Effects of Pomegranate Seed Oil on Lower Extremity Ischemia-Reperfusion Damage: Insights into Oxidative Stress, Inflammation, and Cell Death
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups and Chemicals
2.2. Experimental Procedure
2.3. Biochemical Evaluation
2.3.1. Serum Total Oxidant Status
2.3.2. Serum Total Antioxidant Status
2.3.3. Oxidative Stress Index
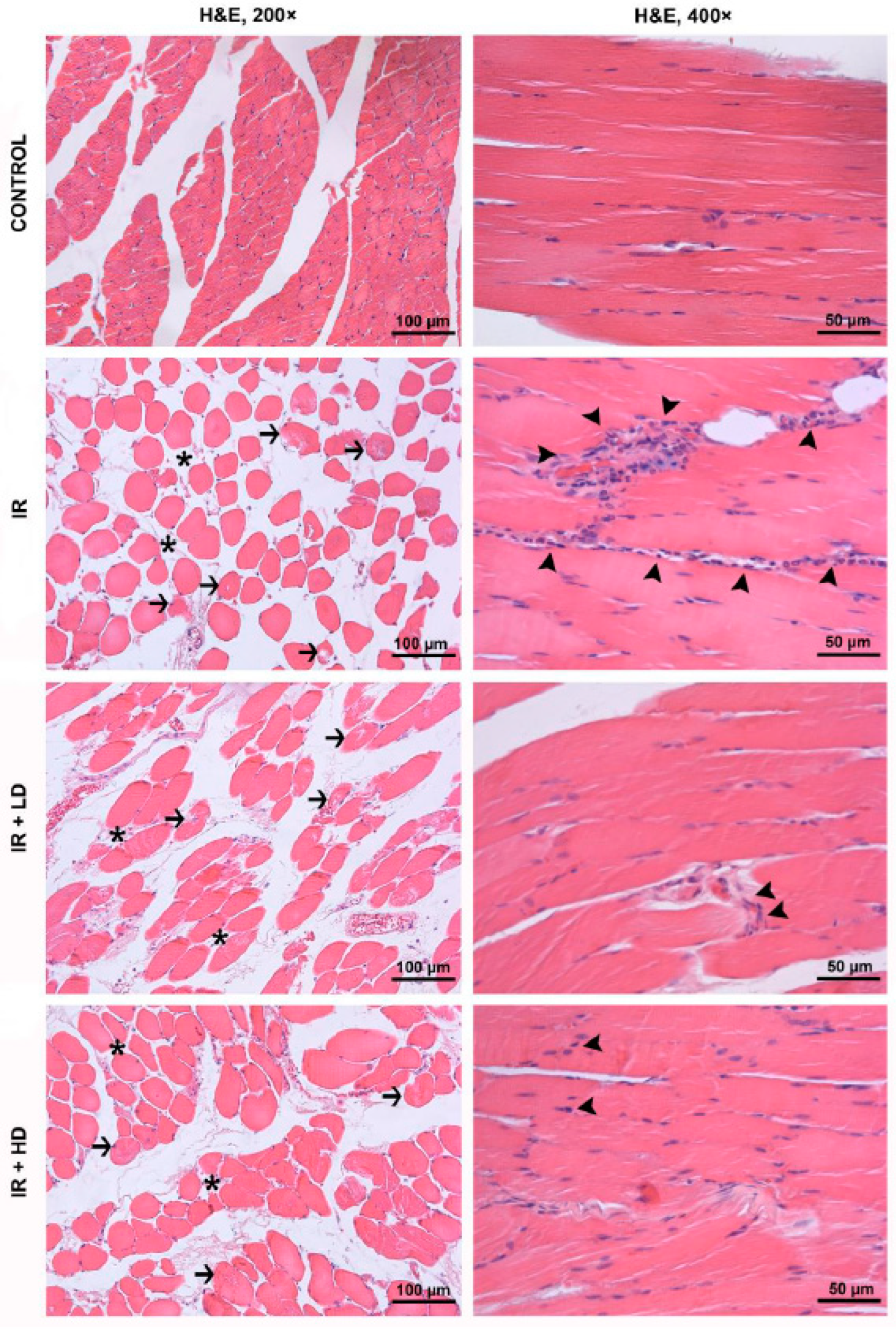
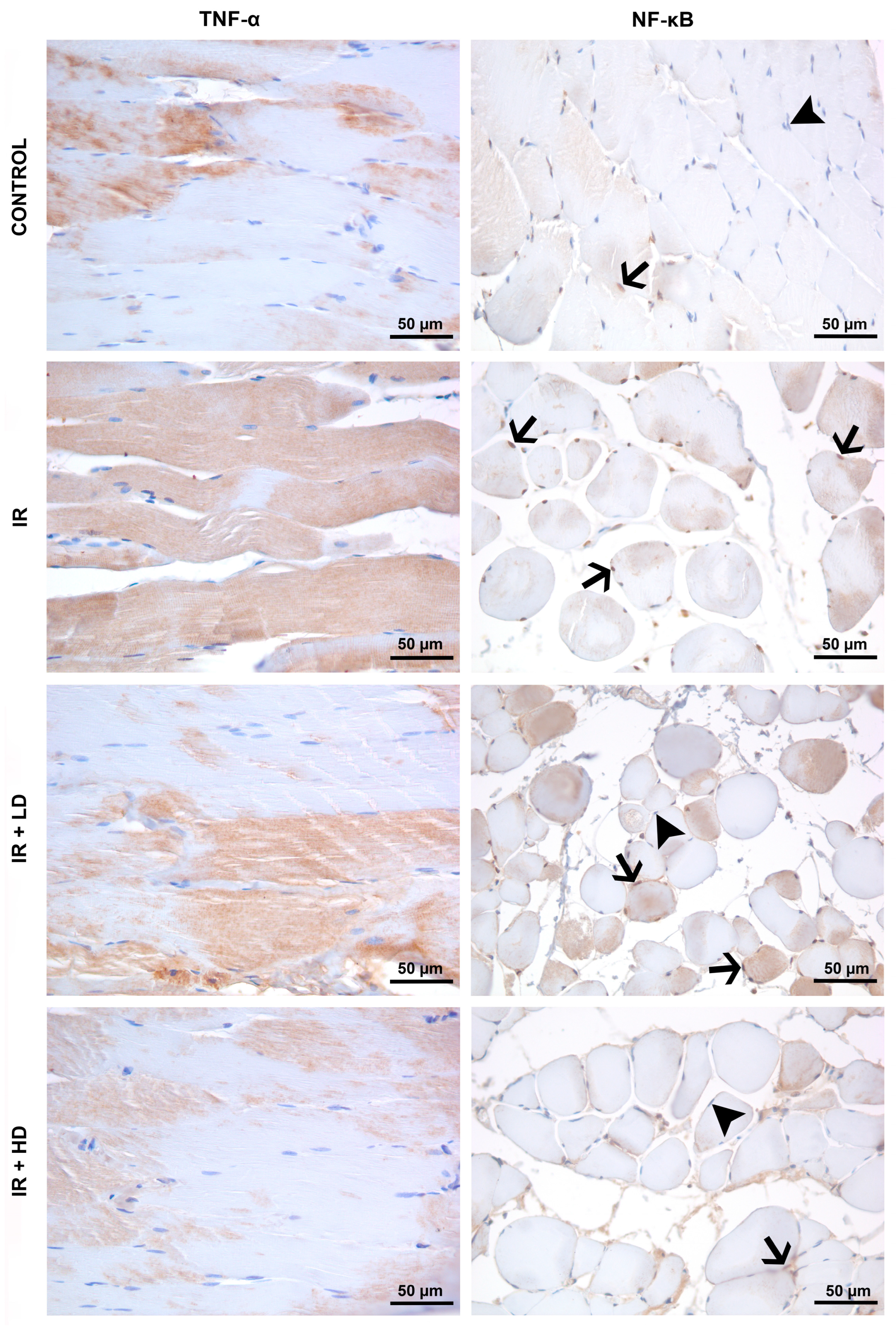
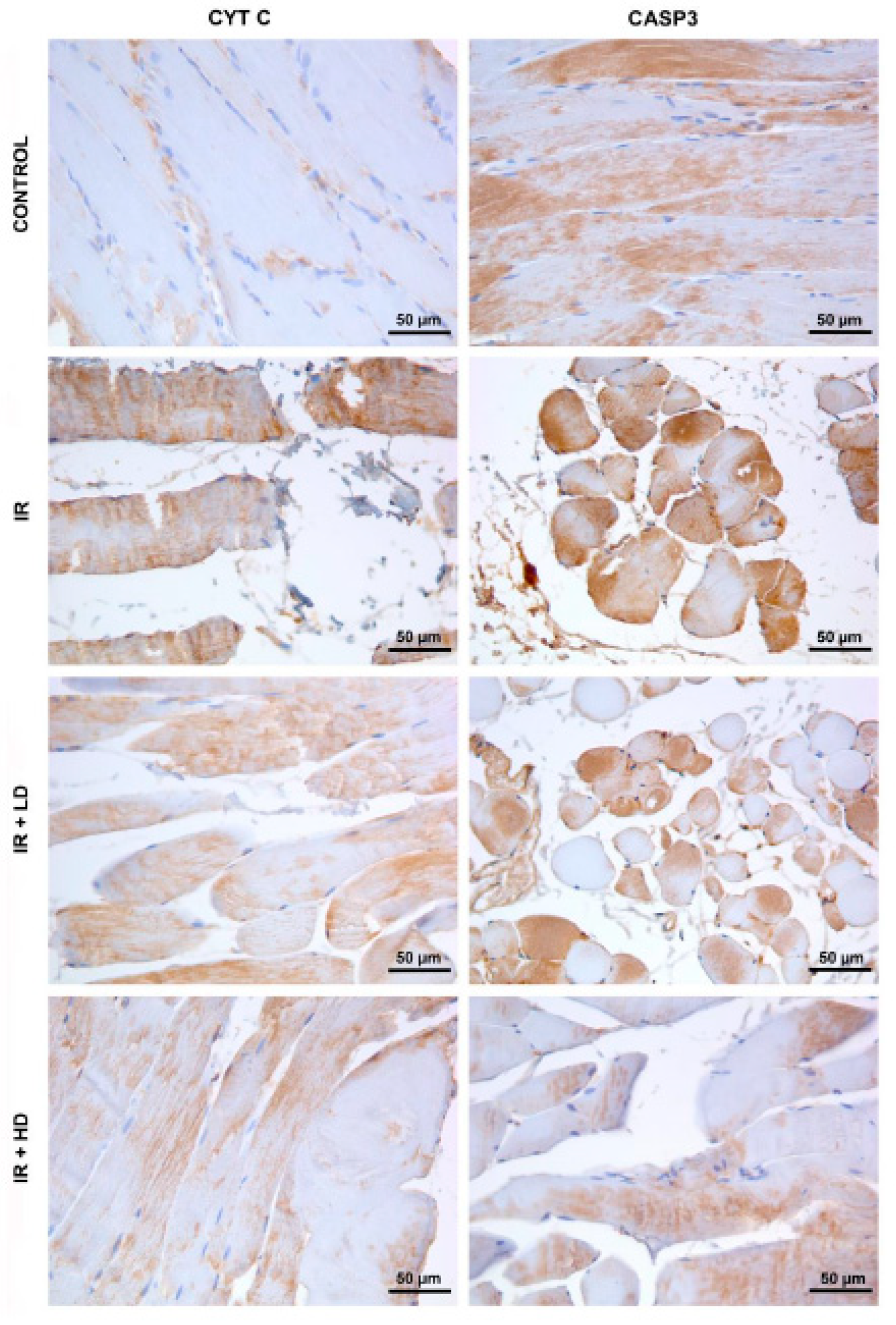
2.4. Histopathological and Immunohistochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Biochemical Results
3.2. Histopathological and Immunohistochemical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.A.; Eid, M.A.; Creager, M.A.; Goodney, P.P. Epidemiology and Risk of Amputation in Patients with Diabetes Mellitus and Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Bozok, Ü.G.; Küçük, A.; Arslan, M. İskemi Reperfüzyon Hasarında Stres ve Hücre Ölümü. Hitit Med. J. 2022, 4, 64–73. [Google Scholar] [CrossRef]
- Bradbury, A.W.; Ruckley, C.V.; Fowkes, F.G.; Forbes, J.F.; Gillespie, I.; Adam, D.J. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): Multicentre, randomised controlled trial. Lancet 2005, 366, 1925–1934. [Google Scholar]
- Gonçalves, R.V.; Costa, A.M.A.; Grzeskowiak, L. Oxidative Stress and Tissue Repair: Mechanism, Biomarkers, and Therapeutics. Oxid. Med. Cell Longev. 2021, 2021, 6204096. [Google Scholar] [CrossRef] [PubMed]
- Pawluk, H.; Woźniak, A.; Grześk, G.; Kołodziejska, R.; Kozakiewicz, M.; Kopkowska, E.; Grzechowiak, E.; Kozera, G. The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clin. Interv. Aging 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Elsayed Abouzed, D.E.; Ezelarab, H.A.A.; Selim, H.M.R.M.; Elsayed, M.M.A.; El Hamd, M.A.; Aboelez, M.O. Multimodal modulation of hepatic ischemia/reperfusion-induced injury by phytochemical agents: A mechanistic evaluation of hepatoprotective potential and safety profiles. Int. Immunopharmacol. 2024, 138, 112445. [Google Scholar] [CrossRef]
- Tastan, O.; Baysal, T. Adulteration Analysis of Pomegranate Juice. In Frontiers in Drug Safety; Shrivastava, A., Ed.; Bentham Science Publishers (Executive Suite Y—2 Building Y Saif Zone Sharjah, U.A.E): Sharjah, United Arab Emirates, 2018; pp. 91–100. Available online: http://www.eurekaselect.com/node/164300 (accessed on 12 November 2024).
- Arslan, M.; Küçük, A.; Bozok, Ü.G.; Ergörün, A.İ.; Sezen, Ş.C.; Yavuz, A.; Kavutçu, M. The role of pomegranate seed oil on kidney and lung tissues in the treatment of sepsis: Animal pre-clinical research. J. Infect. Dev. Ctries 2023, 17, 1791–1797. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Sadeghnia, H.R.; Hoseini, A.; Farzadnia, M.; Boroushaki, M.T. Effects of pomegranate seed oil on oxidative stress markers, serum biochemical parameters and pathological findings in kidney and heart of streptozotocin-induced diabetic rats. Ren. Fail. 2016, 38, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Pantiora, P.D.; Balaouras, A.I.; Mina, I.K.; Freris, C.I.; Pappas, A.C.; Danezis, G.P.; Zoidis, E.; Georgiou, C.A. The Therapeutic Alliance between Pomegranate and Health Emphasizing on Anticancer Properties. Antioxidants 2023, 12, 187. [Google Scholar] [CrossRef]
- Boussetta, T.; Raad, H.; Lettéron, P.; Gougerot-Pocidalo, M.A.; Marie, J.C.; Driss, F.; El-Benna, J. Punicic Acid a Conjugated Linolenic Acid Inhibits TNFα-Induced Neutrophil Hyperactivation and Protects from Experimental Colon Inflammation in Rats. PLoS ONE 2009, 4, e6458. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, A.G.; Cumbul, A.; Akyüz, E.Y.; Noval, M.K.A. Pomegranate seed oil mitigates liver and kidney damage in an experimental colitis model: Modulation of NF-κB activation and apoptosis. Prostaglandins Other Lipid Mediat. 2024, 171, 106804. [Google Scholar] [CrossRef] [PubMed]
- Tuncay, A.; Sivgin, V.; Ozdemirkan, A.; Sezen, S.C.; Boyunaga, H.; Kucuk, A.; Gunes, I.; Arslan, M. The Effect of Cerium Oxide on Lung Tissue in Lower Extremity Ischemia Reperfusion Injury in Sevoflurane Administered Rats. Int. J. Nanomed. 2020, 15, 7481–7489. [Google Scholar] [CrossRef]
- Sezen, Ş.C.; Kucuk, A.; Özer, A.; Kılıç, Y.; Mardin, B.; Alkan, M.; Erkent, F.D.; Arslan, M.; Ünal, Y.; Oktar, G.L.; et al. Assessment of the effects of levosimendan and thymoquinone on lung injury after myocardial ischemia reperfusion in rats. Drug Des. Dev. Ther. 2018, 12, 1347–1352. [Google Scholar] [CrossRef]
- Gram, D.; Atasever, A.; Eren, M. Effect of pomegranate (Punica granatum) seed oil on carbon tetrachloride-induced acute and chronic hepatotoxicity in rats. Pharmacogn. Res. 2018, 10, 124. [Google Scholar] [CrossRef]
- Boroushaki, M.T.; Rajabian, A.; Farzadnia, M.; Hoseini, A.; Poorlashkari, M.; Taghavi, A.; Dolati, K.; Bazmandegan, G. Protective effect of pomegranate seed oil against cisplatin-induced nephrotoxicity in rat. Ren. Fail. 2015, 37, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Küçük, A.; Polat, Y.; Kılıçarslan, A.; Süngü, N.; Kartal, H.; Dursun, A.D.; Arslan, M. Irisin Protects Against Hind Limb Ischemia Reperfusion Injury. Drug Des. Dev. Ther. 2021, 15, 361–368. [Google Scholar] [CrossRef]
- Shih, Y.; Shih, J.; Pai, M.; Hou, Y.; Yeh, C.; Yeh, S. Glutamine Administration After Sublethal Lower Limb Ischemia Reduces Inflammatory Reaction and Offers Organ Protection in Ischemia/Reperfusion Injury. J. Parenter. Enter. Nutr. 2016, 40, 1122–1130. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Bacus, S.; Flowers, J.L.; Press, M.F.; Bacus, J.W.; Mccarty, K.S. The Evaluation of Estrogen Receptor in Primary Breast Carcinoma by Computer-Assisted Image Analysis. Am. J. Clin. Pathol. 1988, 90, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Liu, M.; Chen, M.; Luo, Y.; Wang, C.; Xu, T.; Jiang, Y.; Guo, Y.; Zhang, J.H. Natural medicine in neuroprotection for ischemic stroke: Challenges and prospective. Pharmacol. Ther. 2020, 216, 107695. [Google Scholar] [CrossRef] [PubMed]
- Bihamta, M.; Hosseini, A.; Ghorbani, A.; Boroushaki, M.T. Protective effect of pomegranate seed oil against H2O2-induced oxidative stress in cardiomyocytes. Avicenna J. Phytomed. 2017, 7, 46–53. [Google Scholar]
- Rojo-Gutiérrez, E.; Carrasco-Molinar, O.; Tirado-Gallegos, J.M.; Levario-Gómez, A.; Chávez-González, M.L.; Baeza-Jiménez, R.; Buenrostro-Figueroa, J.J. Evaluation of green extraction processes, lipid composition and antioxidant activity of pomegranate seed oil. J. Food Meas. Charact. 2021, 15, 2098–2107. [Google Scholar] [CrossRef]
- Hayashi, M.; Obara, H.; Matsuda, S.; Homma, K.; Sasaki, J.; Matsubara, K.; Higuchi, M.; Sano, M.; Masugi, Y.; Kitagawa, Y. Protective Effects of Hydrogen Gas Inhalation for Hindlimb Ischaemia–Reperfusion Injury in a Mouse Model. Eur. J. Vasc. Endovasc. Surg. 2024, 68, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Polat, Y.; Şengel, N.; Küçük, A.; Özdemir, Ç.; Yığman, Z.; Balcı, A.B.; Ergörün, A.İ.; Kavutçu, M.; Arslan, M. Effects of sevoflurane and fullerenol C60 on lower limb ischemia–reperfusion injury in streptozocin-induced diabetic mice. Sci. Prog. 2024, 107, 00368504241239444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, S.; Wang, Y. Diannexin alleviates myocardial ischemia–reperfusion injury by orchestrating cardiomyocyte oxidative damage, macrophage polarization and fibrotic process by TLR4-NF-kB-mediated inactivation of NLRP3 inflammasome. Int. Immunopharmacol. 2024, 130, 111668. [Google Scholar] [CrossRef]
- Adams, J.W.; Sakata, Y.; Davis, M.G.; Sah, V.P.; Wang, Y.; Liggett, S.B.; Chien, K.R.; Brown, J.H.; Dorn, G.W. Enhanced Gαq signaling: A common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc. Natl. Acad. Sci. USA 1998, 95, 10140–10145. [Google Scholar] [CrossRef]
- Li, Q.; Ding, J.; Xia, B.; Liu, K.; Zheng, K.; Wu, J.; Huang, C.; Yuan, X.; You, Q. L-theanine alleviates myocardial ischemia/reperfusion injury by suppressing oxidative stress and apoptosis through activation of the JAK2/STAT3 pathway in mice. Mol. Med. 2024, 30, 98. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Gaidano, G. Messengers of cell death: Apoptotic signaling in health and disease. Haematologica 2003, 88, 212–218. [Google Scholar]
- Souza, J.A.C.D.; Junior, C.R.; Garlet, G.P.; Nogueira, A.V.B.; Cirelli, J.A. Modulation of host cell signaling pathways as a therapeutic approach in periodontal disease. J. Appl. Oral Sci. 2012, 20, 128–138. [Google Scholar] [CrossRef]
- Shal, B.; Ding, W.; Ali, H.; Kim, Y.S.; Khan, S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wan, H.; Zhou, H.; Yu, L.; He, Y. Protective effect of hydroxysafflor yellow A alone or in combination with acetylglutamine on cerebral ischemia reperfusion injury in rat: A PET study using 18F-fuorodeoxyglucose. Eur. J. Pharmacol. 2018, 825, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Yang, X. Proteases for Cell Suicide: Functions and Regulation of Caspases. Microbiol. Mol. Biol. Rev. 2000, 64, 821–846. [Google Scholar] [CrossRef]
- Zapata-Lopera, Y.M.; Trejo-Tapia, G.; Cano-Europa, E.; Rodríguez-Hernández, A.A.; Rojas-Franco, P.; Herrera-Ruiz, M.; Jiménez-Ferrer, E. Neuroprotective effect of Bouvardia ternifolia (Cav.) Schltdl via inhibition of TLR4/NF-κB, caspase-3/Bax/Bcl-2 pathways in ischemia/reperfusion injury in rats. Front. Pharmacol. 2024, 15, 1471542. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Khatyan, U.; Sun, J.; Alasmari, A.; Alshahrani, M.Y.; Qazi, I.H.; Wang, T.; Liu, Z.; Zou, H. Mitigating effect of pomegranate peel extract against the furan induced testicular injury by apoptosis, steroidogenic enzymes and oxidative stress. Chemosphere 2024, 358, 142086. [Google Scholar] [CrossRef] [PubMed]
| Group C (n = 8) | Group IR (n = 8) | Group IR + LD (n = 8) | Group IR + HD (n = 8) | p ** | |
|---|---|---|---|---|---|
| TOS (µmol/L) | 6.07 ± 2.65 | 26.36 ± 9.18 * | 12.13 ± 3.49 & | 8.14 ± 3.40 & | <0.0001 |
| TAS (mmol/L) | 1.17 ± 0.17 | 0.64 ± 0.07 * | 0.89 ± 0.09 *, & | 1.10 ± 0.32 & | <0.0001 |
| OSI | 5.72 ± 2.98 | 43.30 ± 13.45 * | 13.57 ± 3.64 & | 8.13 ± 2.74 & | <0.0001 |
| Group C (n = 8) | Group IR (n = 8) | Group IR + LD (n = 8) | Group IR + HD (n = 8) | p ** | |
|---|---|---|---|---|---|
| Disorganization and degeneration of the muscle fibers | 1.00 (0–1) | 2.50 (2–3) * | 1.50 (1–1) *, & | 1.00 (0.75–1.25) & | <0.0001 |
| Inflammatory cell infiltration | 0.00 (0–0) | 2.50 (2–3) * | 2.00 (1–2) *, & | 1.00 (0.75–1.25) *, & | <0.0001 |
| Total injury score | 1.00 (0–1) | 5.00 (4–5.25) * | 3.50 (2–4) *, & | 2.00 (1.5–3) *, &, + | <0.0001 |
| Group C (n = 8) | Group IR (n = 8) | Group IR + LD (n = 8) | Group IR + HD (n = 8) | p ** | |
|---|---|---|---|---|---|
| TNF-α immunostaining intensity | 123.10 ± 16.94 | 145.41 ± 15.80 * | 141.05 ± 14.44 * | 110.89 ± 6.83 &, + | 0.003 |
| % NF-κB immunopositivity | 29.39 ± 7.67 | 60.45 ± 6.74 * | 39.95 ± 9.75 *,& | 37.32 ± 3.72 & | <0.001 |
| CYT C immunostaining intensity | 115.66 ± 5.34 | 160.59 ± 13.39 * | 136.55 ± 18.46 * & | 134.42 ± 16.83 *, & | <0.001 |
| CASP3 immunostaining intensity | 147.62 ± 31.47 | 193.24 ± 17.2 * | 170.20 ± 33.79 | 132.45 ± 10.29 &, + | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozok, Ü.G.; Ergörün, A.İ.; Küçük, A.; Yığman, Z.; Dursun, A.D.; Arslan, M. Effects of Pomegranate Seed Oil on Lower Extremity Ischemia-Reperfusion Damage: Insights into Oxidative Stress, Inflammation, and Cell Death. Medicina 2025, 61, 212. https://doi.org/10.3390/medicina61020212
Bozok ÜG, Ergörün Aİ, Küçük A, Yığman Z, Dursun AD, Arslan M. Effects of Pomegranate Seed Oil on Lower Extremity Ischemia-Reperfusion Damage: Insights into Oxidative Stress, Inflammation, and Cell Death. Medicina. 2025; 61(2):212. https://doi.org/10.3390/medicina61020212
Chicago/Turabian StyleBozok, Ümmü Gülşen, Aydan İremnur Ergörün, Ayşegül Küçük, Zeynep Yığman, Ali Doğan Dursun, and Mustafa Arslan. 2025. "Effects of Pomegranate Seed Oil on Lower Extremity Ischemia-Reperfusion Damage: Insights into Oxidative Stress, Inflammation, and Cell Death" Medicina 61, no. 2: 212. https://doi.org/10.3390/medicina61020212
APA StyleBozok, Ü. G., Ergörün, A. İ., Küçük, A., Yığman, Z., Dursun, A. D., & Arslan, M. (2025). Effects of Pomegranate Seed Oil on Lower Extremity Ischemia-Reperfusion Damage: Insights into Oxidative Stress, Inflammation, and Cell Death. Medicina, 61(2), 212. https://doi.org/10.3390/medicina61020212






