Development of a Short-Term Embolic Agent Based on Cilastatin for Articular Microvessels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Application of Embolic Material
2.3. Animals Study
2.4. Morphological Analysis
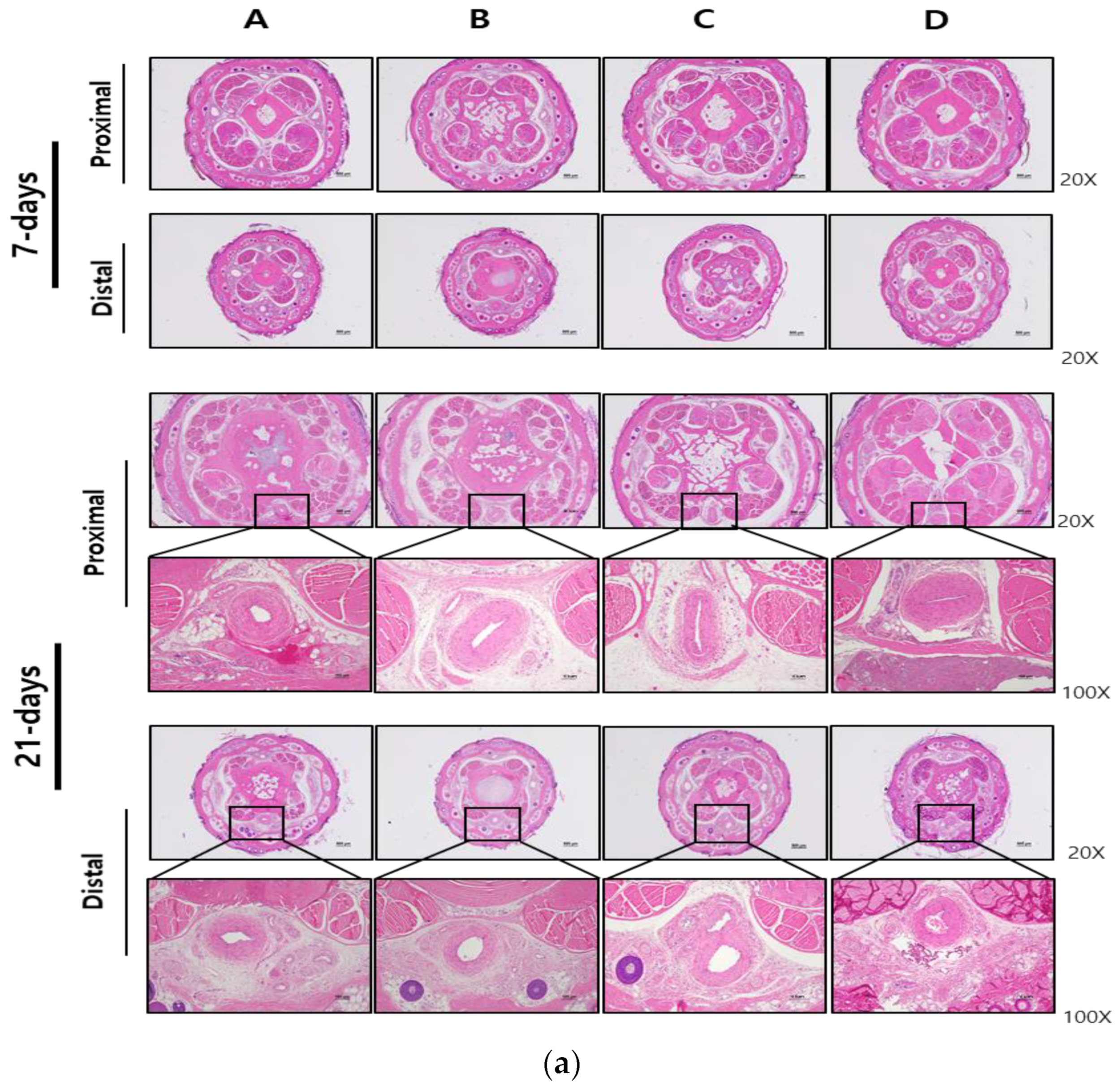
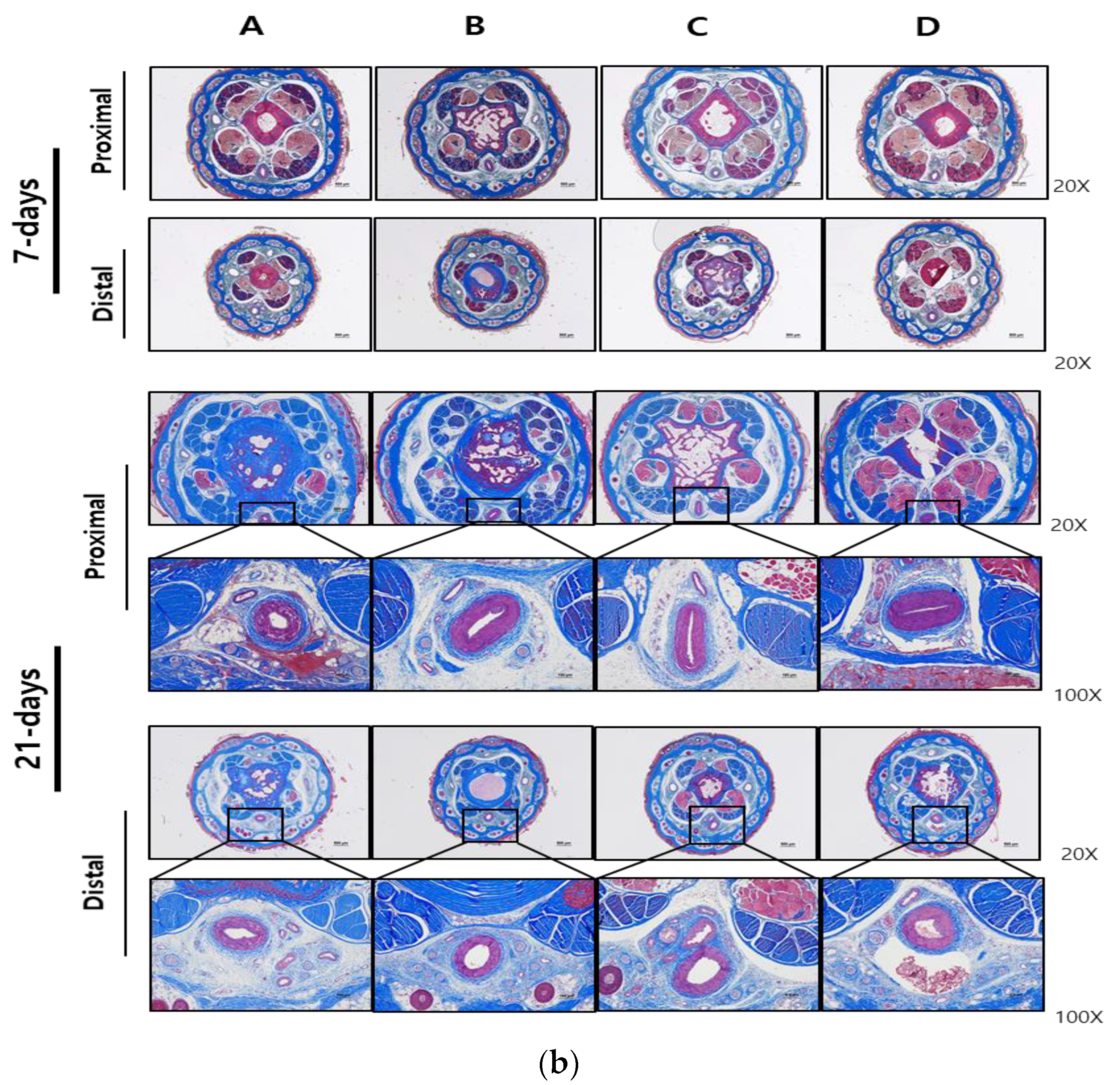
2.5. Histopathologic Analysis
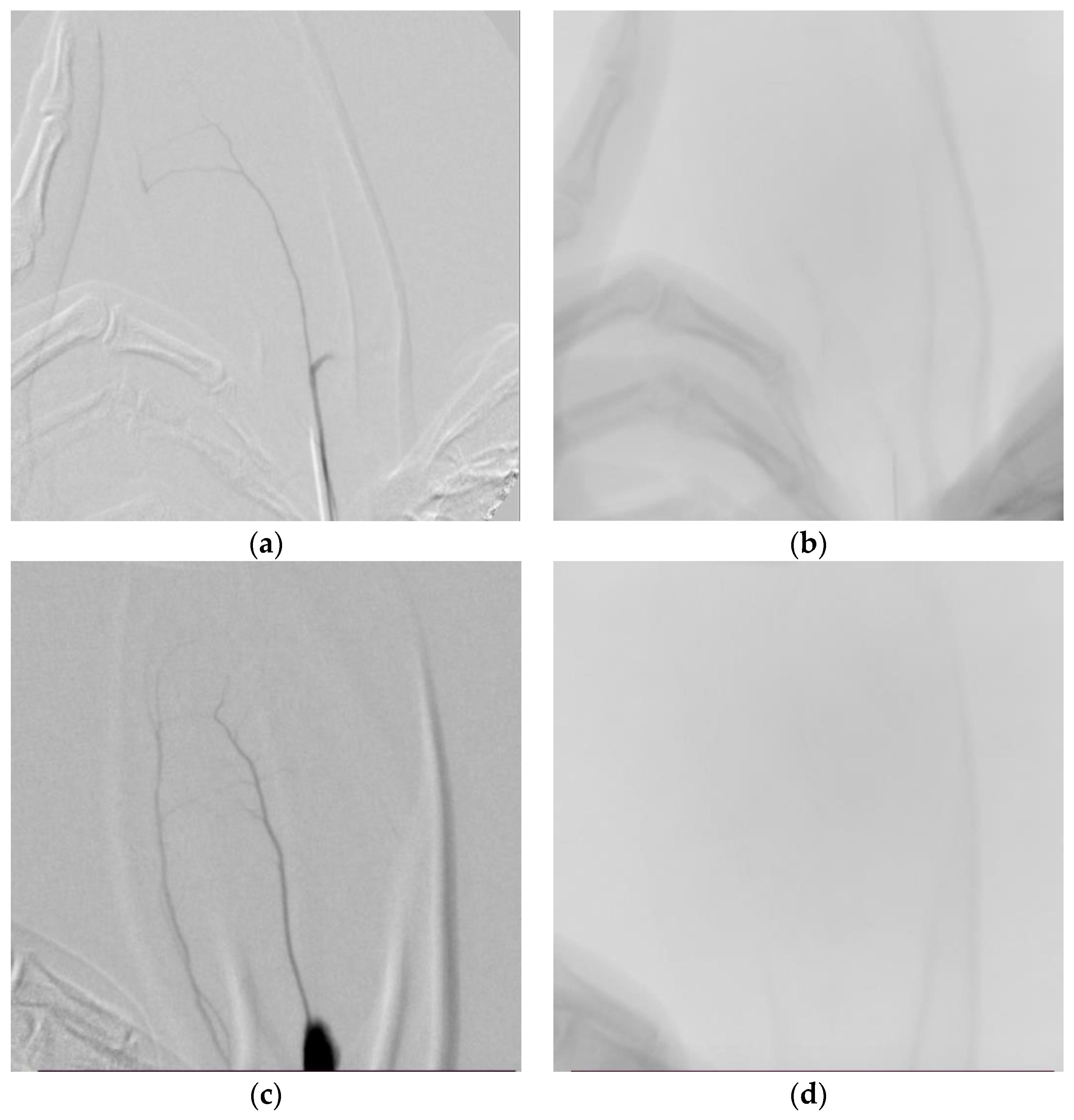
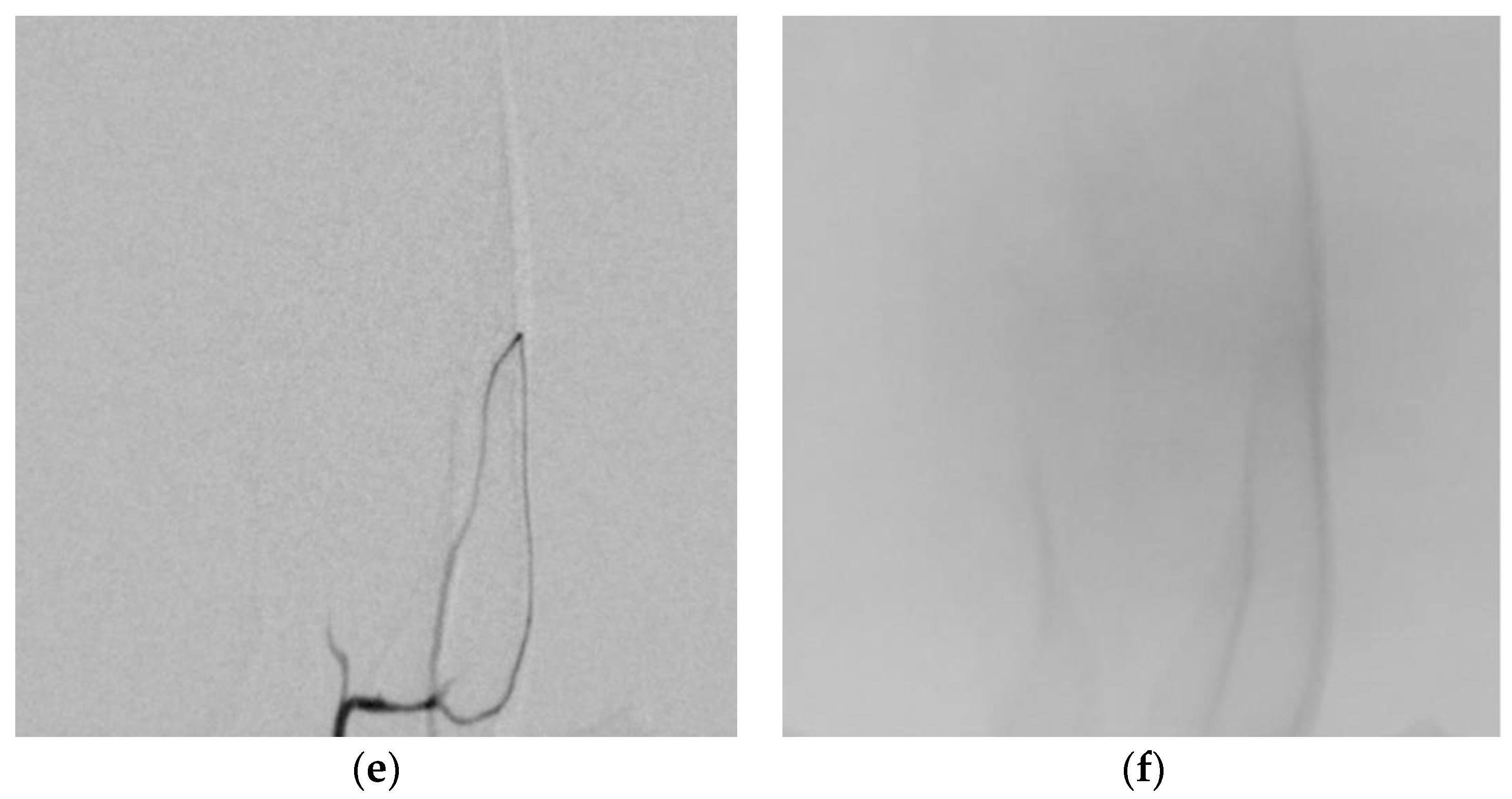
2.6. Angiographic Analysis
3. Results
3.1. Preparation and Comparison of Cilastatin-Based Short-Term Embolization Material
3.2. Local and Systemic Toxicity Evaluation Following Tail Vein Injection of Test Substance
3.3. Angiographic Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mapp, P.I.; Walsh, D.A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.J. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J. Bone Miner. Res. 2016, 31, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.L.; Meng, H.Y.; Wang, Y.C.; Peng, J.; Guo, Q.Y.; Wang, A.Y.; Lu, S.B. Bone–cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014, 22, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.V.; Kwon, S.M. Vascular Endothelial Growth Factor Biology and Its Potential as a Therapeutic Target in Rheumatic Diseases. Int. J. Mol. Sci. 2021, 22, 5387. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.S.; Walsh, D.A. Osteoarthritis, angiogenesis and inflammation. Rheumatology 2004, 44, 7–16. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Liu, S.C.; Su, C.M.; Wang, Y.H.; Tsai, C.H.; Tang, C.H. Implications of Angiogenesis Involvement in Arthritis. Int. J. Mol. Sci. 2018, 19, 2012. [Google Scholar] [CrossRef]
- Henrotin, Y.; Pesesse, L.; Lambert, C. Targeting the synovial angiogenesis as a novel treatment approach to osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2014, 6, 20–34. [Google Scholar] [CrossRef]
- Zhao, T.; Li, X.; Li, H.; Deng, H.; Li, J.; Yang, Z.; He, S.; Jiang, S.; Sui, X.; Guo, Q.; et al. Advancing drug delivery to articular cartilage: From single to multiple strategies. Acta Pharm. Sin. B 2023, 13, 4127–4148. [Google Scholar] [CrossRef]
- Nagai, T.; Sato, M.; Kobayashi, M.; Yokoyama, M.; Tani, Y.; Mochida, J. Bevacizumab, an anti-vascular endothelial growth factor antibody, inhibits osteoarthritis. Arthritis Res. Ther. 2014, 16, 427. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef]
- Ma, K.; Singh, G.; Wang, J.; InSug, O.; Votta-Velis, G.; Bruce, B.; Anbazhagan, A.N.; van Wijnen, A.J.; Im, H.-J. Targeting Vascular Endothelial Growth Factor Receptors as a Therapeutic Strategy for Osteoarthritis and Associated Pain. Int. J. Biol. Sci. 2023, 19, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.A.; Chen, S.; Pei, M. The essential anti-angiogenic strategies in cartilage engineering and osteoarthritic cartilage repair. Cell Mol. Life Sci. 2022, 79, 71. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; Russo, F.; Musumeci, M.; Giacalone, A.; Papalia, R.; Denaro, V. Targeting VEGF-A in cartilage repair and regeneration: State of the art and perspectives. J. Biol. Regul. Homeost. Agents 2018, 32 (Suppl. S1), 217–224. [Google Scholar] [PubMed]
- Wang, L.; Liu, W.Q.; Broussy, S.; Han, B.; Fang, H. Recent advances of anti-angiogenic inhibitors targeting VEGF/VEGFR axis. Front. Pharmacol. 2023, 14, 1307860. [Google Scholar] [CrossRef]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef]
- Sotozawa, M.; Kumagai, K.; Ishikawa, K.; Yamada, S.; Inoue, Y.; Inaba, Y. Bevacizumab suppressed degenerative changes in articular cartilage explants from patients with osteoarthritis of the knee. J. Orthop. Surg. Res. 2023, 18, 25. [Google Scholar] [CrossRef]
- Yamada, K.; Jahangiri, Y.; Li, J.; Gabr, A.; Anoushiravani, A.; Kumagai, K.; Uchida, B.; Farsad, K.; Horikawa, M. Embolic Characteristics of Imipenem-Cilastatin Particles in Vitro and in Vivo: Implications for Transarterial Embolization in Joint Arthropathies. J. Vasc. Interv. Radiol. 2021, 32, 1031–1039. [Google Scholar] [CrossRef]
- Wang, B.; Liang, K.W.; Chen, C.H.; Wang, C.K. Transcatheter Arterial Embolization for Alleviating Chronic Musculoskeletal Pain and Improving Physical Function: A Narrative Review. Diagnostics 2022, 13, 134. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, G.W. The efficacy of transcatheter arterial embolization for knee pain on patients with knee osteoarthritis: A case series. J. Back. Musculoskelet. Rehabil. 2022, 35, 743–748. [Google Scholar] [CrossRef]
- Okuno, Y.; Korchi, A.M.; Shinjo, T.; Kato, S. Transcatheter arterial embolization as a treatment for medial knee pain in patients with mild to moderate osteoarthritis. Cardiovasc. Interv. Radiol. 2015, 38, 336–343. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Piacentino, F.; Pesapane, F.; Carnevale, A.; Curti, M.; Fontana, F.; Venturini, M.; Pinto, A.; Gentili, F.; Guerrini, S.; et al. Basic embolization techniques: Tips and tricks. Acta Biomed. 2020, 91, 71–80. [Google Scholar] [PubMed]
- Goode, J.A.; Matson, M.B. Embolisation of cancer: What is the evidence? Cancer Imaging 2004, 4, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.; Tozer, K.R.; Chen, J. An overview of embolic agents. Semin. Interv. Radiol. 2008, 25, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Medsinge, A.; Zajko, A.; Orons, P.; Amesur, N.; Santos, E. A case-based approach to common embolization agents used in vascular interventional radiology. AJR Am. J. Roentgenol. 2014, 203, 699–708. [Google Scholar] [CrossRef]
- Hu, J.; Albadawi, H.; Chong, B.W.; Deipolyi, A.R.; Sheth, R.A.; Khademhosseini, A.; Oklu, R. Advances in Biomaterials and Technologies for Vascular Embolization. Adv. Mater. 2019, 31, 1901071. [Google Scholar] [CrossRef]
- Thakral, S.; Sonje, J.; Munjal, B.; Bhatnagar, B.; Suryanarayanan, R. Mannitol as an Excipient for Lyophilized Injectable Formulations. J. Pharm. Sci. 2023, 112, 19–35. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Hu, A.; Nie, T.; Cheng, Z.; Liu, W. A Critical Review on Engineering of d-Mannitol Crystals: Properties, Applications, and Polymorphic Control. Crystals 2022, 12, 1080. [Google Scholar] [CrossRef]
- Altay Benetti, A.; Bianchera, A.; Buttini, F.; Bertocchi, L.; Bettini, R. Mannitol Polymorphs as Carrier in DPIs Formulations: Isolation Characterization and Performance. Pharmaceutics 2021, 13, 113. [Google Scholar] [CrossRef]
- Su, W.; Jia, N.; Li, H.; Hao, H.; Li, C. Polymorphism of D-mannitol: Crystal structure and the crystal growth mechanism. Chin. J. Chem. Eng. 2017, 25, 358–362. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Lin, X.; Yang, H.; Li, M.; Lei, L.; Huang, W. Precipitation behavior of δ phase and its effect on stress rupture properties of selective laser-melted Inconel 718 superalloy. Compos. B Eng. 2021, 224, 109202. [Google Scholar] [CrossRef]
- Penha, F.M.; Gopalan, A.; Meijlink, J.C.; Ibis, F.; Eral, H.B. Selective Crystallization of d-Mannitol Polymorphs Using Surfactant Self-Assembly. Cryst. Growth Des. 2021, 21, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Cares-Pacheco, M.G.; Vaca-Medina, G.; Calvet, R.; Espitalier, F.; Letourneau, J.J.; Rouilly, A.; Rodier, E. Physicochemical characterization of D-mannitol polymorphs: The challenging surface energy determination by inverse gas chromatography in the infinite dilution region. Int. J. Pharm. 2014, 475, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Köller, M.; Brom, J.; Raulf, M.; König, W. Cilastatin (MK 0791) is a potent and specific inhibitor of the renal leukotriene D4-dipeptidase. Biochem. Biophys. Res. Commun. 1985, 131, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.A.; Allegretto, N.J.; Hitchcock, M.J. Cilastatin-sensitive dehydropeptidase I enzymes from three sources all catalyze carbapenem hydrolysis and conversion of leukotriene D4 to leukotriene E4. Arch. Biochem. Biophys. 1987, 256, 253–259. [Google Scholar] [CrossRef]
- Rogers, J.D.; Meisinger, M.A.; Ferber, F.; Calandra, G.B.; Demetriades, J.L.; Bland, J.A. Pharmacokinetics of imipenem and cilastatin in volunteers. Rev. Infect. Dis. 1985, 7, S435–S446. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Meng, Q.; Wang, C.; Zhu, Y.; Liu, Z.; Ma, X.; Peng, J.; Sun, H.; Liu, K. Cilastatin protects against imipenem-induced nephrotoxicity via inhibition of renal organic anion transporters (OATs). Acta Pharm. Sin. B 2019, 9, 986–996. [Google Scholar] [CrossRef]
- Buckley, M.M.; Brogden, R.N.; Barradell, L.B.; Goa, K.L. Imipenem/cilastatin. A reappraisal of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1992, 44, 408–444. [Google Scholar] [CrossRef]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef]
- Sahra, S.; Jahangir, A.; Hamadi, R.; Jahangir, A.; Glaser, A. Clinical and Microbiologic Efficacy and Safety of Imipenem/Cilastatin/Relebactam in Complicated Infections: A Meta-analysis. Infect. Chemother. 2021, 53, 271–283. [Google Scholar] [CrossRef]
- Shin, C.I.; Kim, H.C.; Song, Y.S.; Cho, H.R.; Lee, K.B.; Lee, W.; Jae, H.J.; Chung, J.W. Rat model of hindlimb ischemia induced via embolization with polyvinyl alcohol and N-butyl cyanoacrylate. Korean J. Radiol. 2013, 14, 923–930. [Google Scholar] [CrossRef]
- Wang, C.Y.; Hu, J.; Sheth, R.A.; Oklu, R. Emerging embolic agents in endovascular embolization: An overview. Prog. Biomed. Eng. 2020, 2, 012003. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Xie, H.; Wang, G.; An, Y. Risk comparison of filler embolism between polymethyl methacrylate (PMMA) and hyaluronic acid (HA). Aesthetic Plast. Surg. 2019, 43, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, M.; Sendo, T.; Kataoka, Y.; Imafuku, S.; Futagami, K.; Oishi, R. Particulate matter analyses after dissolution of powder antibiotic injections used in the pediatric ward. Jpn. J. Hosp. Pharm. 1998, 24, 389–392. [Google Scholar] [CrossRef]
- Lee, K.S.; Jeong, E.S.; Heo, S.H.; Seo, J.H.; Lee, M.S.; Eom, K.D.; Choi, Y.K. Re-Examination of Blood Vessels of Rat Tail Using Angiographic and Histological Methods. J. Vet. Clin. 2010, 27, 159–162. [Google Scholar]
- Ghaly, E.N.; Gergis, S.W.; Aziz, J.N.; Yassa, H.D.; Hassan, H.A.R. Role of mesenchymal stem cell therapy in cisplatin induced nephrotoxicity in adult albino rats: Ultrastructural & biochemical study. Acta Med. Int. 2014, 1, 57–66. [Google Scholar]

| Component | Concentration (% w/v) |
|---|---|
| Cilastatin sodium salt | 2.5 |
| D-Mannitol | 23 |
| Test Group (n = Number of Subjects) | Day 0 (n = 6) | Day 3 (n = 6) | Day 7 (n = 3) | Day 15 (n = 3) | Day 21 (n = 3) |
|---|---|---|---|---|---|
| A. Cilastatin + D-Mannitol Mixture | 0 | 0 | 0 (0,0,0) 1 | 0 | 0 (0,0,0) 1 |
| B. Iohexol | 0 | 0 | 0 (0,0,0) 1 | 0 | 0 (0,0,0) 1 |
| C. Prepenem | 0 | 0 | 0 (0,0,0) 1 | 0 | 0 (0,0,0) 1 |
| D. EGgel | 0 | 0 | 0 (0,0,0) 1 | 1 | 3 (1,3,3) 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Jeon, A.; Kang, E.K.; An, W.; Lim, S.J.; Shin, K.C.; Shin, D.H.; Hwang, I.; Kang, J.S. Development of a Short-Term Embolic Agent Based on Cilastatin for Articular Microvessels. Medicina 2024, 60, 1538. https://doi.org/10.3390/medicina60091538
Kim HJ, Jeon A, Kang EK, An W, Lim SJ, Shin KC, Shin DH, Hwang I, Kang JS. Development of a Short-Term Embolic Agent Based on Cilastatin for Articular Microvessels. Medicina. 2024; 60(9):1538. https://doi.org/10.3390/medicina60091538
Chicago/Turabian StyleKim, Hyun Jin, Areum Jeon, Eun Kyung Kang, Wen An, So Jung Lim, Kyu Chul Shin, Dong Hun Shin, Inyoung Hwang, and Ju Seop Kang. 2024. "Development of a Short-Term Embolic Agent Based on Cilastatin for Articular Microvessels" Medicina 60, no. 9: 1538. https://doi.org/10.3390/medicina60091538
APA StyleKim, H. J., Jeon, A., Kang, E. K., An, W., Lim, S. J., Shin, K. C., Shin, D. H., Hwang, I., & Kang, J. S. (2024). Development of a Short-Term Embolic Agent Based on Cilastatin for Articular Microvessels. Medicina, 60(9), 1538. https://doi.org/10.3390/medicina60091538







