Effects of Insulin-like Growth Factor 1 on the Maintenance of Cell Viability and Osteogenic Differentiation of Gingiva-Derived Mesenchymal Stem Cell Spheroids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Spheroids of Gingiva-Derived Mesenchymal Stem Cells
2.2. Assessment of Cellular Viability
2.3. Levels of Alkaline Phosphatase Activity and Calcium Deposition
2.4. Total RNA Extraction and Real-Time Polymerase Chain Reaction Quantification of RUNX2 and COL1A1
- RUNX2: Forward 5′-AAT GAT GGT GTT GAC GCT GA-3′; Reverse 5′-TTG ATA CGT GTG GGA TGT GG-3′
- COL1A1: Forward 5′-CCAGAAGAACTGGTACATCAGCAA-3′; Reverse 5′-TGGTTTCTTCTCCTCTGCGC-3′
- β-actin: Forward 5′-TGGCACCCAGCACAATGAA-3′; Reverse 5′-CTAAGTCATAGTCCGCCTAGAAGC-3′
2.5. Statistical Analysis
3. Results
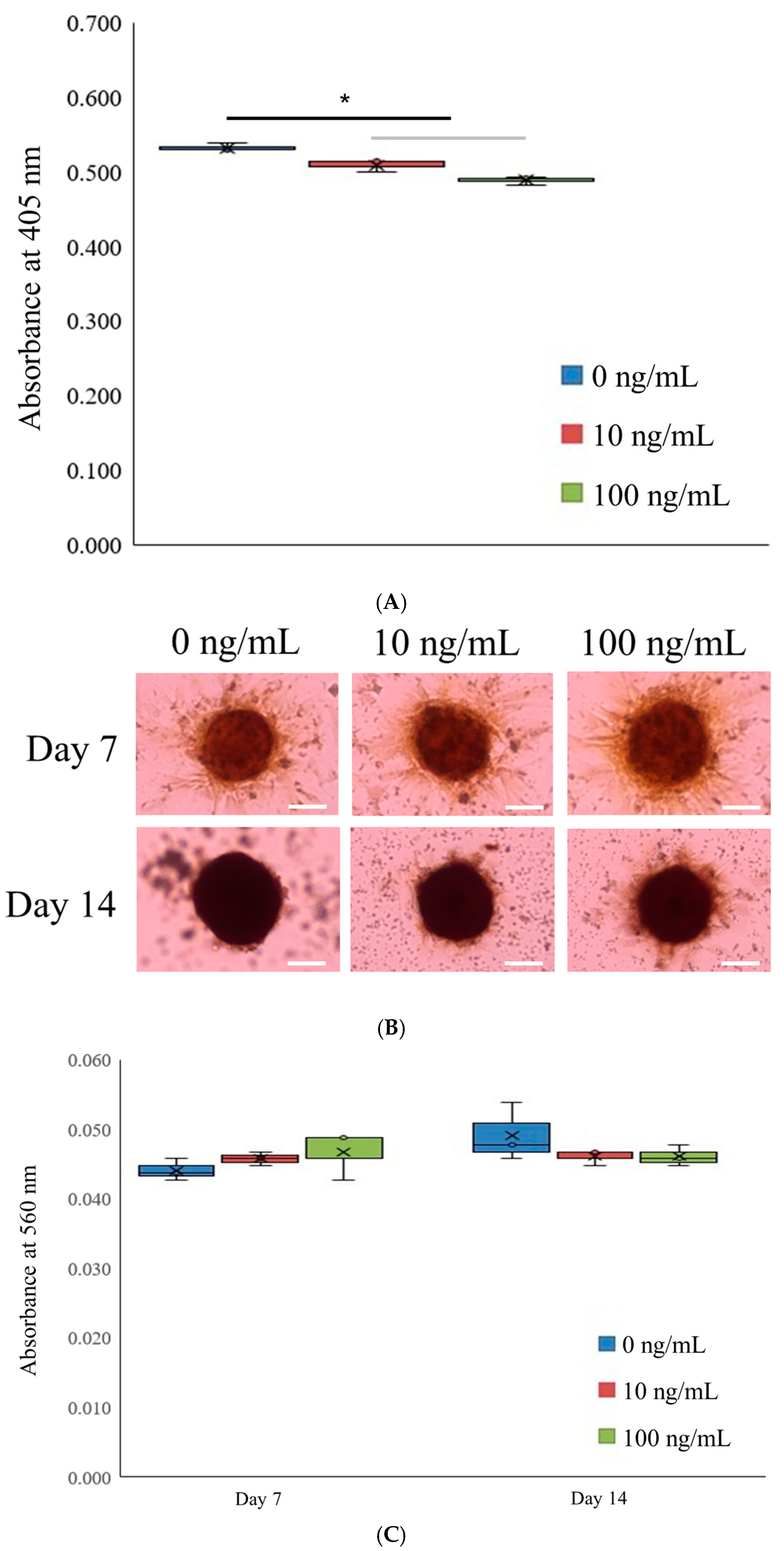
3.1. Generation of Spheroid-Shaped Stem Cell Aggregates
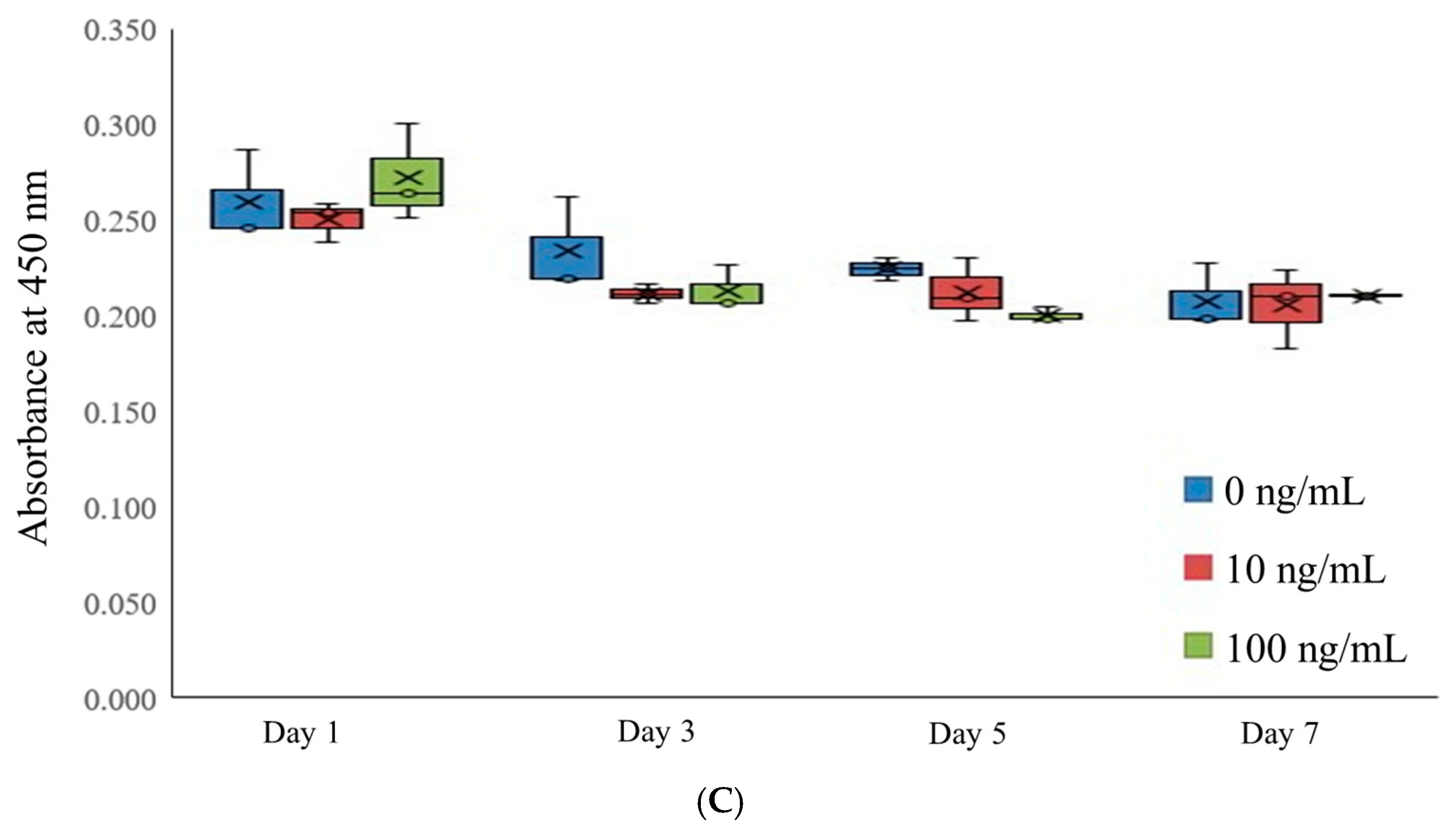
3.2. Assessment of Cell Viability
3.3. Alkaline Phosphatase Activity Levels and Extent of Calcium Deposition
3.4. Quantitative Real-Time Polymerase Chain Reactions of RUNX2 and COL1A1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Zhu, Q.; Cao, D.; Peng, Q.; Zhang, X.; Li, C.; Zhang, C.; Zhou, B.O.; Yue, R. Bone marrow-derived IGF-1 orchestrates maintenance and regeneration of the adult skeleton. Proc. Natl. Acad. Sci. USA 2023, 120, e2203779120. [Google Scholar] [CrossRef]
- Ashour, S.H.; Mudalal, M.; Al-Aroomi, O.A.; Al-Attab, R.; Li, W.; Yin, L. The Effects of Injectable Platelet-Rich Fibrin and Advanced-Platelet Rich Fibrin on Gingival Fibroblast Cell Vitality, Proliferation, Differentiation. Tissue Eng. Regen. Med. 2023, 20, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xing, H.; Qi, F.; Liu, H.; Gao, L.; Wang, X. Local delivery of insulin/IGF-1 for bone regeneration: Carriers, strategies, and effects. Nanotheranostics 2020, 4, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Brill, C.; Blum, W.F.; Brenner, R.E. IGF-I and IGF-II stimulate directed cell migration of bone-marrow-derived human mesenchymal progenitor cells. Biochem. Biophys. Res. Commun. 2006, 345, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Molagoda, I.M.N.; Jayasingha, J.; Choi, Y.H.; Park, E.K.; Jeon, Y.J.; Lee, B.J.; Kim, G.Y. Fermented Oyster Extract Promotes Insulin-Like Growth Factor-1-Mediated Osteogenesis and Growth Rate. Mar. Drugs 2020, 18, 472. [Google Scholar] [CrossRef] [PubMed]
- Luginbuehl, V.; Zoidis, E.; Meinel, L.; von Rechenberg, B.; Gander, B.; Merkle, H.P. Impact of IGF-I release kinetics on bone healing: A preliminary study in sheep. Eur. J. Pharm. Biopharm. 2013, 85, 99–106. [Google Scholar] [CrossRef]
- Cao, W.; Helder, M.N.; Bravenboer, N.; Wu, G.; Jin, J.; Ten Bruggenkate, C.M.; Klein-Nulend, J.; Schulten, E. Is There a Governing Role of Osteocytes in Bone Tissue Regeneration? Curr. Osteoporos. Rep. 2020, 18, 541–550. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, J.; Xia, Y.; Li, Y.; Fu, C. Application of platelet-rich plasma in spinal surgery. Front. Endocrinol. 2023, 14, 1138255. [Google Scholar] [CrossRef]
- Sheng, M.H.; Lau, K.H.; Baylink, D.J. Role of Osteocyte-derived Insulin-Like Growth Factor I in Developmental Growth, Modeling, Remodeling, and Regeneration of the Bone. J. Bone Metab. 2014, 21, 41–54. [Google Scholar] [CrossRef]
- Park, J.; Yan, G.; Kwon, K.C.; Liu, M.; Gonnella, P.A.; Yang, S.; Daniell, H. Oral delivery of novel human IGF-1 bioencapsulated in lettuce cells promotes musculoskeletal cell proliferation, differentiation and diabetic fracture healing. Biomaterials 2020, 233, 119591. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Doll, J.; Tanner, M.; Bruckner, T.; Zimmermann, G.; Helbig, L.; Biglari, B.; Schmidmaier, G.; Moghaddam, A. Quantification of TGF-ß1, PDGF and IGF-1 cytokine expression after fracture treatment vs. non-union therapy via masquelet. Injury 2016, 47, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Sundararaj, S.K.; Cieply, R.D.; Gupta, G.; Milbrandt, T.A.; Puleo, D.A. Treatment of growth plate injury using IGF-I-loaded PLGA scaffolds. J. Tissue Eng. Regen. Med. 2015, 9, E202–E209. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, H.; Okada, S.; Saito, A.; Hoshi, K.; Yamashita, H.; Takato, T.; Azuma, T. Inhibition of insulin-like growth factor-1 (IGF-1) expression by prolonged transforming growth factor-β1 (TGF-β1) administration suppresses osteoblast differentiation. J. Biol. Chem. 2012, 287, 22654–22661. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Kim, I.; Lee, W.; Kim, H. Evaluation of the regenerative capacity of stem cells combined with bone graft material and collagen matrix using a rabbit calvarial defect model. J. Periodontal Implant. Sci. 2023, 53, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.K.; Jou, E.; Lu, V.; Zhang, J.; Chabra, S.; Abishek, J.; Wong, E.; Zeng, X.; Guo, B. Using Pre-Clinical Studies to Explore the Potential Clinical Uses of Exosomes Secreted from Induced Pluripotent Stem Cell-Derived Mesenchymal Stem cells. Tissue Eng. Regen. Med. 2023, 20, 793–809. [Google Scholar] [CrossRef]
- Abuarqoub, D.; Theeb, L.S.; Omari, M.B.; Hamadneh, Y.I.; Alrawabdeh, J.A.; Aslam, N.; Jafar, H.; Awidi, A. The Osteogenic Role of Biomaterials Combined with Human-Derived Dental Stem Cells in Bone Tissue Regeneration. Tissue Eng. Regen. Med. 2023, 20, 251–270. [Google Scholar] [CrossRef]
- Suhandi, C.; Mohammed, A.F.A.; Wilar, G.; El-Rayyes, A.; Wathoni, N. Effectiveness of Mesenchymal Stem Cell Secretome on Wound Healing: A Systematic Review and Meta-analysis. Tissue Eng. Regen. Med. 2023, 20, 1053–1062. [Google Scholar] [CrossRef]
- Kang, Y.; Na, J.; Karima, G.; Amirthalingam, S.; Hwang, N.S.; Kim, H.D. Mesenchymal Stem Cell Spheroids: A Promising Tool for Vascularized Tissue Regeneration. Tissue Eng. Regen. Med. 2024, 21, 673–693. [Google Scholar] [CrossRef] [PubMed]
- Septiana, W.L.; Pawitan, J.A. Potential Use of Organoids in Regenerative Medicine. Tissue Eng. Regen. Med. 2024, 21, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Kim, J.A.; Lim, S.; Lee, S.J.; Kim, C.H.; Bae, J.S.; Boo, Y.C.; Kim, Y.J.; Park, E.K. Glycinamide Facilitates Nanocomplex Formation and Functions Synergistically with Bone Morphogenetic Protein 2 to Promote Osteoblast Differentiation In Vitro and Bone Regeneration in a Mouse Calvarial Defect Model. Tissue Eng. Regen. Med. 2024, 21, 1093–1107. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, K.; Liao, C.; Han, T.; Jiang, F.; Gao, Z.; Yan, J. Exosomes-Shuttled lncRNA SNHG7 by Bone Marrow Mesenchymal Stem Cells Alleviates Osteoarthritis Through Targeting miR-485-5p/FSP1 Axis-Mediated Chondrocytes Ferroptosis and Inflammation. Tissue Eng. Regen. Med. 2024, 21, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Dobson, L.K.; Zeitouni, S.; McNeill, E.P.; Bearden, R.N.; Gregory, C.A.; Saunders, W.B. Canine Mesenchymal Stromal Cell-Mediated Bone Regeneration is Enhanced in the Presence of Sub-Therapeutic Concentrations of BMP-2 in a Murine Calvarial Defect Model. Front. Bioeng. Biotechnol. 2021, 9, 764703. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; López-García, S.; Osorio, R.; Toledano, M.; García-Bernal, D.; Sánchez-Bautista, S.; Rodríguez-Lozano, F.J. Dexamethasone and Doxycycline Doped Nanoparticles Increase the Differentiation Potential of Human Bone Marrow Stem Cells. Pharmaceutics 2022, 14, 1865. [Google Scholar] [CrossRef]
- Reible, B.; Schmidmaier, G.; Moghaddam, A.; Westhauser, F. Insulin-Like Growth Factor-1 as a Possible Alternative to Bone Morphogenetic Protein-7 to Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells in Vitro. Int. J. Mol. Sci. 2018, 19, 1674. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Park, J.B.; Kim, I.; Lee, W.; Kim, H. Assessment of stem cell viability in the initial healing period in rabbits with a cranial bone defect according to the type and form of scaffold. J. Periodontal Implant. Sci. 2019, 49, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.B.; Tae, J.Y.; Ko, Y.; Park, J.B. Lovastatin increases the proliferation and osteoblastic differentiation of human gingiva-derived stem cells in three-dimensional cultures. Exp. Ther. Med. 2019, 18, 3425–3430. [Google Scholar] [CrossRef]
- Xu, A.L.; Han, L.; Yan, J.; Liu, D.; Wang, W. Effects of Mesenchymal Stem Cells-Derived Extracellular Vesicles on Inhibition of Hepatic Fibrosis by Delivering miR-200a. Tissue Eng. Regen. Med. 2024, 21, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Son, J.; Min, S.K.; Na, C.B.; Yi, G.; Koo, H.; Park, J.B. A Study of the Effects of Doxorubicin-Containing Liposomes on Osteogenesis of 3D Stem Cell Spheroids Derived from Gingiva. Materials 2019, 12, 2693. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, J.B. Dimethyl Sulfoxide Leads to Decreased Osteogenic Differentiation of Stem Cells Derived from Gingiva via Runx2 and Collagen I Expression. Eur. J. Dent. 2019, 13, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, H.; Na, C.B.; Park, J.B. The effects of simvastatin on cellular viability, stemness and osteogenic differentiation using 3-dimensional cultures of stem cells and osteoblast-like cells. Adv. Clin. Exp. Med. 2019, 28, 699–706. [Google Scholar] [CrossRef]
- Tang, C.; Li, X.; Wang, F.; Cui, X.; Zhu, Y. Effect of local application of insulin like growth factor-1 gelatin sponge complex on osseointegration around implant in osteoporosis rats. Zhonghua kou qiang yi xue za zhi = Zhonghua kouqiang yixue zazhi = Chin. J. Stomatol. 2015, 50, 418–422. [Google Scholar]
- Vahabzadeh, S.; Bandyopadhyay, A.; Bose, S.; Mandal, R.; Nandi, S.K. IGF-loaded silicon and zinc doped brushite cement: Physico-mechanical characterization and in vivo osteogenesis evaluation. Integr. Biol. 2015, 7, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- López-Quiles, J.; Forteza-López, A.; Montiel, M.; Clemente, C.; Fernández-Tresguerres, J.A.; Fernández-Tresguerres, I. Effects of locally applied Insulin-like Growth Factor-I on osseointegration. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e652–e658. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Milbrandt, T.A.; Hilt, J.Z.; Puleo, D.A. Retention of insulin-like growth factor I bioactivity during the fabrication of sintered polymeric scaffolds. Biomed. Mater. 2014, 9, 025015. [Google Scholar] [CrossRef]
- Lu, L.; Wang, H.; Yang, M.; Wang, L.; Gan, K. Three-dimensional-printed MPBI@β-TCP scaffold promotes bone regeneration and impedes osteosarcoma under near-infrared laser irradiation. FASEB J. 2023, 37, e22924. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Feng, J.; Feng, J.; Huang, X.; Li, L.; Shi, Q. Combined delivery of bone morphogenetic protein-2 and insulin-like growth factor-1 from nano-poly (γ-glutamic acid)/β-tricalcium phosphate-based calcium phosphate cement and its effect on bone regeneration in vitro. J. Biomater. Appl. 2017, 32, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Dixit, J. Clinical Evaluation of Insulin like Growth Factor-I and Vascular Endothelial Growth Factor with Alloplastic Bone Graft Material in the Management of Human Two Wall Intra-Osseous Defects. J. Clin. Diagn. Res. JCDR 2016, 10, ZC41–ZC46. [Google Scholar] [CrossRef]
- Poudel, S.B.; Bhattarai, G.; Kook, S.H.; Shin, Y.J.; Kwon, T.H.; Lee, S.Y.; Lee, J.C. Recombinant human IGF-1 produced by transgenic plant cell suspension culture enhances new bone formation in calvarial defects. Growth Horm. IGF Res. 2017, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gugjoo, M.B.; Amarpal; Abdelbaset-Ismail, A.; Aithal, H.P.; Kinjavdekar, P.; Pawde, A.M.; Kumar, G.S.; Sharma, G.T. Mesenchymal stem cells with IGF-1 and TGF- β1 in laminin gel for osteochondral defects in rabbits. Biomed. Pharmacother. = Biomed. Pharmacotherapie 2017, 93, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Asparuhova, M.B.; Riedwyl, D.; Aizawa, R.; Raabe, C.; Couso-Queiruga, E.; Chappuis, V. Local Concentrations of TGF-β1 and IGF-1 Appear Determinant in Regulating Bone Regeneration in Human Postextraction Tooth Sockets. Int. J. Mol. Sci. 2023, 24, 8239. [Google Scholar] [CrossRef]
- Park, Y.; Lin, S.; Bai, Y.; Moeinzadeh, S.; Kim, S.; Huang, J.; Lee, U.; Huang, N.F.; Yang, Y.P. Dual Delivery of BMP2 and IGF1 Through Injectable Hydrogel Promotes Cranial Bone Defect Healing. Tissue Eng. Part A 2022, 28, 760–769. [Google Scholar] [CrossRef]
- Puig-Herreros, C.; Sanz, J.L.; García-Bernal, D.; Rodríguez-Lozano, F.J.; Murcia, L.; Forner, L.; Ghilotti, J.; Oñate-Sánchez, R.E.; López-García, S. Comparative Cytotoxicity of Menthol and Eucalyptol: An In Vitro Study on Human Gingival Fibroblasts. Pharmaceutics 2024, 16, 521. [Google Scholar] [CrossRef] [PubMed]
- Shindo, S.; Savitri, I.J.; Ishii, T.; Ikeda, A.; Pierrelus, R.; Heidari, A.; Okubo, K.; Nakamura, S.; Kandalam, U.; Rawas-Qalaji, M.; et al. Dual-Function Semaphorin 4D Released by Platelets: Suppression of Osteoblastogenesis and Promotion of Osteoclastogenesis. Int. J. Mol. Sci. 2022, 23, 2938. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, Q.; Chen, C.; Li, J.; Zhang, J.; Qu, S.; Tang, H.; Zeng, H.; Zhang, Y. CD301b(+) macrophage: The new booster for activating bone regeneration in periodontitis treatment. Int. J. Oral Sci. 2023, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Wang, Y. Insulin like growth factor-I: A critical mediator of the skeletal response to parathyroid hormone. Curr. Mol. Pharmacol. 2012, 5, 135–142. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, D.; Wang, M.; Zhang, D.; Xu, Y. Three-Dimensional Printed Titanium Scaffolds Enhance Osteogenic Differentiation and New Bone Formation by Cultured Adipose Tissue-Derived Stem Cells Through the IGF-1R/AKT/Mammalian Target of Rapamycin Complex 1 (mTORC1) Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 8043–8054. [Google Scholar] [CrossRef]
- Li, G.; Wang, Q.; Liu, H.; Yang, Z.; Wu, Y.; He, L.; Deng, X. Fabricating Composite Cell Sheets for Wound Healing: Cell Sheets Based on the Communication Between BMSCs and HFSCs Facilitate Full-Thickness Cutaneous Wound Healing. Tissue Eng. Regen. Med. 2024, 21, 421–435. [Google Scholar] [CrossRef]
- Kizildağ, A.; Çiçek, Y.; Arabaci, T.; Köse, O. The effect of leukocyte-platelet-rich fibrin on bone morphogenetic protein-2 and insulin-like growth factor-1 levels in patients with chronic periodontitis: A randomized split mouth clinical trail. Growth Factors 2018, 36, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Meng, Z. Insulin growth factor-1 promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells through the Wnt/β-catenin pathway. Exp. Ther. Med. 2021, 22, 891. [Google Scholar] [CrossRef]
- Komori, T. Roles of Runx2 in Skeletal Development. Adv. Exp. Med. Biol. 2017, 962, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Devos, H.; Zoidakis, J.; Roubelakis, M.G.; Latosinska, A.; Vlahou, A. Reviewing the Regulators of COL1A1. Int. J. Mol. Sci. 2023, 24, 10004. [Google Scholar] [CrossRef]
- Duruel, T.; Çakmak, A.S.; Akman, A.; Nohutcu, R.M.; Gümüşderelioğlu, M. Sequential IGF-1 and BMP-6 releasing chitosan/alginate/PLGA hybrid scaffolds for periodontal regeneration. Int. J. Biol. Macromol. 2017, 104, 232–241. [Google Scholar] [CrossRef]
- Yin, L.; Yang, S.; He, M.; Chang, Y.; Wang, K.; Zhu, Y.; Liu, Y.; Chang, Y.; Yu, Z. Physicochemical and biological characteristics of BMP-2/IGF-1-loaded three-dimensional coaxial electrospun fibrous membranes for bone defect repair. J. Mater. Sci. Mater. Med. 2017, 28, 94. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, E.; Guerriero, M.; Coli, A.; Di Giannuario, A.; Minniti, G.; Polimeni, A. Effect of PDGF, IGF-1 and PRP on the implant osseointegration. An histological and immunohistochemical study in rabbits. Ann. Stomatol. 2014, 5, 66–68. [Google Scholar] [CrossRef]
- Meesuk, L.; Suwanprateeb, J.; Thammarakcharoen, F.; Tantrawatpan, C.; Kheolamai, P.; Palang, I.; Tantikanlayaporn, D.; Manochantr, S. Osteogenic differentiation and proliferation potentials of human bone marrow and umbilical cord-derived mesenchymal stem cells on the 3D-printed hydroxyapatite scaffolds. Sci. Rep. 2022, 12, 19509. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, H.; Wang, Z.; Shi, J.; Li, J.; Wang, L.; Liao, L.; Ma, S.; Zhang, Y.; Liu, B.; et al. Define of Optimal Addition Period of Osteogenic Peptide to Accelerate the Osteogenic Differentiation of Human Pluripotent Stem Cells. Tissue Eng. Regen. Med. 2024, 21, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Xia, P.; Pan, S.; Zheng, S.; Fu, C.; Chang, Y.; Ma, Y.; Wang, J.; Yang, X. Combined treatment with electrical stimulation and insulin-like growth factor-1 promotes bone regeneration in vitro. PLoS ONE 2018, 13, e0197006. [Google Scholar] [CrossRef] [PubMed]
- Rico-Llanos, G.A.; Becerra, J.; Visser, R. Insulin-like growth factor-1 (IGF-1) enhances the osteogenic activity of bone morphogenetic protein-6 (BMP-6) in vitro and in vivo, and together have a stronger osteogenic effect than when IGF-1 is combined with BMP-2. J. Biomed. Mater. Res. Part A 2017, 105, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Ono, N. The mechanism of bone repair: Stem cells in the periosteum dedicated to bridging a large gap. Cell Rep. Med. 2022, 3, 100807. [Google Scholar] [CrossRef]
- Deng, S.; Zhu, F.; Dai, K.; Wang, J.; Liu, C. Harvest of functional mesenchymal stem cells derived from in vivo osteo-organoids. Biomater. Transl. 2023, 4, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, J.; Wang, M.; Liu, X.; Jiang, Y.; Su, J. Biomaterials regulates BMSCs differentiation via mechanical microenvironment. Biomater. Adv. 2024, 157, 213738. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Wu, Y.; Li, G.; Ji, N.; Han, R.; Tang, H.; Liu, X.; Liu, H.; Wang, C.; et al. Delivery of m7G methylated Runx2 mRNA by bone-targeted lipid nanoparticle promotes osteoblastic bone formation in senile osteoporosis. Nano Today 2024, 54, 102074. [Google Scholar] [CrossRef]
- Triffitt, J.T.; Wang, Q. Stem cell fate and microenvironment. Biomater. Transl. 2022, 3, 1–2. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwa, S.; Lee, H.-J.; Ko, Y.; Park, J.-B. Effects of Insulin-like Growth Factor 1 on the Maintenance of Cell Viability and Osteogenic Differentiation of Gingiva-Derived Mesenchymal Stem Cell Spheroids. Medicina 2025, 61, 76. https://doi.org/10.3390/medicina61010076
Hwa S, Lee H-J, Ko Y, Park J-B. Effects of Insulin-like Growth Factor 1 on the Maintenance of Cell Viability and Osteogenic Differentiation of Gingiva-Derived Mesenchymal Stem Cell Spheroids. Medicina. 2025; 61(1):76. https://doi.org/10.3390/medicina61010076
Chicago/Turabian StyleHwa, Somyeong, Hyun-Jin Lee, Youngkyung Ko, and Jun-Beom Park. 2025. "Effects of Insulin-like Growth Factor 1 on the Maintenance of Cell Viability and Osteogenic Differentiation of Gingiva-Derived Mesenchymal Stem Cell Spheroids" Medicina 61, no. 1: 76. https://doi.org/10.3390/medicina61010076
APA StyleHwa, S., Lee, H.-J., Ko, Y., & Park, J.-B. (2025). Effects of Insulin-like Growth Factor 1 on the Maintenance of Cell Viability and Osteogenic Differentiation of Gingiva-Derived Mesenchymal Stem Cell Spheroids. Medicina, 61(1), 76. https://doi.org/10.3390/medicina61010076






