rubin, G.S.; Akpek, E.K. Functional impairment of reading in patients with dry eye. Br. J. Ophthalmol. 2017, 101, 481–486. [Google Scholar] [CrossRef] [PubMed]
- van Tilborg, M.M.; Murphy, P.J.; Evans, K.S. Impact of Dry Eye Symptoms and Daily Activities in a Modern Office. Optom. Vis. Sci. 2017, 94, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Asche, C.V.; Fairchild, C.J. The economic burden of dry eye disease in the United States: A decision tree analysis. Cornea 2011, 30, 379–387. [Google Scholar] [CrossRef]
- Wang, M.T.M.; Chan, E.; Ea, L.; Kam, C.; Lu, Y.; Misra, S.L.; Craig, J.P. Randomized Trial of Desktop Humidifier for Dry Eye Relief in Computer Users. Optom. Vis. Sci. 2017, 94, 1052–1057. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Wang, M.T.M.; Vidal-Rohr, M.; Menduni, F.; Dhallu, S.; Ipek, T.; Acar, D.; Recchioni, A.; France, A.; Kingsnorth, A.; et al. Demographic and lifestyle risk factors of dry eye disease subtypes: A cross-sectional study. Ocul. Surf. 2021, 21, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; Muntz, A.; Lim, J.; Kim, J.S.; Lacerda, L.; Arora, A.; Craig, J.P. Ageing and the natural history of dry eye disease: A prospective registry-based cross-sectional study. Ocul. Surf. 2020, 18, 736–741. [Google Scholar] [CrossRef]
- Craig, J.P.; Alves, M.; Wolffsohn, J.S.; Downie, L.E.; Efron, N.; Galor, A.; Gomes, J.A.P.; Jones, L.; Markoulli, M.; Stapleton, F.; et al. TFOS Lifestyle Report Executive Summary: A Lifestyle Epidemic—Ocular Surface Disease. Ocul. Surf. 2023, 30, 240–253. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; Diprose, W.K.; Craig, J.P. Epidemiologic Research in Dry Eye Disease and the Utility of Mobile Health Technology. JAMA Ophthalmol. 2020, 138, 69–70. [Google Scholar] [CrossRef]
- Papas, E.B. The global prevalence of dry eye disease: A Bayesian view. Ophthalmic Physiol. Opt. 2021, 41, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, D.A.; Dana, R.; Buring, J.E.; Sullivan, D.A. Prevalence of dry eye disease among US men: Estimates from the Physicians’ Health Studies. Arch. Ophthalmol. 2009, 127, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, D.A.; Sullivan, D.A.; Buring, J.E.; Dana, M.R. Prevalence of dry eye syndrome among US women. Am. J. Ophthalmol. 2003, 136, 318–326. [Google Scholar] [CrossRef]
- Farrand, K.F.; Fridman, M.; Stillman, I.O.; Schaumberg, D.A. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am. J. Ophthalmol. 2017, 182, 90–98. [Google Scholar] [CrossRef]
- Vehof, J.; Snieder, H.; Jansonius, N.; Hammond, C.J. Prevalence and risk factors of dry eye in 79,866 participants of the population-based Lifelines cohort study in the Netherlands. Ocul. Surf. 2021, 19, 83–93. [Google Scholar] [CrossRef]
- Castro, J.S.d.; Selegatto, I.B.; Castro, R.S.d.; Miranda, E.C.M.; de Vasconcelos, J.P.C.; de Carvalho, K.M.; Arieta, C.E.L.; Alves, M. Prevalence and Risk Factors of self-reported dry eye in Brazil using a short symptom questionnaire. Sci. Rep. 2018, 8, 2076. [Google Scholar] [CrossRef]
- Ahn, J.M.; Lee, S.H.; Rim, T.H.; Park, R.J.; Yang, H.S.; Kim, T.I.; Yoon, K.C.; Seo, K.Y.; Epidemiologic Survey Committee of the Korean Ophthalmological Society. Prevalence of and risk factors associated with dry eye: The Korea National Health and Nutrition Examination Survey 2010–2011. Am. J. Ophthalmol. 2014, 158, 1205–1214 e1207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Wu, X. Prevalence and risk factors associated with dry eye syndrome among senior high school students in a county of Shandong Province, China. Ophthalmic Epidemiol. 2012, 19, 226–230. [Google Scholar] [CrossRef]
- Um, S.B.; Kim, N.H.; Lee, H.K.; Song, J.S.; Kim, H.C. Spatial epidemiology of dry eye disease: Findings from South Korea. Int. J. Health Geogr. 2014, 13, 31. [Google Scholar] [CrossRef]
- Lim, E.W.L.; Chee, M.L.; Sabanayagam, C.; Majithia, S.; Tao, Y.; Wong, T.Y.; Cheng, C.-Y.; Tong, L. Relationship Between Sleep and Symptoms of Tear Dysfunction in Singapore Malays and Indians. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1889–1897. [Google Scholar] [CrossRef]
- Man, R.E.K.; Veerappan, A.R.; Tan, S.-P.; Fenwick, E.K.; Sabanayagam, C.; Chua, J.; Leong, Y.-Y.; Wong, T.Y.; Lamoureux, E.L.; Cheng, C.-Y.; et al. Incidence and risk factors of symptomatic dry eye disease in Asian Malays from the Singapore Malay Eye Study. Ocul. Surf. 2017, 15, 742–748. [Google Scholar] [CrossRef]
- Moss, S.E.; Klein, R.; Klein, B.E.K. Prevalence of and Risk Factors for Dry Eye Syndrome. Arch. Ophthalmol. 2000, 118, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Sullivan, R.; Buring, J.E.; Sullivan, D.A.; Dana, R.; Schaumberg, D.A. Validation and repeatability of a short questionnaire for dry eye syndrome. Am. J. Ophthalmol. 2006, 142, 125–131. [Google Scholar] [CrossRef]
- Hashemi, H.; Khabazkhoob, M.; Kheirkhah, A.; Emamian, M.H.; Mehravaran, S.; Shariati, M.; Fotouhi, A. Prevalence of dry eye syndrome in an adult population. Clin. Experiment. Ophthalmol. 2014, 42, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Viso, E.; Rodriguez-Ares, M.T.; Gude, F. Prevalence of and associated factors for dry eye in a Spanish adult population (the Salnes Eye Study). Ophthalmic Epidemiol. 2009, 16, 15–21. [Google Scholar] [CrossRef]
- Malet, F.; Le Goff, M.; Colin, J.; Schweitzer, C.; Delyfer, M.N.; Korobelnik, J.F.; Rougier, M.B.; Radeau, T.; Dartigues, J.F.; Delcourt, C. Dry eye disease in French elderly subjects: The Alienor Study. Acta Ophthalmol. 2014, 92, e429–e436. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.; Kozareva, D.; Hysi, P.G.; Hammond, C.J. Prevalence and risk factors of dry eye disease in a British female cohort. Br. J. Ophthalmol. 2014, 98, 1712–1717. [Google Scholar] [CrossRef]
- Tian, Y.J.; Liu, Y.; Zou, H.D.; Jiang, Y.J.; Liang, X.Q.; Sheng, M.J.; Li, B.; Xu, X. Epidemiologic study of dry eye in populations equal or over 20 years old in Jiangning District of Shanghai. Zhonghua Yan Ke Za Zhi 2009, 45, 486–491. [Google Scholar] [PubMed]
- Jie, Y.; Xu, L.; Wu, Y.Y.; Jonas, J.B. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye 2009, 23, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.Y.; Zhang, F.; Zhou, J.; Li, J.; Zhang, G.H.; Wang, J.L.; Gu, Z.S. Prevalence of Dry Eye in Uyghur and Han Ethnic Groups in Western China. Ophthalmic Epidemiol. 2017, 24, 181–187. [Google Scholar] [CrossRef]
- Viet Vu, C.H.; Uchino, M.; Kawashima, M.; Yuki, K.; Tsubota, K.; Nishi, A.; German, C.A.; Sakata, K.; Tanno, K.; Iso, H.; et al. Lack of social support and social trust as potential risk factors for dry eye disease: JPHC-NEXT study. Ocul. Surf. 2019, 17, 278–284. [Google Scholar] [CrossRef]
- Arita, R.; Mizoguchi, T.; Kawashima, M.; Fukuoka, S.; Koh, S.; Shirakawa, R.; Suzuki, T.; Morishige, N. Meibomian Gland Dysfunction and Dry Eye Are Similar but Different Based on a Population-Based Study: The Hirado-Takushima Study in Japan. Am. J. Ophthalmol. 2019, 207, 410–418. [Google Scholar] [CrossRef]
- Tandon, R.; Vashist, P.; Gupta, N.; Gupta, V.; Sahay, P.; Deka, D.; Singh, S.; Vishwanath, K.; Murthy, G.V.S. Association of dry eye disease and sun exposure in geographically diverse adult (≥40 years) populations of India: The SEED (sun exposure, environment and dry eye disease) study—Second report of the ICMR-EYE SEE study group. Ocul. Surf. 2020, 18, 718–730. [Google Scholar] [CrossRef]
- Ferrero, A.; Alassane, S.; Binquet, C.; Bretillon, L.; Acar, N.; Arnould, L.; Muselier-Mathieu, A.; Delcourt, C.; Bron, A.M.; Creuzot-Garcher, C. Dry eye disease in the elderly in a French population-based study (the Montrachet study: Maculopathy, Optic Nerve, nuTRition, neurovAsCular and HEarT diseases): Prevalence and associated factors. Ocul. Surf. 2018, 16, 112–119. [Google Scholar] [CrossRef]
- Olaniyan, S.I.; Fasina, O.; Bekibele, C.O.; Ogundipe, A.O. Dry eye disease in an adult population in South-West Nigeria. Contact Lens Anterior Eye 2016, 39, 359–364. [Google Scholar] [CrossRef]
- Viso, E.; Rodriguez-Ares, M.T.; Abelenda, D.; Oubina, B.; Gude, F. Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2601–2606. [Google Scholar] [CrossRef]
- Viso, E.; Rodriguez-Ares, M.T.; Boveda, F.J.; Tourino, R.; Gude, F. Prevalence of conjunctival shrinkage and its association with dry eye disease: Results from a population-based study in Spain. Cornea 2014, 33, 442–447. [Google Scholar] [CrossRef]
- Han, S.B.; Hyon, J.Y.; Woo, S.J.; Lee, J.J.; Kim, T.H.; Kim, K.W. Prevalence of dry eye disease in an elderly Korean population. Arch. Ophthalmol. 2011, 129, 633–638. [Google Scholar] [CrossRef]
- Siak, J.J.; Tong, L.; Wong, W.L.; Cajucom-Uy, H.; Rosman, M.; Saw, S.M.; Wong, T.Y. Prevalence and risk factors of meibomian gland dysfunction: The Singapore Malay eye study. Cornea 2012, 31, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Tsai, S.Y.; Cheng, C.Y.; Liu, J.H.; Chou, P.; Hsu, W.M. Prevalence of dry eye among an elderly Chinese population in Taiwan: The Shihpai Eye Study. Ophthalmology 2003, 110, 1096–1101. [Google Scholar] [CrossRef]
- Hashemi, H.; Rastad, H.; Emamian, M.H.; Fotouhi, A. Meibomian gland dysfunction and its determinants in Iranian adults: A population-based study. Contact Lens Anterior Eye 2017, 40, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Asharlous, A.; Aghamirsalim, M.; Yekta, A.; Pourmatin, R.; Sajjadi, M.; Pakbin, M.; Asadollahi, M.; Khabazkhoob, M. Meibomian gland dysfunction in geriatric population: Tehran geriatric eye study. Int. Ophthalmol. 2021, 41, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; Craig, J.P. Natural history of dry eye disease: Perspectives from inter-ethnic comparison studies. Ocul. Surf. 2019, 17, 424–433. [Google Scholar] [CrossRef]
- Yu, W.; Mingzhou, Z.; Xuemin, L. The global prevalence of dry eye disease and its association with economy: A systematic review. Res. Sq. 2021; unpublished. [Google Scholar] [CrossRef]
- Stapleton, F.; Abad, J.C.; Barabino, S.; Burnett, A.; Iyer, G.; Lekhanont, K.; Li, T.; Liu, Y.; Navas, A.; Obinwanne, C.J.; et al. TFOS lifestyle: Impact of societal challenges on the ocular surface. Ocul. Surf. 2023, 28, 165–199. [Google Scholar] [CrossRef]
- Dana, R.; Bradley, J.L.; Guerin, A.; Pivneva, I.; Stillman, I.; Evans, A.M.; Schaumberg, D.A. Estimated Prevalence and Incidence of Dry Eye Disease Based on Coding Analysis of a Large, All-age United States Health Care System. Am. J. Ophthalmol. 2019, 202, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Rico-Del-Viejo, L.; Lorente-Velazquez, A.; Hernandez-Verdejo, J.L.; Garcia-Mata, R.; Benitez-Del-Castillo, J.M.; Madrid-Costa, D. The effect of ageing on the ocular surface parameters. Contact Lens Anterior Eye 2018, 41, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Velez, F.G.; Lau, C.; Wolffsohn, J.S. Dry eye disease in the young: A narrative review. Ocul. Surf. 2024, 31, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Donthineni, P.R.; Das, A.V.; Basu, S. Dry eye disease in children and adolescents in India. Ocul. Surf. 2020, 18, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Kazanci, B.; Eroglu, F.C. The Effects of Daily Digital Device Use on the Ocular Surface in Healthy Children. Optom. Vis. Sci. 2022, 99, 167–171. [Google Scholar] [CrossRef]
- Uchino, M.; Schaumberg, D.A.; Dogru, M.; Uchino, Y.; Fukagawa, K.; Shimmura, S.; Satoh, T.; Takebayashi, T.; Tsubota, K. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology 2008, 115, 1982–1988. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Rocha, E.M.; Aragona, P.; Clayton, J.A.; Ding, J.; Golebiowski, B.; Hampel, U.; McDermott, A.M.; Schaumberg, D.A.; Srinivasan, S.; et al. TFOS DEWS II Sex, Gender, and Hormones Report. Ocul. Surf. 2017, 15, 284–333. [Google Scholar] [CrossRef]
- Craig, J.P.; Wang, M.T.M.; Ambler, A.; Cheyne, K.; Wilson, G.A. Characterising the ocular surface and tear film in a population-based birth cohort of 45-year old New Zealand men and women. Ocul. Surf. 2020, 18, 808–813. [Google Scholar] [CrossRef]
- Vehof, J.; Wang, B.; Kozareva, D.; Hysi, P.G.; Snieder, H.; Hammond, C.J. The Heritability of Dry Eye Disease in a Female Twin Cohort. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7278–7283. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Chuang, H.-K.; Hsiao, Y.-J.; Chiang, P.-H.; Chen, S.-W.; Luo, W.-T.; Yang, Y.-P.; Tsai, P.-H.; Chen, S.-J.; Hsieh, A.-R.; et al. Predicting Risks of Dry Eye Disease Development Using a Genome-Wide Polygenic Risk Score Model. Transl. Vision Sci. Technol. 2024, 13, 13. [Google Scholar] [CrossRef]
- Huang, J.J.; Gorman, B.; Barr, P.B.; Halladay, C.W.; Nealon, C.L.; Chatzinakos, C.; Francis, M.; Greenberg, P.B.; Wu, W.-C.; Pyarajan, S.; et al. Multi-ancestry genome-wide association study of dry eye disease in the Million Veteran Program. Investig. Ophthalmol. Vis. Sci. 2024, 65, 1013. [Google Scholar]
- Lee, L.; Garrett, Q.; Flanagan, J.; Chakrabarti, S.; Papas, E. Genetic factors and molecular mechanisms in dry eye disease. Ocul. Surf. 2018, 16, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Lim, J.; Han, A.; Tien, L.; Xue, A.L.; Wang, M.T.M. Ethnic differences between the Asian and Caucasian ocular surface: A co-located adult migrant population cohort study. Ocul. Surf. 2019, 17, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Wang, M.T.; Kim, D.; Lee, J.M. Exploring the Predisposition of the Asian Eye to Development of Dry Eye. Ocul. Surf. 2016, 14, 385–392. [Google Scholar] [CrossRef]
- Kim, J.S.; Wang, M.T.M.; Craig, J.P. Exploring the Asian ethnic predisposition to dry eye disease in a pediatric population. Ocul. Surf. 2019, 17, 70–77. [Google Scholar] [CrossRef]
- Wang, M.T.M.; Tien, L.; Han, A.; Lee, J.M.; Kim, D.; Markoulli, M.; Craig, J.P. Impact of blinking on ocular surface and tear film parameters. Ocul. Surf. 2018, 16, 424–429. [Google Scholar] [CrossRef]
- Kim, A.D.; Muntz, A.; Lee, J.; Wang, M.T.M.; Craig, J.P. Therapeutic benefits of blinking exercises in dry eye disease. Contact Lens Anterior Eye 2021, 44, 101329. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Nichols, J.J.; Papas, E.B.; Tong, L.; Uchino, M.; Nichols, K.K. The international workshop on meibomian gland dysfunction: Report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1994–2005. [Google Scholar] [CrossRef]
- Wang, M.T.M.; Vidal-Rohr, M.; Muntz, A.; Diprose, W.K.; Ormonde, S.E.; Wolffsohn, J.S.; Craig, J.P. Systemic risk factors of dry eye disease subtypes: A New Zealand cross-sectional study. Ocul. Surf. 2020, 18, 374–380. [Google Scholar] [CrossRef]
- Ismail, O.M.; Poole, Z.B.; Bierly, S.L.; Van Buren, E.D.; Lin, F.C.; Meyer, J.J.; Davis, R.M. Association Between Dry Eye Disease and Migraine Headaches in a Large Population-Based Study. JAMA Ophthalmol. 2019, 137, 532–536. [Google Scholar] [CrossRef]
- Yoo, T.K.; Oh, E. Diabetes mellitus is associated with dry eye syndrome: A meta-analysis. Int. Ophthalmol. 2019, 39, 2611–2620. [Google Scholar] [CrossRef]
- Wan, K.H.; Chen, L.J.; Young, A.L. Depression and anxiety in dry eye disease: A systematic review and meta-analysis. Eye 2016, 30, 1558–1567. [Google Scholar] [CrossRef]
- Wong, J.; Lan, W.; Ong, L.M.; Tong, L. Non-hormonal systemic medications and dry eye. Ocul. Surf. 2011, 9, 212–226. [Google Scholar] [CrossRef]
- Galor, A.; Britten-Jones, A.C.; Feng, Y.; Ferrari, G.; Goldblum, D.; Gupta, P.K.; Merayo-Lloves, J.; Na, K.S.; Naroo, S.A.; Nichols, K.K.; et al. TFOS Lifestyle: Impact of lifestyle challenges on the ocular surface. Ocul. Surf. 2023, 28, 262–303. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.P.; Azar, D.T.; Baudouin, C.; Bitton, E.; Chen, W.; Hafezi, F.; Hamrah, P.; Hogg, R.E.; Horwath-Winter, J.; Kontadakis, G.A.; et al. TFOS Lifestyle: Impact of elective medications and procedures on the ocular surface. Ocul. Surf. 2023, 29, 331–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, P.B.; Chen, T.; Zou, J.; Li, Y.J.; Ran, X.F.; Li, Q.X. Analysis of Clinical Characteristics of Immune-Related Dry Eye. J. Ophthalmol. 2017, 2017, 8532397. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.M.; Thomson, W.M.; Craig, J.P. Association between symptoms of xerostomia and dry eye in older people. Contact Lens Anterior Eye 2020, 43, 99–102. [Google Scholar] [CrossRef]
- Achtsidis, V.; Eleftheriadou, I.; Kozanidou, E.; Voumvourakis, K.I.; Stamboulis, E.; Theodosiadis, P.G.; Tentolouris, N. Dry eye syndrome in subjects with diabetes and association with neuropathy. Diabetes Care 2014, 37, e210–e211. [Google Scholar] [CrossRef]
- Lv, H.; Li, A.; Zhang, X.; Xu, M.; Qiao, Y.; Zhang, J.; Yu, L. Meta-analysis and review on the changes of tear function and corneal sensitivity in diabetic patients. Acta Ophthalmol. 2014, 92, e96–e104. [Google Scholar] [CrossRef]
- Lyu, Y.; Zeng, X.; Li, F.; Zhao, S. The effect of the duration of diabetes on dry eye and corneal nerves. Contact Lens Anterior Eye 2019, 42, 380–385. [Google Scholar] [CrossRef]
- Ma, A.; Mak, M.S.; Shih, K.C.; Tsui, C.K.; Cheung, R.K.; Lee, S.H.; Leung, H.; Leung, J.N.; Leung, J.T.; Van-Boswell, M.Z.; et al. Association of long-term glycaemic control on tear break-up times and dry eye symptoms in Chinese patients with type 2 diabetes. Clin. Exp. Ophthalmol. 2018, 46, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Britten-Jones, A.C.; Craig, J.P.; Anderson, A.J.; Downie, L.E. Association between systemic omega-3 polyunsaturated fatty acid levels, and corneal nerve structure and function. Eye 2023, 37, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.C.; Cheng, H.T.; Stables, C.L.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012, 11, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Shih, K.C.; Lam, K.S.; Tong, L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr. Diabetes 2017, 7, e251. [Google Scholar] [CrossRef] [PubMed]
- Markoulli, M.; Flanagan, J.; Tummanapalli, S.S.; Wu, J.; Willcox, M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul. Surf. 2018, 16, 45–57. [Google Scholar] [CrossRef]
- Tummanapalli, S.S.; Willcox, M.D.P.; Issar, T.; Yan, A.; Pisarcikova, J.; Kwai, N.; Poynten, A.M.; Krishnan, A.V.; Markoulli, M. Tear film substance P: A potential biomarker for diabetic peripheral neuropathy. Ocul. Surf. 2019. [Google Scholar] [CrossRef]
- Sullivan, B.D.; Evans, J.E.; Cermak, J.M.; Krenzer, K.L.; Dana, M.R.; Sullivan, D.A. Complete androgen insensitivity syndrome: Effect on human meibomian gland secretions. Arch. Ophthalmol. 2002, 120, 1689–1699. [Google Scholar] [CrossRef]
- Krenzer, K.L.; Dana, M.R.; Ullman, M.D.; Cermak, J.M.; Tolls, D.B.; Evans, J.E.; Sullivan, D.A. Effect of androgen deficiency on the human meibomian gland and ocular surface. J. Clin. Endocrinol. Metab. 2000, 85, 4874–4882. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, X.; Lin, X.; Lin, H. The Prevalence of Depression and Depressive Symptoms among Eye Disease Patients: A Systematic Review and Meta-analysis. Sci. Rep. 2017, 7, 46453. [Google Scholar] [CrossRef]
- Kitazawa, M.; Sakamoto, C.; Yoshimura, M.; Kawashima, M.; Inoue, S.; Mimura, M.; Tsubota, K.; Negishi, K.; Kishimoto, T. The Relationship of Dry Eye Disease with Depression and Anxiety: A Naturalistic Observational Study. Transl. Vis. Sci. Technol. 2018, 7, 35. [Google Scholar] [CrossRef]
- Ong, E.S.; Alghamdi, Y.A.; Levitt, R.C.; McClellan, A.L.; Lewis, G.; Sarantopoulos, C.D.; Felix, E.R.; Galor, A. Longitudinal Examination of Frequency of and Risk Factors for Severe Dry Eye Symptoms in US Veterans. JAMA Ophthalmol. 2017, 135, 116–123. [Google Scholar] [CrossRef]
- Galor, A.; Levitt, R.C.; McManus, K.T.; Kalangara, J.P.; Seiden, B.E.; Park, J.J.; Covington, D.B.; Sarantopoulos, C.D.; Felix, E.R. Assessment of Somatosensory Function in Patients With Idiopathic Dry Eye Symptoms. JAMA Ophthalmol. 2016, 134, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Kocer, E.; Kocer, A.; Ozsutcu, M.; Dursun, A.E.; Krpnar, I. Dry Eye Related to Commonly Used New Antidepressants. J. Clin. Psychopharmacol. 2015, 35, 411–413. [Google Scholar] [CrossRef]
- Egger, S.F.; Huber-Spitzy, V.; Böhler, K.; Scholda, C. Isotretinoin administration in treatment of acne vulgaris. A prospective study of the kind and extent of ocular complications. Ophthalmologe 1995, 92, 17–20. [Google Scholar] [PubMed]
- Schaumberg, D.A.; Buring, J.E.; Sullivan, D.A.; Dana, M.R. Hormone replacement therapy and dry eye syndrome. JAMA 2001, 286, 2114–2119. [Google Scholar] [CrossRef]
- Acan, D.; Kurtgoz, P. Influence of selective serotonin reuptake inhibitors on ocular surface. Clin. Exp. Optom. 2017, 100, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Chhadva, P.; Lee, T.; Sarantopoulos, C.D.; Hackam, A.S.; McClellan, A.L.; Felix, E.R.; Levitt, R.C.; Galor, A. Human Tear Serotonin Levels Correlate with Symptoms and Signs of Dry Eye. Ophthalmology 2015, 122, 1675–1680. [Google Scholar] [CrossRef]
- Chia, E.M.; Mitchell, P.; Rochtchina, E.; Lee, A.J.; Maroun, R.; Wang, J.J. Prevalence and associations of dry eye syndrome in an older population: The Blue Mountains Eye Study. Clin. Exp. Ophthalmol. 2003, 31, 229–232. [Google Scholar] [CrossRef]
- Golebiowski, B.; Badarudin, N.; Eden, J.; You, J.; Hampel, U.; Stapleton, F. Does endogenous serum oestrogen play a role in meibomian gland dysfunction in postmenopausal women with dry eye? Br. J. Ophthalmol. 2017, 101, 218–222. [Google Scholar] [CrossRef]
- Uncu, G.; Avci, R.; Uncu, Y.; Kaymaz, C.; Develioğlu, O. The effects of different hormone replacement therapy regimens on tear function, intraocular pressure and lens opacity. Gynecol. Endocrinol. 2006, 22, 501–505. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Karpecki, P.M.; Perez, V.L. Treatment of blepharitis: Recent clinical trials. Ocul. Surf. 2014, 12, 273–284. [Google Scholar] [CrossRef]
- Craig, J.P.; Rupenthal, I.D.; Seyfoddin, A.; Cheung, I.M.Y.; Uy, B.; Wang, M.T.M.; Watters, G.A.; Swift, S. Preclinical development of MGO Manuka Honey microemulsion for blepharitis management. BMJ Open Ophthalmol. 2017, 1, e000065. [Google Scholar] [CrossRef]
- Dougherty, J.M.; McCulley, J.P.; Silvany, R.E.; Meyer, D.R. The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Invest. Ophthalmol. Vis. Sci. 1991, 32, 2970–2975. [Google Scholar]
- Zhang, A.C.; Muntz, A.; Wang, M.T.M.; Craig, J.P.; Downie, L.E. Ocular Demodex: A systematic review of the clinical literature. Ophthalmic Physiol. Opt. 2020, 40, 389–432. [Google Scholar] [CrossRef]
- Liu, J.; Sheha, H.; Tseng, S.C. Pathogenic role of Demodex mites in blepharitis. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 505–510. [Google Scholar] [CrossRef]
- English, F.P.; Nutting, W.B. Feeding characteristics in demodectic mites of the eyelid. Aust. J. Ophthalmol. 1981, 9, 311–313. [Google Scholar] [CrossRef]
- Kim, J.H.; Chun, Y.S.; Kim, J.C. Clinical and immunological responses in ocular demodecosis. J. Korean Med. Sci. 2011, 26, 1231–1237. [Google Scholar] [CrossRef]
- Jones, L.; Efron, N.; Bandamwar, K.; Barnett, M.; Jacobs, D.S.; Jalbert, I.; Pult, H.; Rhee, M.K.; Sheardown, H.; Shovlin, J.P.; et al. TFOS Lifestyle: Impact of contact lenses on the ocular surface. Ocul. Surf. 2023, 29, 175–219. [Google Scholar] [CrossRef]
- Nichols, J.J.; Sinnott, L.T. Tear Film, Contact Lens, and Patient-Related Factors Associated with Contact Lens–Related Dry Eye. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1319–1328. [Google Scholar] [CrossRef]
- Kojima, T.; Matsumoto, Y.; Ibrahim, O.M.A.; Wakamatsu, T.H.; Uchino, M.; Fukagawa, K.; Ogawa, J.; Dogru, M.; Negishi, K.; Tsubota, K. Effect of Controlled Adverse Chamber Environment Exposure on Tear Functions in Silicon Hydrogel and Hydrogel Soft Contact Lens Wearers. Invest. Ophthalmol. Vis. Sci. 2011, 52, 8811–8817. [Google Scholar] [CrossRef]
- Efron, N.; Brennan, N.A.; Morgan, P.B.; Wilson, T. Lid wiper epitheliopathy. Prog. Retin. Eye Res. 2016, 53, 140–174. [Google Scholar] [CrossRef]
- Paulsen, A.J.; Cruickshanks, K.J.; Fischer, M.E.; Huang, G.-H.; Klein, B.E.K.; Klein, R.; Dalton, D.S. Dry eye in the beaver dam offspring study: Prevalence, risk factors, and health-related quality of life. Am. J. Ophthalmol. 2014, 157, 799–806. [Google Scholar] [CrossRef]
- Doughty, M.J.; Fonn, D.; Richter, D.; Simpson, T.; Caffery, B.; Gordon, K. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom. Vis. Sci. 1997, 74, 624–631. [Google Scholar] [CrossRef]
- Dumbleton, K.; Caffery, B.; Dogru, M.; Hickson-Curran, S.; Kern, J.; Kojima, T.; Morgan, P.B.; Purslow, C.; Robertson, D.M.; Nelson, J.D. The TFOS International Workshop on Contact Lens Discomfort: Report of the Subcommittee on Epidemiology. Invest. Ophthalmol. Vis. Sci. 2013, 54, TFOS20–TFOS36. [Google Scholar] [CrossRef]
- Dumbleton, K.; Woods, C.A.; Jones, L.W.; Fonn, D. The impact of contemporary contact lenses on contact lens discontinuation. Eye Contact Lens Sci. Clin. Pract. 2013, 39, 93–99. [Google Scholar] [CrossRef]
- Lee, B.H.; McLaren, J.W.; Erie, J.C.; Hodge, D.O.; Bourne, W.M. Reinnervation in the cornea after LASIK. Invest. Ophthalmol. Vis. Sci. 2002, 43, 3660–3664. [Google Scholar]
- Tervo, T.; Moilanen, J. In vivo confocal microscopy for evaluation of wound healing following corneal refractive surgery. Prog. Retin. Eye Res. 2003, 22, 339–358. [Google Scholar] [CrossRef]
- Denoyer, A.; Landman, E.; Trinh, L.; Faure, J.F.; Auclin, F.; Baudouin, C. Dry eye disease after refractive surgery: Comparative outcomes of small incision lenticule extraction versus LASIK. Ophthalmology 2015, 122, 669–676. [Google Scholar] [CrossRef]
- Braun, R.J.; King-Smith, P.E.; Begley, C.G.; Li, L.; Gewecke, N.R. Dynamics and function of the tear film in relation to the blink cycle. Prog. Retin. Eye Res. 2015, 45, 132–164. [Google Scholar] [CrossRef]
- Craig, J.P.; Tomlinson, A. Importance of the lipid layer in human tear film stability and evaporation. Optom. Vis. Sci. 1997, 74, 8–13. [Google Scholar] [CrossRef]
- Hamawy, A.H.; Farkas, J.P.; Fagien, S.; Rohrich, R.J. Preventing and managing dry eyes after periorbital surgery: A retrospective review. Plast. Reconstr. Surg. 2009, 123, 353–359. [Google Scholar] [CrossRef]
- Kiang, L.; Deptula, P.; Mazhar, M.; Murariu, D.; Parsa, F.D. Muscle-sparing blepharoplasty: A prospective left-right comparative study. Arch. Plast. Surg. 2014, 41, 576–583. [Google Scholar] [CrossRef]
- Wang, M.T.; Kersey, T.L.; Sloan, B.H.; Craig, J.P. Evaporative dry eye following modified Hughes flap reconstruction. Clin. Exp. Ophthalmol. 2018, 46, 700–702. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Azar, D.T.; Baudouin, C.; Efron, N.; Hirayama, M.; Horwath-Winter, J.; Kim, T.; Mehta, J.S.; Messmer, E.M.; Pepose, J.S.; et al. TFOS DEWS II iatrogenic report. Ocul. Surf. 2017, 15, 511–538. [Google Scholar] [CrossRef]
- Arita, R.; Itoh, K.; Maeda, S.; Maeda, K.; Furuta, A.; Tomidokoro, A.; Aihara, M.; Amano, S. Effects of long-term topical anti-glaucoma medications on meibomian glands. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1181–1185. [Google Scholar] [CrossRef]
- Wong, A.B.C.; Wang, M.T.M.; Liu, K.; Prime, Z.J.; Danesh-Meyer, H.V.; Craig, J.P. Exploring topical anti-glaucoma medication effects on the ocular surface in the context of the current understanding of dry eye. Ocul. Surf. 2018, 16, 289–293. [Google Scholar] [CrossRef]
- Barabino, S.; Antonelli, S.; Cimbolini, N.; Mauro, V.; Bouzin, M. The effect of preservatives and antiglaucoma treatments on the ocular surface of mice with dry eye. Invest. Ophthalmol. Vis. Sci. 2014, 55, 6499–6504. [Google Scholar] [CrossRef]
- Baudouin, C.; Labbé, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef]
- Avunduk, A.M.; Avunduk, M.C.; Varnell, E.D.; Kaufman, H.E. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: A clinical and immunocytochemical study. Am. J. Ophthalmol. 2003, 136, 593–602. [Google Scholar] [CrossRef]
- Singer, D.D.; Kennedy, J.; Wittpenn, J.R. Topical NSAIDs effect on corneal sensitivity. Cornea 2015, 34, 541–543. [Google Scholar] [CrossRef]
- Guidera, A.C.; Luchs, J.I.; Udell, I.J. Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology 2001, 108, 936–944. [Google Scholar] [CrossRef]
- Alves, M.; Asbell, P.; Dogru, M.; Giannaccare, G.; Grau, A.; Gregory, D.; Kim, D.H.; Marini, M.C.; Ngo, W.; Nowinska, A.; et al. TFOS Lifestyle Report: Impact of environmental conditions on the ocular surface. Ocul. Surf. 2023, 29, 1–52. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Lingham, G.; Downie, L.E.; Huntjens, B.; Inomata, T.; Jivraj, S.; Kobia-Acquah, E.; Muntz, A.; Mohamed-Noriega, K.; Plainis, S.; et al. TFOS Lifestyle: Impact of the digital environment on the ocular surface. Ocul. Surf. 2023, 28, 213–252. [Google Scholar] [CrossRef]
- Markoulli, M.; Ahmad, S.; Arcot, J.; Arita, R.; Benitez-Del-Castillo, J.; Caffery, B.; Downie, L.E.; Edwards, K.; Flanagan, J.; Labetoulle, M.; et al. TFOS Lifestyle: Impact of nutrition on the ocular surface. Ocul. Surf. 2023, 29, 226–271. [Google Scholar] [CrossRef]
- Sullivan, D.A.; da Costa, A.X.; Del Duca, E.; Doll, T.; Grupcheva, C.N.; Lazreg, S.; Liu, S.H.; McGee, S.R.; Murthy, R.; Narang, P.; et al. TFOS Lifestyle: Impact of cosmetics on the ocular surface. Ocul. Surf. 2023, 29, 77–130. [Google Scholar] [CrossRef]
- Hwang, S.H.; Choi, Y.-H.; Paik, H.J.; Wee, W.R.; Kim, M.K.; Kim, D.H. Potential Importance of Ozone in the Association Between Outdoor Air Pollution and Dry Eye Disease in South Korea. JAMA Ophthalmol. 2016, 134, 503–510. [Google Scholar] [CrossRef]
- Berg, E.J.; Ying, G.-s.; Maguire, M.G.; Sheffield, P.E.; Szczotka-Flynn, L.B.; Asbell, P.A.; Shen, J.F.; The DREAM Study Research Group. Climatic and Environmental Correlates of Dry Eye Disease Severity: A Report From the Dry Eye Assessment and Management (DREAM) Study. Transl. Vis. Sci. Technol. 2020, 9, 25. [Google Scholar] [CrossRef]
- Galor, A.; Kumar, N.; Feuer, W.; Lee, D.J. Environmental Factors Affect the Risk of Dry Eye Syndrome in a United States Veteran Population. Ophthalmology 2014, 121, 972–973.e971. [Google Scholar] [CrossRef]
- McCulley, J.P.; Aronowicz, J.D.; Uchiyama, E.; Shine, W.E.; Butovich, I.A. Correlations in a change in aqueous tear evaporation with a change in relative humidity and the impact. Am. J. Ophthalmol. 2006, 141, 758–760. [Google Scholar] [CrossRef]
- Hanyuda, A.; Sawada, N.; Uchino, M.; Kawashima, M.; Yuki, K.; Tsubota, K.; Yamagishi, K.; Iso, H.; Yasuda, N.; Saito, I.; et al. Physical inactivity, prolonged sedentary behaviors, and use of visual display terminals as potential risk factors for dry eye disease: JPHC-NEXT study. Ocul. Surf. 2020, 18, 56–63. [Google Scholar] [CrossRef]
- Wang, M.T.M.; Muntz, A.; Mamidi, B.; Wolffsohn, J.S.; Craig, J.P. Modifiable lifestyle risk factors for dry eye disease. Contact Lens Anterior Eye 2021, 101409. [Google Scholar] [CrossRef]
- Himebaugh, N.L.; Begley, C.G.; Bradley, A.; Wilkinson, J.A. Blinking and tear break-up during four visual tasks. Optom. Vis. Sci. 2009, 86, E106–E114. [Google Scholar] [CrossRef]
- Argilés, M.; Cardona, G.; Pérez-Cabré, E.; Rodríguez, M. Blink Rate and Incomplete Blinks in Six Different Controlled Hard-Copy and Electronic Reading Conditions. Invest. Ophthalmol. Vis. Sci. 2015, 56, 6679–6685. [Google Scholar] [CrossRef]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Anatomy, Physiology, and Pathophysiology of the Meibomian Gland. Invest. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef]
- Pansell, T.; Porsblad, M.; Abdi, S. The effect of vertical gaze position on ocular tear film stability. Clin. Exp. Optom 2007, 90, 176–181. [Google Scholar] [CrossRef]
- Lee, W.; Lim, S.S.; Won, J.U.; Roh, J.; Lee, J.H.; Seok, H.; Yoon, J.H. The association between sleep duration and dry eye syndrome among Korean adults. Sleep Med. 2015, 16, 1327–1331. [Google Scholar] [CrossRef]
- Magno, M.S.; Utheim, T.P.; Snieder, H.; Hammond, C.J.; Vehof, J. The relationship between dry eye and sleep quality. Ocul. Surf. 2021, 20, 13–19. [Google Scholar] [CrossRef]
- Lee, Y.B.; Koh, J.W.; Hyon, J.Y.; Wee, W.R.; Kim, J.J.; Shin, Y.J. Sleep Deprivation Reduces Tear Secretion and Impairs the Tear Film. Invest. Ophthalmol. Vis. Sci. 2014, 55, 3525–3531. [Google Scholar] [CrossRef]
- Schmid, S.M.; Hallschmid, M.; Jauch-Chara, K.; Lehnert, H.; Schultes, B. Sleep timing may modulate the effect of sleep loss on testosterone. Clin. Endocrinol. 2012, 77, 749–754. [Google Scholar] [CrossRef]
- Mahler, B.; Kamperis, K.; Schroeder, M.; Frøkiær, J.; Djurhuus, J.C.; Rittig, S. Sleep deprivation induces excess diuresis and natriuresis in healthy children. Am. J. Physiol.-Ren. Physiol. 2012, 302, F236–F243. [Google Scholar] [CrossRef]
- Sommer, A. Xerophthalmia and vitamin a status. Prog. Retin. Eye Res. 1998, 17, 9–31. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Nutrition and Nutrition-Related Health and Development Data: Vitamin A Deficiency. Available online: www.who.int/data/nutrition/nlis/info/vitamin-a-deficiency (accessed on 4 July 2021).
- Feranchak, A.P.; Gralla, J.; King, R.; Ramirez, R.O.; Corkill, M.; Narkewicz, M.R.; Sokol, R.J. Comparison of indices of vitamin A status in children with chronic liver disease. Hepatology 2005, 42, 782–792. [Google Scholar] [CrossRef]
- Chiu, M.; Dillon, A.; Watson, S. Vitamin A deficiency and xerophthalmia in children of a developed country. J. Paediatr. Child Health 2016, 52, 699–703. [Google Scholar] [CrossRef]
- Ziemanski, J.F.; Wolters, L.R.; Jones-Jordan, L.; Nichols, J.J.; Nichols, K.K. Relation Between Dietary Essential Fatty Acid Intake and Dry Eye Disease and Meibomian Gland Dysfunction in Postmenopausal Women. Am. J. Ophthalmol. 2018, 189, 29–40. [Google Scholar] [CrossRef]
- Miljanovic, B.; Trivedi, K.A.; Dana, M.R.; Gilbard, J.P.; Buring, J.E.; Schaumberg, D.A. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am. J. Clin. Nutr. 2005, 82, 887–893. [Google Scholar] [CrossRef]
- Deinema, L.A.; Vingrys, A.J.; Wong, C.Y.; Jackson, D.C.; Chinnery, H.R.; Downie, L.E. A Randomized, Double-Masked, Placebo-Controlled Clinical Trial of Two Forms of Omega-3 Supplements for Treating Dry Eye Disease. Ophthalmology 2017, 124, 43–52. [Google Scholar] [CrossRef]
- Walter, S.D.; Gronert, K.; McClellan, A.L.; Levitt, R.C.; Sarantopoulos, K.D.; Galor, A. ω-3 Tear Film Lipids Correlate With Clinical Measures of Dry Eye. Invest. Ophthalmol. Vis. Sci. 2016, 57, 2472–2478. [Google Scholar] [CrossRef]
- Kangari, H.; Eftekhari, M.H.; Sardari, S.; Hashemi, H.; Salamzadeh, J.; Ghassemi-Broumand, M.; Khabazkhoob, M. Short-term consumption of oral omega-3 and dry eye Syndrome. Ophthalmology 2013, 120, 2191–2196. [Google Scholar] [CrossRef]
- Downie, L.E.; Ng, S.M.; Lindsley, K.B.; Akpek, E.K. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Zhang, A.C.; Singh, S.; Craig, J.P.; Downie, L.E. Omega-3 Fatty Acids and Eye Health: Opinions and Self-Reported Practice Behaviors of Optometrists in Australia and New Zealand. Nutrients 2020, 12, 1179. [Google Scholar] [CrossRef]
- Ng, A.; Evans, K.; North, R.V.; Purslow, C. Migration of Cosmetic Products into the Tear Film. Eye Contact Lens Sci. Clin. Pract. 2015, 41, 304–309. [Google Scholar] [CrossRef]
- Malik, A.; Claoué, C. Transport and interaction of cosmetic product material within the ocular surface: Beauty and the beastly symptoms of toxic tears. Contact Lens Anterior Eye 2012, 35, 247–259. [Google Scholar] [CrossRef]
- Wang, M.T.; Craig, J.P. Investigating the effect of eye cosmetics on the tear film: Current insights. Clin. Optom. 2018, 10, 33–40. [Google Scholar] [CrossRef]
- Chen, X.; Sullivan, D.A.; Sullivan, A.G.; Kam, W.R.; Liu, Y. Toxicity of cosmetic preservatives on human ocular surface and adnexal cells. Exp. Eye Res. 2018, 170, 188–197. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Kam, W.R.; Li, Y.; Sullivan, D.A. Toxicity of the cosmetic preservatives parabens, phenoxyethanol and chlorphenesin on human meibomian gland epithelial cells. Exp. Eye Res. 2020, 196, 108057. [Google Scholar] [CrossRef]
- Canavez, A.; de Oliveira Prado Corrêa, G.; Isaac, V.L.B.; Schuck, D.C.; Lorencini, M. Integrated approaches to testing and assessment as a tool for the hazard assessment and risk characterization of cosmetic preservatives. J. Appl. Toxicol. 2021, 41, 1687–1699. [Google Scholar] [CrossRef]
- Troutman, J.A.; Rick, D.L.; Stuard, S.B.; Fisher, J.; Bartels, M.J. Development of a physiologically-based pharmacokinetic model of 2-phenoxyethanol and its metabolite phenoxyacetic acid in rats and humans to address toxicokinetic uncertainty in risk assessment. Regul. Toxicol. Pharmacol. 2015, 73, 530–543. [Google Scholar] [CrossRef]
- Amano, Y.; Sugimoto, Y.; Sugita, M. Ocular disorders due to eyelash extensions. Cornea 2012, 31, 121–125. [Google Scholar] [CrossRef]
- Ding, J.; Kam, W.R.; Dieckow, J.; Sullivan, D.A. The influence of 13-cis retinoic acid on human meibomian gland epithelial cells. Invest. Ophthalmol. Vis. Sci. 2013, 54, 4341–4350. [Google Scholar] [CrossRef]
- Choi, M.G.; Yeo, J.H.; Kang, J.W.; Chun, Y.S.; Lee, J.K.; Kim, J.C. Effects of botulinum toxin type A on the treatment of dry eye disease and tear cytokines. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 331–338. [Google Scholar] [CrossRef]
- Diel, R.J.; Kroeger, Z.A.; Levitt, R.C.; Sarantopoulos, C.; Sered, H.; Martinez-Barrizonte, J.; Galor, A. Botulinum Toxin A for the Treatment of Photophobia and Dry Eye. Ophthalmology 2018, 125, 139–140. [Google Scholar] [CrossRef]
- Ho, M.-C.; Hsu, W.-C.; Hsieh, Y.-T. Botulinum Toxin Type A Injection for Lateral Canthal Rhytids: Effect on Tear Film Stability and Tear Production. JAMA Ophthalmol. 2014, 132, 332–337. [Google Scholar] [CrossRef]
- Ozgur, O.K.; Murariu, D.; Parsa, A.A.; Parsa, F.D. Dry eye syndrome due to botulinum toxin type-A injection: Guideline for prevention. Hawai’i J. Med. Public Health 2012, 71, 120–123. [Google Scholar]
| Systemic Conditions |
|---|
| Androgen deficiency Anxiety Atopy Benign prostate hyperplasia Chronic pain condition, including back pain and other pain syndromes Cicatricial pemphigoid Connective tissue disease Depression Diabetes Ectodermal dysplasia syndrome Fibromyalgia Hematopoietic stem cell transplantation Irritable bowel syndrome Menopause Migraine headaches Parkinson’s disease Pemphigoid Polycystic ovarian syndrome Psoriasis Rosacea Sjögren syndrome Sleep disorders Stevens-Johnson syndrome Thyroid disease Toxic epidermal necrolysis Turner syndrome |
| Systemic medications |
| Anti-androgens Antidepressants Antihistamines Anxiolytics Hormone replacement therapy Isotretinoin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Britten-Jones, A.C.; Wang, M.T.M.; Samuels, I.; Jennings, C.; Stapleton, F.; Craig, J.P. Epidemiology and Risk Factors of Dry Eye Disease: Considerations for Clinical Management. Medicina 2024, 60, 1458. https://doi.org/10.3390/medicina60091458
Britten-Jones AC, Wang MTM, Samuels I, Jennings C, Stapleton F, Craig JP. Epidemiology and Risk Factors of Dry Eye Disease: Considerations for Clinical Management. Medicina. 2024; 60(9):1458. https://doi.org/10.3390/medicina60091458
Chicago/Turabian StyleBritten-Jones, Alexis Ceecee, Michael T. M. Wang, Isaac Samuels, Catherine Jennings, Fiona Stapleton, and Jennifer P. Craig. 2024. "Epidemiology and Risk Factors of Dry Eye Disease: Considerations for Clinical Management" Medicina 60, no. 9: 1458. https://doi.org/10.3390/medicina60091458








