Echocardiographic Indices in Patients with End-Stage Renal Disease and Their Association with Hemodialysis-to-Hemodiafiltration Transfer: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical and Reporting Considerations
2.2. Study Design and Participants
2.3. Data Sources
2.4. Conventional Hemodialysis
2.5. Hemodiafiltration
2.6. Comprehensive Echocardiographic Assessment
2.7. Outcomes
2.8. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
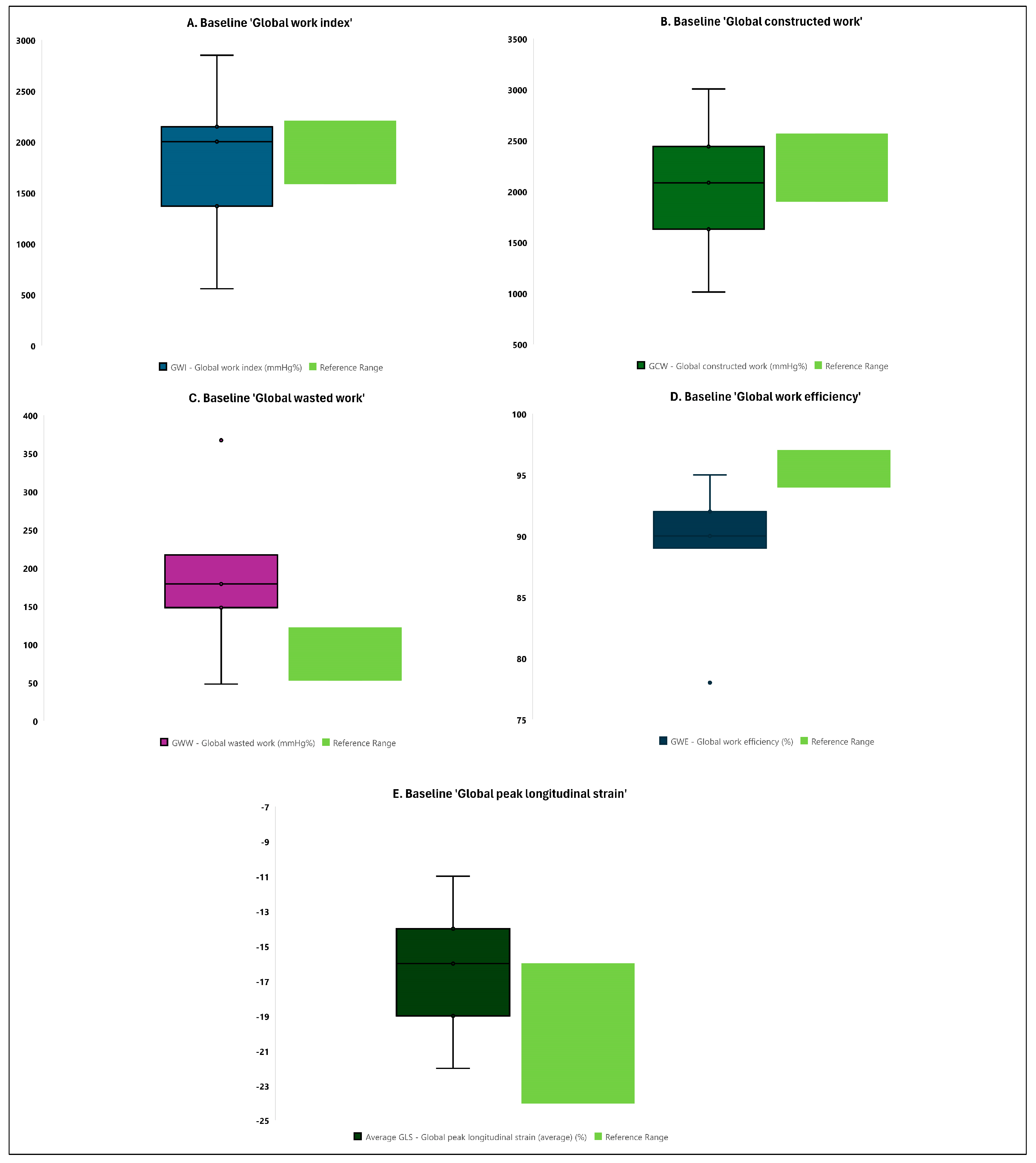
3.2. Comparison of Baseline Echocardiographic Measurements with Reference Values
3.3. Echocardiographic Parameters Following the Hemodialysis-to-Hemodiafiltration Transfer
3.4. Evaluation of the Laboratory Parameters and the Efficacy of Dialysis during the 3-Month Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canaud, B.; Blankestijn, P.J.; Grooteman, M.P.C.; Davenport, A. Why and how high volume hemodiafiltration may reduce cardiovascular mortality in stage 5 chronic kidney disease dialysis patients? A comprehensive literature review on mechanisms involved. Semin. Dial. 2022, 35, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhao, S. Risk factors for mortality in patients undergoing hemodialysis: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 238, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, A.; Wei, F.; Chen, H. Cardiac valve calcification and risk of cardiovascular or all-cause mortality in dialysis patients: A meta-analysis. BMC Cardiovasc. Disord. 2018, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Assa, S.; Hummel, Y.M.; Voors, A.A.; Kuipers, J.; Westerhuis, R.; de Jong, P.E.; Franssen, C.F.M. Hemodialysis-Induced Regional Left Ventricular Systolic Dysfunction: Prevalence, Patient and Dialysis Treatment-Related Factors, and Prognostic Significance. Clin. J. Am. Soc. Nephrol. 2012, 7, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Kott, J.; Reichek, N.; Butler, J.; Arbeit, L.; Mallipattu, S.K. Cardiac Imaging in Dialysis Patients. Kidney Med. 2020, 2, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.O.; Jefferies, H.J.; Selby, N.M.; McIntyre, C.W. Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin. J. Am. Soc. Nephrol. 2009, 4, 914–920. [Google Scholar] [CrossRef]
- Ohtake, T.; Oka, M.; Ishioka, K.; Honda, K.; Mochida, Y.; Maesato, K.; Moriya, H.; Hidaka, S.; Kobayashi, S. Cardiovascular protective effects of on-line hemodiafiltration: Comparison with conventional hemodialysis. Ther. Apher. Dial. 2012, 16, 181–188. [Google Scholar] [CrossRef]
- Blankestijn, P.J.; Vernooij, R.W.M.; Hockham, C.; Strippoli, G.F.M.; Canaud, B.; Hegbrant, J.; Barth, C.; Covic, A.; Cromm, K.; Cucui, A.; et al. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N. Engl. J. Med. 2023, 389, 700–709. [Google Scholar] [CrossRef]
- Wolley, M.; Jardine, M.; Hutchison, C.A. Exploring the Clinical Relevance of Providing Increased Removal of Large Middle Molecules. Clin. J. Am. Soc. Nephrol. 2018, 13, 805–814. [Google Scholar] [CrossRef]
- Lang, T.; Zawada, A.M.; Theis, L.; Braun, J.; Ottillinger, B.; Kopperschmidt, P.; Gagel, A.; Kotanko, P.; Stauss-Grabo, M.; Kennedy, J.P.; et al. Hemodiafiltration: Technical and Medical Insights. Bioengineering 2023, 10, 145. [Google Scholar] [CrossRef]
- Hensen, L.C.R.; Goossens, K.; Delgado, V.; Rotmans, J.I.; Jukema, J.W.; Bax, J.J. Prognostic Implications of Left Ventricular Global Longitudinal Strain in Predialysis and Dialysis Patients. Am. J. Cardiol. 2017, 120, 500–504. [Google Scholar] [CrossRef]
- Liu, Y.W.; Su, C.T.; Sung, J.M.; Wang, S.P.; Su, Y.R.; Yang, C.S.; Tsai, L.M.; Chen, J.H.; Tsai, W.C. Association of left ventricular longitudinal strain with mortality among stable hemodialysis patients with preserved left ventricular ejection fraction. Clin. J. Am. Soc. Nephrol. 2013, 8, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Kramann, R.; Erpenbeck, J.; Schneider, R.K.; Röhl, A.B.; Hein, M.; Brandenburg, V.M.; van Diepen, M.; Dekker, F.; Marx, N.; Floege, J.; et al. Speckle tracking echocardiography detects uremic cardiomyopathy early and predicts cardiovascular mortality in ESRD. J. Am. Soc. Nephrol. 2014, 25, 2351–2365. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy, R.; Isbel, N.M.; Hawley, C.M.; Pascoe, E.M.; Burrage, M.; Leano, R.; Haluska, B.A.; Marwick, T.H.; Stanton, T. Left Ventricular Global Longitudinal Strain (GLS) Is a Superior Predictor of All-Cause and Cardiovascular Mortality When Compared to Ejection Fraction in Advanced Chronic Kidney Disease. PLoS ONE 2015, 10, e0127044. [Google Scholar] [CrossRef] [PubMed]
- WHO Consortium. Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 1995, 854, 1–452. [Google Scholar]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [PubMed]
- Roemer, S.; Jaglan, A.; Santos, D.; Umland, M.; Jain, R.; Tajik, A.J.; Khandheria, B.K. The Utility of Myocardial Work in Clinical Practice. J. Am. Soc. Echocardiogr. 2021, 34, 807–818. [Google Scholar] [CrossRef]
- Chan, J.; Edwards, N.F.A.; Scalia, G.M.; Khandheria, B.K. Myocardial Work: A New Type of Strain Imaging? J. Am. Soc. Echocardiogr. 2020, 33, 1209–1211. [Google Scholar] [CrossRef]
- Charfeddine, S.; Abid, L.; Hammami, R.; Bahloul, A.; Triki, F.; Kammoun, S. Left ventricular myocardial function in hemodialysis patients: The effects of preload decrease in conventional, Doppler and speckle tracking echocardiography parameters. Pan Afr. Med. J. 2021, 38, 45. [Google Scholar] [CrossRef]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar] [CrossRef]
- Nyberg, J.; Jakobsen, E.O.; Østvik, A.; Holte, E.; Stølen, S.; Lovstakken, L.; Grenne, B.; Dalen, H. Echocardiographic Reference Ranges of Global Longitudinal Strain for All Cardiac Chambers Using Guideline-Directed Dedicated Views. Cardiovasc. Imaging 2023, 16, 1516–1531. [Google Scholar] [CrossRef]
- Kou, S.; Caballero, L.; Dulgheru, R.; Voilliot, D.; De Sousa, C.; Kacharava, G.; Athanassopoulos, G.D.; Barone, D.; Baroni, M.; Cardim, N.; et al. Echocardiographic reference ranges for normal cardiac chamber size: Results from the NORRE study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 680–690. [Google Scholar] [CrossRef]
- Dutta, T.; Aronow, W.S. Echocardiographic evaluation of the right ventricle: Clinical implications. Clin. Cardiol. 2017, 40, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, G.; Marsan, N.A.; Gripari, P.; Maffessanti, F.; Brusoni, D.; Muratori, M.; Caiani, E.G.; Fiorentini, C.; Pepi, M. Reference values for right ventricular volumes and ejection fraction with real-time three-dimensional echocardiography: Evaluation in a large series of normal subjects. J. Am. Soc. Echocardiogr. 2010, 23, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Akhaladze, N.; Athanassopoulos, G.D.; Barone, D.; et al. Echocardiographic reference ranges for normal left ventricular 2D strain: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Mostovaya, I.M.; Bots, M.L.; van den Dorpel, M.A.; Grooteman, M.P.; Kamp, O.; Levesque, R.; Ter Wee, P.M.; Nubé, M.J.; Blankestijn, P.J. A randomized trial of hemodiafiltration and change in cardiovascular parameters. Clin. J. Am. Soc. Nephrol. 2014, 9, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Czifra, A.; Páll, A.; Kulcsár, J.; Barta, K.; Kertész, A.; Paragh, G.; Lőrincz, I.; Jenei, Z.; Agarwal, A.; Zarjou, A.; et al. Hemodialysis and hemodiafiltration differently modulate left ventricular diastolic function. BMC Nephrol. 2013, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Ethier, I.; Auger, D.; Beaulieu, M.; Wesolowska, E.; Lévesque, R. Evolution of high-sensitivity troponin-T and echocardiography parameters in patients undergoing high efficiency on-line hemodiafiltration versus conventional low-flux hemodialysis. PLoS ONE 2019, 14, e0223957. [Google Scholar] [CrossRef]
- Hameed, I.; Gaudino, M.; Naik, A.; Rahouma, M.; Robinson, N.B.; Ruan, Y.; Demetres, M.; Bossola, M. Comparison of the effects of hemodialysis and hemodiafiltration on left ventricular hypertrophy in end-stage renal disease patients: A systematic review and meta-analysis. Semin. Dial. 2020, 33, 120–126. [Google Scholar] [CrossRef]
- Ravera, M.; Rosa, G.M.; Fontanive, P.; Bussalino, E.; Dorighi, U.; Picciotto, D.; Di Lullo, L.; Dini, F.L.; Paoletti, E. Impaired Left Ventricular Global Longitudinal Strain among Patients with Chronic Kidney Disease and End-Stage Renal Disease and Renal Transplant Recipients. Cardiorenal Med. 2018, 9, 61–68. [Google Scholar] [CrossRef]
- Sun, M.; Kang, Y.; Cheng, L.; Pan, C.; Cao, X.; Yao, H.; Dong, L.; Shu, X. Global longitudinal strain is an independent predictor of cardiovascular events in patients with maintenance hemodialysis: A prospective study using three-dimensional speckle tracking echocardiography. Int. J. Cardiovasc. Imaging 2016, 32, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Tamulėnaitė, E.; Žvirblytė, R.; Ereminienė, R.; Žiginskienė, E.; Ereminienė, E. Changes of Left and Right Ventricle Mechanics and Function in Patients with End-Stage Renal Disease Undergoing Haemodialysis. Medicina 2018, 54, 87. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.S.; Crowley, L.E.; Jefferies, H.J.; Eldehni, M.T.; Odudu, A.; McIntyre, C.W. The Impact of Hemodialysis on Segmental and Global Longitudinal Myocardial Strain. Can. J. Cardiol. 2014, 30, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Hamano, T.; Wada, A.; Nakai, S.; Masakane, I. Predilution online hemodiafiltration is associated with improved survival compared with hemodialysis. Kidney Int. 2019, 95, 929–938. [Google Scholar] [CrossRef]
- Takayama, F.; Miyazaki, S.; Morita, T.; Hirasawa, Y.; Niwa, T. Dialysis-related amyloidosis of the heart in long-term hemodialysis patients. Kidney Int. 2001, 78, S172–S176. [Google Scholar] [CrossRef] [PubMed]
- Zito, C.; Longobardo, L.; Citro, R.; Alderisi, M.; Oreto, L.; Carerj, M.L.; Manganaro, R.; Cusmà-Piccione, M.; Todaro, M.C.; Di Bella, G.; et al. Ten Years of 2D Longitudinal Strain for Early Myocardial Dysfunction Detection: A Clinical Overview. BioMed Res. Int. 2018, 2018, 8979407. [Google Scholar] [CrossRef]
- Chen, K.W.; Hsieh, W.T.; Huang, C.Y.; Huang, C.C.; Liang, H.Y.; Wang, G.J. Estimated left ventricular pressure-myocardial strain loop as an index of cardiac work predicts all-cause mortality in patients receiving regular hemodialysis. J. Diabetes Complicat. 2021, 35, 107890. [Google Scholar] [CrossRef]
- Kamal, A.; Ahmed, N.; Zahran, A.; Ebrahim, D. Assessment of left ventricular function in patients with chronic renal failure undergoing hemodialysis. Menoufia Med. J. 2021, 34, 433. [Google Scholar]
- Boe, E.; Skulstad, H.; Smiseth, O.A. Myocardial work by echocardiography: A novel method ready for clinical testing. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 18–20. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, X.; Li, Z.; Dai, C.; Yang, Q.; Nie, Y.; Cao, X.; Shu, X.; Pan, C.; Sun, M. Global left ventricular myocardial work: A novel method to assess left ventricular myocardial function and predict major adverse cardiovascular events in maintenance hemodialysis patients. J. Clin. Ultrasound 2024, 1–10. [Google Scholar] [CrossRef]
- Paneni, F.; Gregori, M.; Ciavarella, G.M.; Sciarretta, S.; De Biase, L.; Marino, L.; Tocci, G.; Principe, F.; Domenici, A.; Luciani, R.; et al. Right ventricular dysfunction in patients with end-stage renal disease. Am. J. Nephrol. 2010, 32, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Benedetto, F.A.; Mallamaci, F.; Ripepi, G.; Giacone, G.; Cataliotti, A.; Seminara, G.; Stancanelli, B.; Malatino, L.S. Prognostic impact of the indexation of left ventricular mass in patients undergoing dialysis. J. Am. Soc. Nephrol. 2001, 12, 2768–2774. [Google Scholar] [CrossRef] [PubMed]
- Mostovaya, I.M.; Bots, M.L.; van den Dorpel, M.A.; Goldschmeding, R.; den Hoedt, C.H.; Kamp, O.; Levesque, R.; Mazairac, A.H.; Penne, E.L.; Swinkels, D.W.; et al. Left Ventricular Mass in Dialysis Patients, Determinants and Relation with Outcome. Results from the COnvective TRansport STudy (CONTRAST). PLoS ONE 2014, 9, e84587. [Google Scholar] [CrossRef] [PubMed]
- Francisco, R.C.; Aloha, M.; Ramón, P.S. Effects of high-efficiency postdilution online hemodiafiltration and high-flux hemodialysis on serum phosphorus and cardiac structure and function in patients with end-stage renal disease. Int. Urol. Nephrol. 2013, 45, 1373–1378. [Google Scholar] [CrossRef]
- Schiffl, H.; Lang, S.M.; Fischer, R. Effects of high efficiency post-dilution on-line hemodiafiltration or conventional hemodialysis on residual renal function and left ventricular hypertrophy. Int. Urol. Nephrol. 2013, 45, 1389–1396. [Google Scholar] [CrossRef]
| Echocardiographic Parameters | Study Sample (Baseline—Hemodialysis Treatment) | Reference Values |
|---|---|---|
| LAd—Left atrial diameter (mm) | 39.0 (35.0–42.0) * | 29.3–37.9 |
| LAVi—Left atrial volume indexed (mL/m2) | 26.0 (19.3–28.15) | 18.0–31.6 |
| LVEDV BiP—Left ventricular end-diastolic volume [bi-plane] (mL) | 92.5 (67.0–106.0) | 68.0–117.6 |
| LVESV BiP—Left ventricular end-systolic volume [bi-plane] (mL) | 41.2 (25.0–48.0) | 22.8–44.6 |
| LVEF BiP—Left ventricular ejection fraction [bi-plane] (%) | 57.0 (53.0–63.0) | 59.0–68.8 |
| LVEDVi—Left ventricular end-diastolic volume indexed (mL/m2) | 114.3 (84.0–125.0) * | 40.0–62.8 |
| LVESVi—Left ventricular end-systolic volume indexed (mL/m2) | 55.7 (37.0–69.0) * | 13.4–23.8 |
| IVSd—Interventricular septal wall thickness (mm) | 10.0 (9.0–11.0) | 7.0–10.2 |
| LVPWd—Left ventricular posterior wall thickness (mm) | 11.0 (10.0–11.0) | 7.3–10.3 |
| LVIDd—Left ventricular internal end-diastolic diameter (mm) | 51.0 (46.0–54.0) | 39.5–49.1 |
| LVIDs—Left ventricular internal end-systolic diameter (mm) | 33.0 (26.0–38.0) | 25.2–34.6 |
| LVM—Left ventricular mass (g) | 240.4 (185.1–280.4) * | 89.4–164.2 |
| LVMi—Left ventricular mass indexed (g/m2) | 122.9 (90.6–153.9) | 52.4–87.4 |
| Echocardiographic Parameters | Study Sample (Baseline—Hemodialysis Treatment) | Reference Values |
|---|---|---|
| RVEDV—Right ventricular end-diastolic volume (mL) | 65.0 (53.0–66.0) | 65.0–107.0 |
| RVESV—Right ventricular end-systolic volume (mL) | 34.4 (29.0–40.0) | 18.0–40.0 |
| 3D RVEF (%)—3D right ventricular ejection fraction (%) | 45.9 (41.8–51.2) * | >45.0 |
| RV Dd base—Right ventricular diastolic diameter—base (mm) | 37.1 (32.0–40.0) | 28.7–39.7 |
| RV Dd mid—Right ventricular diastolic diameter—mid (mm) | 26.7 (23.0–28.0) | 22.5–33.5 |
| RV Ld—Right ventricular longitudinal diameter (mm) | 63.1 (59.0–67.0) | 59.8–75.8 |
| TAPSE—Tricuspid annular plane systolic excursion (mm) | 12.2 (9.0–16.0) * | ≥17.0 |
| RV FAC—Right ventricular fractional area change (%) | 36.6 (31.2–36.6) * | 41.3–58.1 |
| Echocardiographic Parameters | Study Sample (Baseline—Hemodialysis Treatment) | Reference Values |
|---|---|---|
| MV E vel—Transmitral E wave velocity (cm/s) | 80.0 (70.0–100.0) * | <50.0 |
| MV DecT—Mitral valve deceleration time (ms) | 291.0 (257.0–356.0) * | 160.0–220.0 |
| MV E/A Ratio—Mitral valve E/A Ratio | 0.8 (0.6–1.0) | 0.8–2.0 |
| e’ Sept—Septal annular e′ velocity (cm/s) | 9.8 (5.2–11.3) | >7.0 |
| e’ lat—Lateral annular e′ velocity (cm/s) | 10.1 (8.5–11.8) | >10.0 |
| E/e’ average ratio | 10.8 (8.7–12.6) | <14.0 |
| PV AccT—Pulmonic valve acceleration time (ms) | 155.0 (135.0–166.0) | >120.0 |
| TV S’—Peak systolic tissue velocity (S’ wave) at the tricuspid annulus (cm/s) | 10.0 (9.3–11.2) | >9.5 |
| Echocardiographic Parameters | Baseline (Hemodialysis Treatment) | 3-Month Follow-Up (Hemodiafiltration Treatment) | p-Value |
|---|---|---|---|
| GWI—Global work index (mmHg%) | 2001.0 (1368.0–2148.0) | 1897.5 (1669.0–2396.0) | 0.637 |
| GCW—Global constructed work (mmHg%) | 2084.5 (1628.0–2440.0) | 1939.5 (1793.0–2387.0) | 0.886 |
| GWW—Global wasted work (mmHg%) | 179.0 (148.0–217.0) | 233.5 (159.0–315.0) | 0.037 |
| GWE—Global work efficiency (%) | 90.0 (89.0–92.0) | 89.0 (87.0–91.0) | 0.250 |
| AVO—Aortic valve opening (ms) | 94.0 (63.0–115.7) | 70.0 (50.0–93.0) | 0.038 |
| AVC—Aortic valve closure (ms) | 361.0 (298.0–384.0) | 347.0 (330.0–371.0) | 0.422 |
| MVO—Mitral valve opening (ms) | 449.7 (411.0–483.0) | 453.6 (424.0–471.0) | 0.817 |
| MVC—Mitral valve closure (ms) | 329.0 (19.0–674.0) | 706.0 (53.0–849.0) | 0.063 |
| Longitudinal peak strain dispersion (ms) | 64.0 (51.0–70.0) | 58.1 (51.0–65.0) | 0.385 |
| APLAX GLS—Global peak longitudinal strain [APLAX] (%) | −16.0 (−18.0–[−14.0]) | −17.1 (−18.0–[−16.0]) | 0.293 |
| A4C GSL (A4C)—Global peak longitudinal strain [A4C] (%) | −16.0 (−19.0–[−14.0]) | −17.0 (−18.0–[−16.0]) | 0.376 |
| A2C GLS (A2C)—Global peak longitudinal strain [A2C] (%) | −18.0 (−20.0–[−14.0]) | −18.2 (−20.0–[−17.0]) | 0.440 |
| Average GLS—Global peak longitudinal strain (average) (%) | −16.0 (−19.0–[−14.0]) | −17.5 (−19.0–[−17.0]) | 0.258 |
| BA PSSL Full (%)—Basal anterior peak systolic longitudinal strain (%) | −13.8 (−18.0–[−9.0]) | −15.0 (−16.0–[−12.0]) | 0.516 |
| BI PSSL Full (%)—Basal inferior peak systolic longitudinal strain (%) | −15.0 (−17.0–[−10.0]) | −15.8 (−18.0–[−14.0]) | 0.117 |
| MA PSSL Full (%)—Mid-anterior peak systolic longitudinal strain (%) | −15.0 (−19.0–[−9.0]) | −15.0 (−18.0–[−13.0]) | 0.826 |
| MI PSSL Full (%)—Mid-inferior peak systolic longitudinal strain (%) | −17.0 (−19.0–[–11.0]) | −18.7 (−20.0–[−18.0]) | 0.016 |
| AA PSSL Full (%)—Apical anterior peak systolic longitudinal strain (%) | −21.0 (−29.0–[−18.0]) | −21.0 (−24.0–[−17.0]) | 0.590 |
| AI PSSL Full (%)—Apical inferior peak systolic longitudinal strain (%) | −22.0 (−32.0–[−18.0]) | −25.5 (−31.0–[−23.0]) | 0.379 |
| BAS PSSL Full (%)—Basal anteroseptal peak systolic longitudinal strain (%) | −12.0 (−14.0–[−8.0]) | −13.0 (−16.0–−10.0]) | 0.107 |
| BP PSSL Full (%)—Basal posterior peak systolic longitudinal strain (%) | −14.0 (−18.0–[−10.0]) | −15.3 (−17.0–[−12.0]) | 0.467 |
| MAS PSSL Full (%)—Mid-anteroseptal peak systolic longitudinal strain (%) | −20.0 (−23.0–[−15.0]) | −20.1 (−23.0–[−18.0]) | 0.441 |
| MP PSSL Full (%)—Mid-posterior peak systolic longitudinal strain (%) | −17.0 (−19.0–[−13.0]) | −16.1 (−19.0–[−12.0]) | 0.930 |
| AS PSSL Full (%)—Apico-anteroseptal peak systolic longitudinal strain (%) | −24.0 (−29.0–−14.0]) | −23.0 (−27.0–[−21.0]) | 0.733 |
| AP PSSL Full (%)—Apical posterior peak systolic longitudinal strain (%) | −20.0 (−28.0–[−14.0]) | −18.3 (−20.0–[−16.0]) | 0.590 |
| BS PSSL Full (%)—Basal septal peak systolic longitudinal strain (%) | −11.0 (−15.0–[−6.0]) | −11.7 (−13.0–[−10.0]) | 0.567 |
| BL PSSL Full (%)—Basal lateral peak systolic longitudinal strain (%) | −13.0 (−17.0–[−11.0]) | −14.0 (−17.0–[−12.0]) | 0.800 |
| MS PSSL Full (%)—Mid-septal peak systolic longitudinal strain (%) | −18.0 (−21.0–[−14.0]) | −19.0 (−21.0–[−17.0]) | 0.454 |
| ML PSSL Full (%)—Mid-lateral peak systolic longitudinal strain (%) | −17.0 (−19.0–[−12.0]) | −16.6 (−19.0–[−14.0]) | 0.692 |
| AS PSSL Full (%)—Apical septal peak systolic longitudinal strain (%) | −23.0 (−29.0–[−16.0]) | −23.0 (−26.0–[−21.0]) | 0.912 |
| AL PSSL Full (%)—Apical lateral peak systolic longitudinal strain (%) | −21.0 (−27.0–[−12.0]) | −18.5 (−23.0–[−14.0]) | 0.545 |
| Echocardiographic Parameters | Baseline (Hemodialysis Treatment) | 3-Month Follow-Up (Hemodiafiltration Treatment) | p-Value |
|---|---|---|---|
| Left atrium | |||
| LALs A2C—Left atrial length—systolic [A2C] (mm) | 53.0 (41.0–57.0) | 54.0 (48.0–59.0) | 0.190 |
| LAESV A-L A2C—Left atrial end-systolic volume, area-length [A2C] (mL) | 49.0 (32.0–68.0) | 52.0 (45.0–71.0) | 0.333 |
| LAESV MOD A2C—Left atrial end-systolic volume, method of discs [A2C] (mL) | 47.0 (31.0–67.0) | 54.0 (42.0–68.0) | 0.291 |
| LALs A4C—Left atrial length—systolic [A4C] (mm) | 56.0 (50.0–62.0) | 56.0 (54.0–62.0) | 0.620 |
| LAESV A-L A4C—Left atrial end-systolic volume, area-length [A4C] (mL) | 44.5 (35.0–60.0) | 52.0 (43.0–57.0) | 0.692 |
| LAESV MOD A4C—Left atrial end-systolic volume, method of discs [A4C] (mL) | 44.0 (33.0–60.0) | 51.0 (40.0–58.0) | 0.590 |
| LAESV AL—Left atrial end-systolic volume, area-length [derived] (mL) | 51.0 (39.0–65.0) | 55.0 (45.0–61.0) | 0.409 |
| LAVi—Left atrial volume indexed (mL/m2) | 26.0 (19.3–28.15) | 28.1 (23.5–30.6) | 0.214 |
| Left ventricle | |||
| LVVED 4Ch—Left ventricular end-diastolic volume [4Ch] (mL) | 76.0 (59.0–103.0) | 77.3 (68.0–84.0) | 0.826 |
| LVVES 4Ch—Left ventricular end-systolic volume [4Ch] (mL) | 32.0 (20.0–44.0) | 36.4 (30.0–45.0) | 0.575 |
| LVEF 4Ch—Left ventricular ejection fraction [4Ch] (%) | 57.2 (50.0–66.0) | 53.1 (51.0–56.0) | 0.153 |
| LVSV 4Ch—Left ventricular stroke volume [4Ch] (mL) | 42.0 (39.0–53.0) | 40.8 (28.0–47.0) | 0.190 |
| LVCO 4Ch—Left ventricular cardiac output [4Ch] (L/min) | 3.6 (2.6–4.1) | (2.8–3.4) | 0.053 |
| LVLs 4Ch—Left ventricular length in systole [4Ch] (mm) | 61.0 (55.0–66.0) | 61.0 (58.0–67.0) | 0.523 |
| LVLd 4Ch—Left ventricular length in diastole [4Ch] (mm) | 72.0 (67.0–77.0) | 73.0 (72.0–77.0) | 0.461 |
| LVVED 2Ch—Left ventricular end-diastolic volume [2Ch] (mL) | 89.0 (56.0–107.0) | 85.0 (74.0–90.0) | 0.982 |
| LVVES 2Ch—Left ventricular end-systolic volume [2Ch] (mL) | 32.0 (24.0–48.0) | 35.3 (27.0–40.0) | 0.886 |
| LVEF 2Ch—Left ventricular ejection fraction [2Ch] (%) | 57.0 (54.0–60.0) | 60.2 (57.0–52.0) | 0.088 |
| LVSV 2Ch—Left ventricular stroke volume [2Ch] (mL) | 47.0 (35.0–61.0) | 51.0 (42.0–55.0) | 0.410 |
| LVCO 2Ch—Left ventricular cardiac output [2Ch] (L/min) | 3.4 (2.5–4.3) | 3.5 (3.1–3.8) | 0.676 |
| LVLs 2Ch—Left ventricular length in systole [2Ch] (mm) | 6.3 (5.7–6.9) | 6.4 (5.9–6.7) | 0.582 |
| LVLs 2Ch—Left ventricular length in diastole [2Ch] (mm) | 7.4 (6.6–8.0) | 7.7 (7.1–8.1) | 0.317 |
| LVVED BiP—Left ventricular end-diastolic volume [biplane] (mL) | 92.5 (67.0–106.0) | 82.8 (72.0–97.0) | 0.367 |
| LVVES BiP—Left ventricular end-systolic volume [biplane] (mL) | 41.2 (25.0–48.0) | 36.6 (30.0–39.0) | 0.488 |
| LVEF BiP -Left ventricular ejection fraction [biplane] (%) | 57.0 (53.0–63.0) | 56.5 (55.0–60.0) | 0.644 |
| LVSV BiP—Left ventricular stroke volume [biplane] (mL) | 49.0 (38.0–58.0) | 46.2 (42.0–29.0) | 0.530 |
| LVCO BiP—Left ventricular cardiac output [biplane] (L/min) | 3.7 (3.0–4.0) | 3.2 (3.0–3.4) | 0.052 |
| LVVED 4D—Left ventricular end-diastolic volume [4-dimensional] (mL) | 114.3 (84.0 –125.0) | 103.6 (73.0–122.0) | 0.404 |
| LVVES 4D—Left ventricular end-systolic volume [4-dimensional] (mL) | 55.7 (37.0–69.0) | 46.9 (34.0–54.0) | 0.126 |
| CO 4D—Cardiac output [4-dimensional] (L/min) | 4.0 (3.2–4.5) | 3.7 (2.9–4.6) | 0.344 |
| Spl—Sphericity index | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.455 |
| IVSd—Interventricular septal thickness in diastole (mm) | 10.0 (9.0–11.0) | 9.0 (8.0–10.0) | 0.013 |
| LVIDd—Left ventricular internal end-diastolic diameter (mm) | 51.0 (46.0–54.0) | 51.0 (48.0–55.0) | 0.860 |
| LVPWd—Left ventricular posterior wall thickness in diastole (mm) | 11.0 (10.0–11.0) | 9.0 (8.0–10.0) | 0.004 |
| IVSs—Interventricular septal thickness in systole (mm) | 13.0 (11.0–14.0) | 12.0 (11.0–13.0) | 0.062 |
| LVIDs—Left ventricular internal end-systolic diameter (mm) | 33.0 (26.0–38.0) | 33.0 (31.0–36.0) | 0.461 |
| LVPWs—Left ventricular posterior wall thickness in systole (mm) | 14.0 (13.0–15.0) | 13.0 (12.0–14.0) | 0.028 |
| EDV Teich—Left ventricular end-diastolic volume [Teicholz] (mL) | 127.1 (96.0–140.0) | 127.0 (105.0–147.0) | 0.904 |
| ESV Teich—Left ventricular end-systolic volume [Teicholz] (mL) | 46.9 (24.0–61.0) | 47.0 (38.0–54.0) | 0.538 |
| LVEF Teich—Left ventricular ejection fraction [Teicholz] (%) | 64.6 (59.0–73.0) | 63.1 (61.0–66.0) | 0.553 |
| %FS—Left ventricular fractional shortening (%) | 36.0 (32.0–42.0) | 35.0 (33.0–37.0) | 0.434 |
| LVSV Teich—Left ventricular stroke volume [Teicholz] (mL) | 80.0 (57.0–107.0) | 79.0 (68.0–93.0) | 0.965 |
| LVd Mass—Left ventricular mass (g) | 240.4 (185.1–280.4) | 211.4 (165.1–258.0) | 0.150 |
| LVMi—Left ventricular mass indexed (g/m2) | 122.9 (90.6–153.9) | 103.6 (81.6–155.5) | 0.123 |
| Ao diam—Aortic diameter (mm) | 33.0 (30.0–36.0) | 32.0 (30.0–35.0) | 0.620 |
| LA diam—Left atrial diameter (mm) | 39.0 (35.0–42.0) | 40.0 (37.0–43.0) | 0.628 |
| LA/Ao—Left atrium/aorta ratio | 1.2 (1.1–1.2) | 1.2 (1.1–1.4) | 0.129 |
| Echocardiographic Parameters | Baseline (Hemodialysis Treatment) | 3-Month Follow-Up (Hemodiafiltration Treatment) | p-Value |
|---|---|---|---|
| MV E vel—Mitral valve E wave velocity (m/s) | 0.8 (0.7–1.0) | 0.9 (0.7–1.0) | 0.397 |
| MV DecT—Mitral valve deceleration time (ms) | 291.0 (257.0–356.0) | 306.5 (244.0–346.0) | 0.660 |
| MV Dec Slope—Mitral valve deceleration slope (m/s2) | 2.8 (2.1–3.4) | 3.3 (2.3–3.7) | 0.361 |
| MV A Vel—Mitral valve A wave velocity (m/s) | 1.0 (0.8–1.1) | 1.0 (0.9–1.2) | 0.428 |
| MV E/A Ratio—Mitral valve E/A wave ratio | 0.8 (0.6–1.0) | 0.9 (0.7–1.0) | 0.397 |
| Average septal annular e′ velocity (cm/s) | 9.8 (5.2–11.3) | 9.6 (5.3–10.9) | 0.190 |
| E/e’ Sept—E to e’ wave ratio at septal mitral annulus | 13.4 (11.2–15.7) | 13.7 (11.8–15.6) | 0.982 |
| Average lateral annular e′ velocity (cm/s) | 10.1 (8.5–11.8) | 9.7 (8.4–11.3) | 0.525 |
| E/e’ Lat—E to e’ wave ratio at lateral mitral annulus | 8.9 (7.0–10.4) | 9.3 (8.0–9.6) | 0.613 |
| E/e’ Avg—E/e’ average ratio | 10.8 (8.7–12.6) | 11.1 (9.7–11.3) | 0.939 |
| AV Vmax—Aortic valve maximal velocity (m/s) | 1.5 (1.2–1.8) | 1.6 (1.3–1.7) | 0.403 |
| AV Vmean—Aortic valve mean velocity (m/s) | 1.2 (0.8–1.3) | 1.1 (0.9–1.1) | 0.684 |
| AV maxPG—Aortic valve maximal pressure gradient (mmHg) | 10.6 (6.7–12.6) | 11.2 (7.2–11.9) | 0.921 |
| AV meanPG—Aortic valve mean pressure gradient (mmHg) | 6.4 (3.1–7.0) | 6.2 (4.0–6.4) | 0.809 |
| AV Env. Ti—Aortic valve envelope time (ms) | 313.5 (262.0–352.0) | 310.7 (304.0–343.0) | 0.636 |
| AV VTI—Aortic valve velocity-time integral (cm) | 33.1 (26.5–35.3) | 36.6 (30.0–36.8) | 0.146 |
| PV AccT—Pulmonic valve acceleration time (ms) | 155.0 (135.0–166.0) | 151.0 (138.0–156.0) | 0.684 |
| PV Acc Slope—Pulmonic valve acceleration slope (m/s2) | 5.9 (4.8–6.9) | 6.2 (5.0–6.7) | 0.947 |
| Peak systolic tissue velocity (S’ wave) at the tricuspid annulus (cm/s) | 10.0 (9.3–11.2) | 10.4 (9.1–12.2) | 0.273 |
| Echocardiographic Parameters | Baseline (Hemodialysis Treatment) | 3-Month Follow-Up (Hemodiafiltration Treatment) | p-Value |
|---|---|---|---|
| RVEDV—Right ventricular end-diastolic volume (mL) | 65.0 (53.0–66.0) | 59.9 (47.0–68.0) | 0.361 |
| RVESV—Right ventricular end-systolic volume (mL) | 34.4 (29.0–40.0) | 32.5 (29.0–35.0) | 0.305 |
| 3D RVEF (%)—3D right ventricular ejection fraction (%) | 45.9 (41.8–51.2) | 45.9 (38.2–48.6) | 0.434 |
| RV SV—Right ventricular stroke volume (mL) | 29.9 (24.0–33.0) | 27.7 (21.0–32.0) | 0.494 |
| RV Dd base—Right ventricular diastolic diameter—base (mm) | 37.1 (32.0–40.0) | 35.6 (34.0–37.0) | 0.152 |
| RV Dd mid—Right ventricular diastolic diameter—mid (mm) | 26.7 (23.0–28.0) | 28.9 (27.0–31.0) | 0.042 |
| RVLd—Right ventricular longitudinal diameter (mm) | 63.1 (59.0–67.0) | 60.5 (58.0–61.0) | 0.134 |
| TAPSE—Tricuspid annular plane systolic excursion (mm) | 12.2 (9.0–16.0) | 12.4 (10.0–14.0) | 0.886 |
| RV FAC—Right ventricular fractional area change (%) | 36.6 (31.2–36.6) | 39.5 (31.0–42.4) | 0.182 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domjanović Matetić, J.; Baković Kramarić, D.; Domjanović Škopinić, T.; Jeličić, I.; Borić Škaro, D.; Božić, J.; Matetic, A. Echocardiographic Indices in Patients with End-Stage Renal Disease and Their Association with Hemodialysis-to-Hemodiafiltration Transfer: A Prospective Observational Study. Medicina 2024, 60, 1537. https://doi.org/10.3390/medicina60091537
Domjanović Matetić J, Baković Kramarić D, Domjanović Škopinić T, Jeličić I, Borić Škaro D, Božić J, Matetic A. Echocardiographic Indices in Patients with End-Stage Renal Disease and Their Association with Hemodialysis-to-Hemodiafiltration Transfer: A Prospective Observational Study. Medicina. 2024; 60(9):1537. https://doi.org/10.3390/medicina60091537
Chicago/Turabian StyleDomjanović Matetić, Josipa, Darija Baković Kramarić, Tea Domjanović Škopinić, Ivo Jeličić, Dijana Borić Škaro, Joško Božić, and Andrija Matetic. 2024. "Echocardiographic Indices in Patients with End-Stage Renal Disease and Their Association with Hemodialysis-to-Hemodiafiltration Transfer: A Prospective Observational Study" Medicina 60, no. 9: 1537. https://doi.org/10.3390/medicina60091537
APA StyleDomjanović Matetić, J., Baković Kramarić, D., Domjanović Škopinić, T., Jeličić, I., Borić Škaro, D., Božić, J., & Matetic, A. (2024). Echocardiographic Indices in Patients with End-Stage Renal Disease and Their Association with Hemodialysis-to-Hemodiafiltration Transfer: A Prospective Observational Study. Medicina, 60(9), 1537. https://doi.org/10.3390/medicina60091537









