Saliva as a Diagnostic Tool for Systemic Diseases—A Narrative Review
Abstract
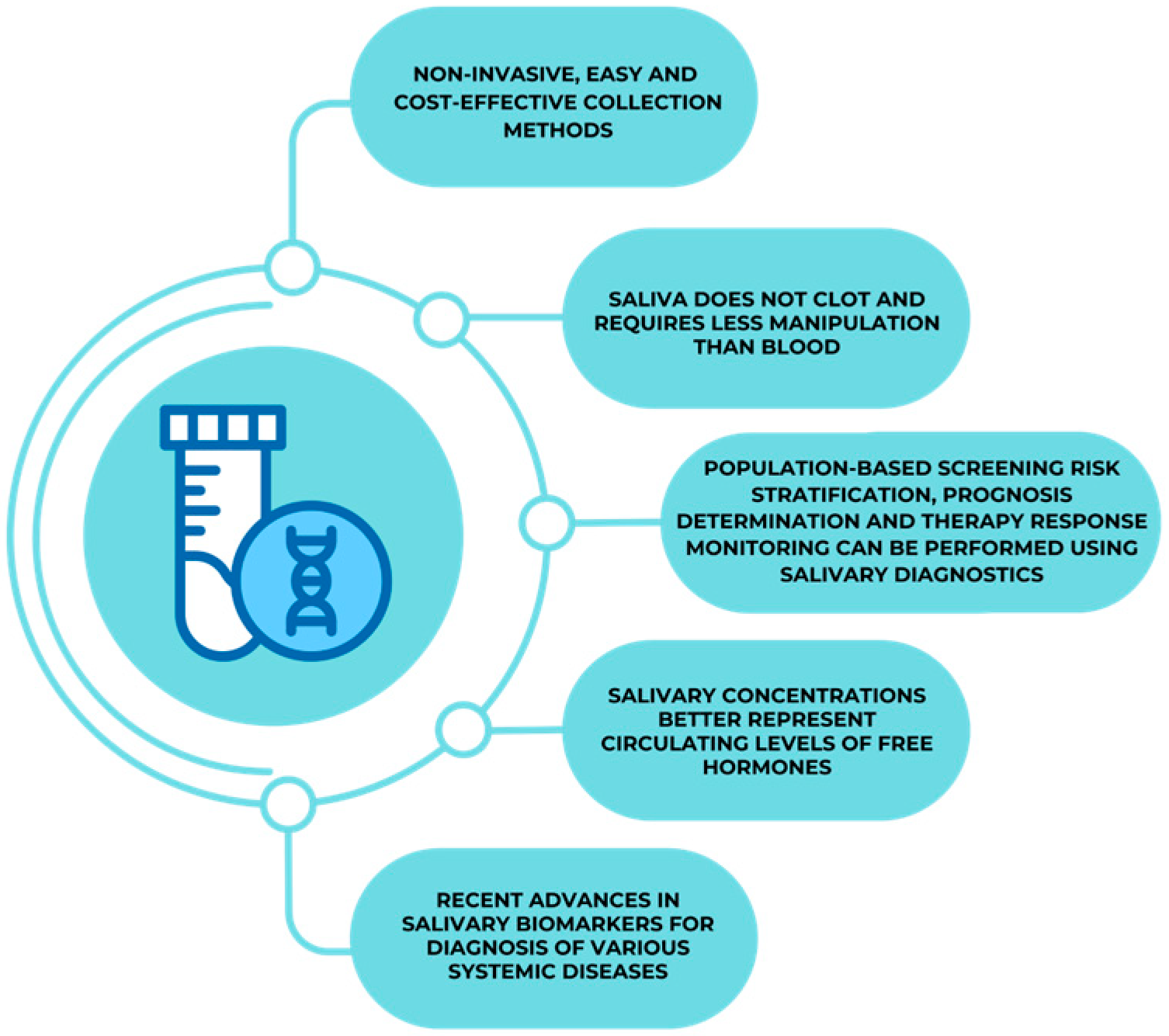
:1. Introduction
2. Salivary Composition and Secretion
3. Salivary Secretion Disorders
- ➢
- Xerostomia
- ➢
- Sialorrhea
4. Role of Saliva
4.1. Immunological Functions
4.2. Lubricant Functions
4.3. Taste and Digestive Functions
4.4. Saliva Proteome Functions
5. Saliva as a Diagnostic Tool
5.1. Oncology
5.2. Cardiac Diseases
5.3. Immune Mediated and Inflammatory Skin Diseases
5.4. Infectious Diseases
5.5. Diabetes Mellitus
5.6. Endocrinological Disorders
5.7. Renal Disorders
5.8. Psychiatric Disorders
5.9. Neurological Disorders
5.10. Forensic Medicine
5.11. Gastrointestinal Diseases
5.12. Saliva in Chronobiology
6. Salivary Diagnostic Technology
7. Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekström, J. Salivary secretion in health and disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef]
- Woo, J.S.; Lu, D.Y. Procurement, Transportation, and Storage of Saliva, Buccal Swab, and Oral Wash Specimens. Methods Mol. Biol. 2019, 1897, 99–105. [Google Scholar]
- Goto, T.; Kishimoto, T.; Iwawaki, Y.; Fujimoto, K.; Ishida, Y.; Watanabe, M.; Nagao, K.; Ichikawa, T. Reliability of Screening Methods to Diagnose Oral Dryness and Evaluate Saliva Secretion. Dent. J. 2020, 8, 102. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Cheng, X.Q.; Li, J.Y.; Zhang, P.; Yi, P.; Xu, X.; Zhou, X.D. Saliva in the diagnosis of diseases. Int. J. Oral Sci. 2016, 8, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Dave, P.K.; Rojas-Cessa, R.; Dong, Z.; Umpaichitra, V. Survey of Saliva Components and Virus Sensors for Prevention of COVID-19 and Infectious Diseases. Biosensors 2021, 11, 14. [Google Scholar] [CrossRef]
- Hamid, H.; Khurshid, Z.; Adanir, N.; Zafar, M.S.; Zohaib, S. COVID-19 Pandemic and Role of Human Saliva as a Testing Biofluid in Point-of-Care Technology. Eur. J. Dent. 2020, 14 (Suppl. S1), S123–S129. [Google Scholar] [CrossRef]
- Roca, C.; Alkhateeb, A.A.; Deanhardt, B.K.; Macdonald, J.K.; Chi, D.L.; Wang, J.R.; Wolfgang, M.C. Saliva sampling method influences oral microbiome composition and taxa distribution associated with oral diseases. PLoS ONE 2024, 19, e0301016. [Google Scholar] [CrossRef]
- McGeachan, A.J.; Mcdermott, C.J. Management of oral secretions in neurological disease. Pract. Neurol. 2017, 17, 96–103. [Google Scholar] [CrossRef]
- Lamy, E.; Capela-Silva, F.; Tvarijonaviciute, A. Research on Saliva Secretion and Composition. Biomed. Res. Int. 2018, 2018, 7406312. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, E.J.; Oppenheim, F.G. Saliva: A dynamic proteome. J. Dent. Res. 2007, 86, 680–693. [Google Scholar] [CrossRef]
- Ghods, K.; Motamed, B.; Alaee, A.; Farrokhnia, T. Review of Oral Saliva Measurement Standard Methods. J. Res. Dent. Sci. 2021, 18, 150–160. [Google Scholar] [CrossRef]
- Bozorgi, C.; Holleufer, C.; Wendin, K. Saliva Secretion and Swallowing—The Impact of Different Types of Food and Drink on Subsequent Intake. Nutrients 2020, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Shin, Y.H.; Namkoong, E.; Hwang, S.M.; Cong, X.; Yu, G.; Park, K. TRPV1 in Salivary Gland Epithelial Cells Is Not Involved in Salivary Secretion via Transcellular Pathway. Korean J. Physiol. Pharmacol. 2014, 18, 525–530. [Google Scholar] [CrossRef]
- Suzuki, A.; Ogata, K.; Iwata, J. Cell signaling regulation in salivary gland development. Cell. Mol. Life Sci. 2021, 78, 3299–3315. [Google Scholar] [CrossRef]
- Wei, F.; Wei, M.X.; Murakami, M. Mechanism involved in Danshen-induced fluid secretion in salivary glands. World J. Gastroenterol. 2015, 21, 1444–1456. [Google Scholar] [CrossRef]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A Review of Selected Studies That Determine the Physical and Chemical Properties of Saliva in the Field of Dental Treatment. Biomed. Res. Int. 2018, 2018, 6572381. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, M.; Asli, H.N.; Mohamadi, M.H. Comparison of Salivary Calcium Level in Dentulous and Edentulous Patients. Eur. J. Dent. 2019, 13, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, K.S.; Zareena; Hegde, S.; Arun Kumar, M.S. Assessment of salivary calcium, phosphate, magnesium, pH, and flow rate in healthy subjects, periodontitis, and dental caries. Contemp. Clin. Dent. 2015, 6, 461–465. [Google Scholar] [PubMed]
- Fiyaz, M.; Ramesh, A.; Ramalingam, K.; Thomas, B.; Shetty, S.; Prakash, P. Association of salivary calcium, phosphate, pH and flow rate on oral health: A study on 90 subjects. J. Indian Soc. Periodontol. 2013, 17, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Orihara, K.; Kamagata, M.; Hama, K.; Sasaki, H.; Haraguchi, A.; Miyakawa, H.; Nakao, A.; Shibata, S. Circadian clock-dependent increase in salivary IgA secretion modulated by sympathetic receptor activation in mice. Sci. Rep. 2017, 7, 8802. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, Y.; Fu, B.; Yonemoto, K.; Akifusa, S.; Shibata, Y.; Takeshita, T.; Ninomiya, T.; Kiyohara, Y.; Yamashita, Y. Stimulated salivary flow rate and oral health status. J. Oral Sci. 2017, 59, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Theda, C.; Hwang, S.H.; Czajko, A.; Loke, Y.J.; Leong, P.; Craig, J.M. Quantitation of the cellular content of saliva and buccal swab samples. Sci. Rep. 2018, 8, 6944. [Google Scholar] [CrossRef]
- Budala, D.G.; Balcos, C.; Bida, F.C.; Virvescu, D.I.; Baciu, E.R.; Surlari, Z. Changes in saliva secretion in the context of teeth loss. Rom. J. Oral Rehab. 2021, 13, 103–108. [Google Scholar]
- Kanehira, T.; Hongou, H.; Asano, K.; Morita, M.; Maeshima, E.; Matsuda, A.; Sakamoto, W. A simple test for salivary gland function measuring resting and stimulated submandibular and sublingual secretions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 197–203. [Google Scholar] [CrossRef]
- Proctor, G.B. The physiology of salivary secretion. Periodontol. 2000 2016, 70, 11–25. [Google Scholar] [CrossRef]
- Wilson, K.F.; Meier, J.D.; Ward, P.D. Salivary gland disorders. Am. Fam. Physician 2014, 89, 882–888. [Google Scholar] [PubMed]
- Swid, M.A.; Li, L.; Drahnak, E.M.; Idom, H.; Quinones, W. Updated Salivary Gland Immunohistochemistry: A Review. Arch. Pathol. Lab Med. 2023, 147, 1383–1389. [Google Scholar] [CrossRef]
- Hosoi, K.; Yao, C.; Hasegawa, T.; Yoshimura, H.; Akamatsu, T. Dynamics of Salivary Gland AQP5 under Normal and Pathologic Conditions. Int. J. Mol. Sci. 2020, 21, 1182. [Google Scholar] [CrossRef] [PubMed]
- Tanasiewicz, M.; Hildebrandt, T.; Obersztyn, I. Xerostomia of Various Etiologies: A Review of the Literature. Adv. Clin. Exp. Med. 2016, 25, 199–206. [Google Scholar] [CrossRef]
- Porcheri, C.; Mitsiadis, T.A. Physiology, Pathology and Regeneration of Salivary Glands. Cells 2019, 8, 976. [Google Scholar] [CrossRef]
- Tan, E.C.K.; Lexomboon, D.; Sandborgh-Englund, G.; Haasum, Y. Medications That Cause Dry Mouth as an Adverse Effect in Older People: A Systematic Review and Metaanalysis. J. Am. Geriatr. Soc. 2018, 66, 76–84. [Google Scholar] [CrossRef] [PubMed]
- López-Jornet, P.; Collado, Y.; Zambudio, A.; Pons-Fuster, E.; Castillo Felipe, C.; Tvarijonaviciute, A. Chemosensory Function in Burning Mouth Syndrome a Comparative Cross-Sectional Study. Nutrients 2021, 13, 722. [Google Scholar] [CrossRef]
- Toan, N.; Ahn, S.-G. Aging-Related Metabolic Dysfunction in the Salivary Gland: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 5835. [Google Scholar] [CrossRef]
- Kapourani, A.; Kontogiannopoulos, K.N.; Manioudaki, A.-E.; Poulopoulos, A.K.; Tsalikis, L.; Assimopoulou, A.N.; Barmpalexis, P. A Review on Xerostomia and Its Various Management Strategies: The Role of Advanced Polymeric Materials in the Treatment Approaches. Polymers 2022, 14, 850. [Google Scholar] [CrossRef]
- Mortazavi, H.; Baharvand, M.; Movahhedian, A.; Mohammadi, M.; Khodadoustan, A. Xerostomia due to systemic disease: A review of 20 conditions and mechanisms. Ann. Med. Health Sci. Res. 2014, 4, 503–510. [Google Scholar] [PubMed]
- Ugga, L.; Ravanelli, M.; Pallottino, A.A.; Farina, D.; Maroldi, R. Diagnostic work-up in obstructive and inflammatory salivary gland disorders. Acta Otorhinolaryngol. Ital. 2017, 37, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W.M.; Smith, M.B.; Ferguson, C.A.; Moses, G. The Challenge of Medication-Induced Dry Mouth in Residential Aged Care. Pharmacy 2021, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Abati, S. Risk factors and symptoms associated with xerostomia: A cross-sectional study. Aust. Dent. J. 2011, 56, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Hast, M.A.; Kong, A.M.; Abdelhadi, J.; Shah, R.; Szendrey, A.; Holmes, J. Real-World Observational Analysis of Clinical Characteristics and Treatment Patterns of Patients with Chronic Sialorrhea. Toxins 2024, 16, 366. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H.; Bäumer, T.; Laskawi, R.; Slawek, J.; Spittau, B.; Steffen, A.; Winterholler, M.; Bavikatte, G. Therapy of Sialorrhea with Botulinum Neurotoxin. Neurol. Ther. 2019, 8, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Joshi, R.K.; Ekström, J.; Aframian, D.; Pedersen, A.M.; Proctor, G.; Narayana, N.; Villa, A.; Sia, Y.W.; Aliko, A.; et al. A Guide to Medications Inducing Salivary Gland Dysfunction, Xerostomia, and Subjective Sialorrhea: A Systematic Review Sponsored by the World Workshop on Oral Medicine VI. Drugs R D 2017, 17, 1–28. [Google Scholar] [CrossRef]
- Vila, T.; Rizk, A.M.; Sultan, A.S.; Jabra-Rizk, M.A. The power of saliva: Antimicrobial and beyond. PLoS Pathog. 2019, 15, e1008058. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W.; de Szalay, S. Innate Antimicrobial Defense of Skin and Oral Mucosa. Antibiotics 2020, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Matczuk, J.; Żendzian-Piotrowska, M.; Maciejczyk, M.; Kurek, K. Salivary lipids: A review. Adv. Clin. Exp. Med. 2017, 26, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, E.S.; Ribbeck, K. Salivary mucins in host defense and disease prevention. J. Oral Microbiol. 2015, 7, 29759. [Google Scholar] [CrossRef]
- Uchida, H.; Ovitt, C.E. Novel impacts of saliva with regard to oral health. J. Prosthet. Dent. 2022, 127, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M. Science behind human saliva. J. Nat. Sci. Biol. Med. 2011, 2, 53–58. [Google Scholar] [CrossRef]
- Song, M.; Bai, H.; Zhang, P.; Zhou, X.; Ying, B. Promising applications of human-derived saliva biomarker testing in clinical diagnostics. Int. J. Oral Sci. 2023, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Kraaij, S.; de Visscher, J.G.A.M.; Apperloo, R.C.; Nazmi, K.; Bikker, F.J.; Brand, H.S. Lactoferrin and the development of salivary stones: A pilot study. Biometals 2023, 36, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, Y.; Wang, Y.; Cui, J.; Liu, M.; Du, W.; Xu, Y. Computational prediction of human salivary proteins from blood circulation and application to diagnostic biomarker identification. PLoS ONE 2013, 8, e80211. [Google Scholar] [CrossRef] [PubMed]
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary Defense Proteins: Their Network and Role in Innate and Acquired Oral Immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ouyang, X.; Chen, J.; Zhang, P.; Feng, Y. A Review on Salivary Proteomics for Oral Cancer Screening. Curr. Issues Mol. Biol. 2020, 37, 47–56. [Google Scholar] [CrossRef]
- Chibly, A.M.; Aure, M.H.; Patel, V.N.; Hoffman, M.P. Salivary gland function, development, and regeneration. Physiol. Rev. 2022, 102, 1495–1552. [Google Scholar] [CrossRef]
- Kroese, F.G.M.; Haacke, E.A.; Bombardieri, M. The role of salivary gland histopathology in primary Sjögren’s syndrome: Promises and pitfalls. Clin. Exp. Rheumatol. 2018, 36 (Suppl. S112), 222–233. [Google Scholar] [PubMed]
- Nonaka, T.; Wong, D.T.W. Saliva Diagnostics. Annu. Rev. Anal. Chem. 2022, 15, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.E.; Gutierrez, V.A.; Torregrossa, A.M. The role of saliva in taste and food intake. Physiol. Behav. 2023, 262, 114109. [Google Scholar] [CrossRef]
- Feng, Y.; Licandro, H.; Martin, C.; Septier, C.; Zhao, M.; Neyraud, E.; Morzel, M. The Associations between Biochemical and Microbiological Variables and Taste Differ in Whole Saliva and in the Film Lining the Tongue. Biomed. Res. Int. 2018, 2018, 2838052. [Google Scholar] [CrossRef] [PubMed]
- López-Dávalos, P.C.; Requena, T.; Pozo-Bayón, M.Á.; Muñoz-González, C. Decreased retronasal olfaction and taste perception in obesity are related to saliva biochemical and microbiota composition. Food. Res. Int. 2023, 167, 112660. [Google Scholar] [CrossRef] [PubMed]
- Błochowiak, K. Smell and Taste Function and Their Disturbances in Sjögren’s Syndrome. Int. J. Environ. Res. Public Health 2022, 19, 12472. [Google Scholar] [CrossRef]
- Fábián, T.K.; Beck, A.; Fejérdy, P.; Hermann, P.; Fábián, G. Molecular Mechanisms of Taste Recognition: Considerations about the Role of Saliva. Int. J. Mol. Sci. 2015, 16, 5945–5974. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, A.; Narayanan, R. Comparison of Salivary Flow Rate, pH, Buffering Capacity, and Secretory Immunoglobulin A Levels between Children with Early Childhood Caries and Caries-free Children. Int. J. Clin. Pediatr. Dent. 2024, 17, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Madariaga, V.I.; Pereira-Cenci, T.; Walboomers, X.F.; Loomans, B.A.C. Association between salivary characteristics and tooth wear: A systematic review and meta-analysis. J. Dent. 2023, 138, 104692. [Google Scholar] [CrossRef] [PubMed]
- Andrioaie, I.M.; Luchian, I.; Dămian, C.; Nichitean, G.; Andrese, E.P.; Pantilimonescu, T.F.; Trandabăț, B.; Prisacariu, L.J.; Budală, D.G.; Dimitriu, D.C.; et al. The Clinical Utility of Circulating HPV DNA Biomarker in Oropharyngeal, Cervical, Anal, and Skin HPV-Related Cancers: A Review. Pathogens 2023, 12, 908. [Google Scholar] [CrossRef] [PubMed]
- Schweigel, H.; Wicht, M.; Schwendicke, F. Salivary and pellicle proteome: A datamining analysis. Sci. Rep. 2016, 6, 38882. [Google Scholar] [CrossRef]
- Miller, C.S.; Foley, J.D.; Bailey, A.L.; Campell, C.L.; Humphries, R.L.; Christodoulides, N.; Floriano, P.N.; Simmons, G.; Bhagwandin, B.; Jacobson, J.W.; et al. Current developments in salivary diagnostics. Biomark. Med. 2010, 4, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the oral microbiome by whole-genome sequencing and resistome analysis: The complexity of the healthy picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef]
- Roi, A.; Rusu, L.C.; Roi, C.I.; Luca, R.E.; Boia, S.; Munteanu, R.I. A New Approach for the Diagnosis of Systemic and Oral Diseases Based on Salivary Biomolecules. Dis. Markers 2019, 2019, 8761860. [Google Scholar] [CrossRef]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef]
- Humeau, M.; Vignolle-Vidoni, A.; Sicard, F.; Martins, F.; Bournet, B.; Buscail, L.; Torrisani, J.; Cordelier, P. Salivary MicroRNA in pancreatic cancer patients. PLoS ONE 2015, 10, e0130996. [Google Scholar] [CrossRef]
- Wei, A.L.; Li, M.; Li, G.Q.; Wang, X.; Hu, W.M.; Li, Z.L.; Yuan, J.; Liu, H.Y.; Zhou, L.L.; Li, K.; et al. Oral microbiome and pancreatic cancer. World J. Gastroenterol. 2020, 26, 7679–7692. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Zhao, H.P.; Yu, Y.; Wang, J.H.; Guo, L.; Liu, J.Y.; Pu, J.; Lv, J. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J. Gastroenterol. 2023, 29, 2452–2468. [Google Scholar] [CrossRef] [PubMed]
- Maconi, G.; Manes, G.; Porro, G.B. Role of symptoms in diagnosis and outcome of gastric cancer. World J. Gastroenterol. 2008, 14, 1149–1155. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, Y.; Kim, Y.; Kim, S.; Kim, J.J.; Kim, K.M.; Yoshizawa, J.; Fan, L.Y.; Cao, C.X.; Wong, D.T. Differential proteomic analysis of human saliva using tandem mass tags quantification for gastric cancer detection. Sci. Rep. 2016, 6, 22165. [Google Scholar] [CrossRef] [PubMed]
- Laidi, F.; Bouziane, A.; Lakhdar, A.; Khabouze, S.; Amrani, M.; Rhrab, B.; Zaoui, F. Significant correlation between salivary and serum Ca 15-3 in healthy women and breast cancer patients. Asian Pac. J. Cancer Prev. 2014, 15, 4659–4662. [Google Scholar] [CrossRef]
- Wood, N.; Streckfus, C.F. The Expression of Lung Resistance Protein in Saliva: A Novel Prognostic Indicator Protein for Carcinoma of the Breast. Cancer Investig. 2015, 33, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Arantes, L.M.; de Carvalho, A.C.; Melendez, M.E.; Carvalho, A.L.; Goloni-Bertollo, E.M. Methylation as a biomarker for head and neck cancer. Oral Oncol. 2014, 50, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Dhanuthai, K.; Rojanawatsirivej, S.; Thosaporn, W.; Kintarak, S.; Subarnbhesaj, A.; Darling, M.; Kryshtalskyj, E.; Chiang, C.P.; Shin, H.I.; Choi, S.Y.; et al. Oral cancer: A multicenter study. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e23–e29. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, D.A.; Cooper, M.A.; Pandit, P.; Coman, W.B.; Cooper-White, J.J.; Keith, P.; Wolvetang, E.J.; Slowey, P.D.; Punyadeera, C. Tumor-suppressor Gene Promoter Hypermethylation in Saliva of Head and Neck Cancer Patients. Transl. Oncol. 2012, 5, 321–326. [Google Scholar] [CrossRef]
- Park, N.J.; Zhou, H.; Elashoff, D.; Henson, B.S.; Kastratovic, D.A.; Abemayor, E.; Wong, D.T. Salivary microRNA: Discovery, characterization, and clinical utility for oral cancer detection. Clin. Cancer Res. 2009, 15, 5473–5477. [Google Scholar] [CrossRef]
- Liu, C.J.; Lin, S.C.; Yang, C.C.; Cheng, H.W.; Chang, K.W. Exploiting salivary miR-31 as a clinical biomarker of oral squamous cell carcinoma. Head Neck. 2012, 34, 219–224. [Google Scholar] [CrossRef]
- Duz, M.B.; Karatas, O.F.; Guzel, E.; Turgut, N.F.; Yilmaz, M.; Creighton, C.J.; Ozen, M. Identification of miR-139-5p as a saliva biomarker for tongue squamous cell carcinoma: A pilot study. Cell. Oncol. 2016, 39, 187–193. [Google Scholar] [CrossRef]
- Wong, D.T. Salivary diagnostics. Oper. Dent. 2012, 37, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; Ahmed, S.S.; Ali, A.; Khan, F.A.; Zulfiqar, G.; Iqbal, J.; Khan, A.A.; Shoaib, M. Salivary Immunosuppressive Cytokines IL-10 and IL-13 Are Significantly Elevated in Oral Squamous Cell Carcinoma Patients. Cancer Invest. 2015, 33, 318–328. [Google Scholar] [CrossRef]
- Allegra, E.; Trapasso, S.; La Boria, A.; Aragona, T.; Pisani, D.; Belfiore, A.; Garozzo, A. Prognostic role of salivary CD44sol levels in the follow-up of laryngeal carcinomas. J. Oral Pathol. Med. 2014, 43, 276–281. [Google Scholar] [CrossRef]
- Yuvaraj, M.; Udayakumar, K.; Jayanth, V.; Prakasa Rao, A.; Bharanidharan, G.; Koteeswaran, D.; Munusamy, B.D.; Murali Krishna, C.; Ganesan, S. Fluorescence spectroscopic characterization of salivary metabolites of oral cancer patients. J. Photochem. Photobiol. B. 2014, 130, 153–160. [Google Scholar] [CrossRef]
- Tantray, S.; Sharma, S.; Prabhat, K.; Nasrullah, N.; Gupta, M. Salivary metabolite signatures of oral cancer and leukoplakia through gas chromatography-mass spectrometry. J. Oral Maxillofac. Pathol. 2022, 26, 31–37. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef]
- Guo, Z.W.; Xiao, W.W.; Yang, X.X.; Yang, X.; Cai, G.X.; Wang, X.J.; Han, B.W.; Li, K.; Zhai, X.M.; Li, F.X.; et al. Noninvasive prediction of response to cancer therapy using promoter profiling of circulating cell-free DNA. Clin. Transl. Med. 2020, 10, e174. [Google Scholar] [CrossRef]
- Spindler, K.L.; Pallisgaard, N.; Vogelius, I.; Jakobsen, A. Quantitative cell-free DNA, KRAS, and BRAF mutations in plasma from patients with metastatic colorectal cancer during treatment with cetuximab and irinotecan. Clin. Cancer Res. 2012, 18, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A., Jr.; Williams, R.T.; Wu, J.; Kinde, I.; Hecht, J.R.; Berlin, J.; Allen, B.; Bozic, I.; Reiter, J.G.; Nowak, M.A.; et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012, 486, 537–540. [Google Scholar] [CrossRef]
- Ozbay, Y.; Aydin, S.; Dagli, A.F.; Akbulut, M.; Dagli, N.; Kilic, N.; Rahman, A.; Sahin, I.; Polat, V.; Ozercan, H.I.; et al. Obestatin is present in saliva: Alterations in obestatin and ghrelin levels of saliva and serum in ischemic heart disease. BMB Rep. 2008, 41, 55–61. [Google Scholar] [CrossRef]
- Miller, C.S.; Foley, J.D., 3rd; Floriano, P.N.; Christodoulides, N.; Ebersole, J.L.; Campbell, C.L.; Bailey, A.L.; Rose, B.G.; Kinane, D.F.; Novak, M.J.; et al. Utility of salivary biomarkers for demonstrating acute myocardial infarction. J. Dent. Res. 2014, 93, 72S–79S. [Google Scholar] [CrossRef]
- Shen, Y.S.; Chen, W.L.; Chang, H.Y.; Kuo, H.Y.; Chang, Y.C.; Chu, H. Diagnostic performance of initial salivary alpha-amylase activity for acute myocardial infarction in patients with acute chest pain. J. Emerg. Med. 2012, 43, 553–560. [Google Scholar] [CrossRef]
- Floriano, P.N.; Christodoulides, N.; Millerm, C.S.; Ebersole, J.L.; Spertus, J.; Rose, B.G.; Kinane, D.F.; Novak, M.J.; Steinhubl, S.; Acosta, S.; et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: A feasibility study. Clin. Chem. 2009, 55, 1530–1538. [Google Scholar] [CrossRef]
- Zheng, H.; Li, R.; Zhang, J.; Zhou, S.; Ma, Q.; Zhou, Y.; Chen, F.; Lin, J. Salivary biomarkers indicate obstructive sleep apnea patients with cardiovascular diseases. Sci. Rep. 2014, 4, 7046. [Google Scholar] [CrossRef]
- Giannoni, M.; Consales, V.; Campanati, A.; Ganzetti, G.; Giuliodori, K.; Postacchini, V.; Liberati, G.; Azzaretto, L.; Vichi, S.; Guanciarossa, F.; et al. Homocysteine plasma levels in psoriasis patients: Our experience and review of the literature. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1781–1785. [Google Scholar] [CrossRef]
- Luchetti, M.M.; Benfaremo, D.; Campanati, A.; Molinelli, E.; Ciferri, M.; Cataldi, S.; Capeci, W.; Di Carlo, M.; Offidani, A.M.; Salaffi, F.; et al. Clinical outcomes and feasibility of the multidisciplinary management of patients with psoriatic arthritis: Two-year clinical experience of a dermo-rheumatologic clinic. Clin. Rheumatol. 2018, 37, 2741–2749. [Google Scholar] [CrossRef]
- Asa’ad, F.; Fiore, M.; Alfieri, A.; Pigatto, P.D.M.; Franchi, C.; Berti, E.; Maiorana, C.; Damiani, G. Saliva as a Future Field in Psoriasis Research. BioMed. Res. Int. 2018, 2018, 7290913. [Google Scholar] [CrossRef]
- Soudan, R.A.; Daoud, S.A.; Mashlah, A.M. Study of some salivary changes in cutaneous psoriatic patients. Saudi Med. J. 2011, 32, 386–389. [Google Scholar]
- Belstrøm, D.; Eiberg, J.M.; Enevold, C.; Grande, M.A.; Jensen, C.A.J.; Skov, L.; Hansen, P.R. Salivary microbiota and inflammation-related proteins in patients with psoriasis. Oral Dis. 2020, 26, 677–687. [Google Scholar] [CrossRef]
- Luchian, I.; Martu, I.; Ioanid, N.; Goriuc, A.; Vata, I.; Martu Stefanache, A.; Hurjui, L.; Tatarciuc, M.; Matei, M.N.; Martu, S. Salivary interleukin-1β: A biochemical marker that predicts periodontal disease in orthodontic treatment. Rev. Chim. 2016, 67, 2479–2483. [Google Scholar]
- Ganzetti, G.; Campanati, A.; Santarelli, A.; Pozzi, V.; Molinelli, E.; Minnetti, I.; Brisigotti, V.; Procaccini, M.; Emanuelli, M.; Offidani, A. Involvement of the oral cavity in psoriasis: Results of a clinical study. Br. J. Dermatol. 2015, 172, 282–285. [Google Scholar] [CrossRef]
- Andreadis, D.; Lorenzini, G.; Drakoulakos, D.; Belazi, M.; Mihailidou, E.; Velkos, G.; Mourellou-Tsatsou, O.; Antoniades, D. Detection of pemphigus desmoglein 1 and desmoglein 3 autoantibodies and pemphigoid BP180 autoantibodies in saliva and comparison with serum values. Eur. J. Oral Sci. 2006, 114, 374–380. [Google Scholar] [CrossRef]
- Campanati, A.; Martina, E.; Diotallevi, F.; Radi, G.; Marani, A.; Sartini, D.; Emanuelli, M.; Kontochristopoulos, G.; Rigopoulos, D.; Gregoriou, S.; et al. Saliva Proteomics as Fluid Signature of Inflammatory and Immune-Mediated Skin Diseases. Int. J. Mol. Sci. 2021, 22, 7018. [Google Scholar] [CrossRef]
- Fang, Y.; Xie, H.; Fan, C. Association of hypertension with Helicobacter pylori: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0268686. [Google Scholar]
- Gisbert, J.P. Helicobacter pylori-associated diseases. Gastroenterol. Hepatol. 2015, 38, 39–48. [Google Scholar] [CrossRef]
- Young, S.H.; Luo, J.C. Will saliva test be a good method to detect Helicobacter pylori in H. pylori-infected patients? J. Chin. Med. Assoc. 2016, 79, 351–352. [Google Scholar] [CrossRef]
- Corstjens, P.L.; Abrams, W.R.; Malamud, D. Saliva and viral infections. Periodontol 2000 2016, 70, 93–110. [Google Scholar] [CrossRef]
- Oliveira Neto, N.F.D.; Caixeta, R.A.V.; Zerbinati, R.M.; Zarpellon, A.C.; Caetano, M.W.; Pallos, D.; Junges, R.; Costa, A.L.F.; Aitken-Saavedra, J.; Giannecchini, S.; et al. The Emergence of Saliva as a Diagnostic and Prognostic Tool for Viral Infections. Viruses 2024, 16, 1759. [Google Scholar] [CrossRef]
- Nefzi, F.; Ben Salem, N.A.; Khelif, A.; Feki, S.; Aouni, M.; Gautheret-Dejean, A. Quantitative analysis of human herpesvirus-6 and human cytomegalovirus in blood and saliva from patients with acute leukemia. J. Med. Virol. 2015, 87, 451–460. [Google Scholar] [CrossRef]
- Sheikhakbari, S.; Mokhtari-Azad, T.; Salimi, V.; Norouzbabaei, Z.; Abbasi, S.; Zahraei, S.M.; Shahmahmoodi, S. The use of oral fluid samples spotted on filter paper for the detection of measles virus using nested rt-PCR. J. Clin. Lab. Anal. 2012, 26, 215–222. [Google Scholar] [CrossRef]
- Laxton, C.S.; Peno, C.; Hahn, A.M.; Allicock, O.M.; Perniciaro, S.; Wyllie, A.L. The potential of saliva as an accessible and sensitive sample type for the detection of respiratory pathogens and host immunity. Lancet Microbe 2023, 4, e837–e850. [Google Scholar] [CrossRef]
- Tobik, E.R.; Kitfield-Vernon, L.B.; Thomas, R.J.; Steel, S.A.; Tan, S.H.; Allicock, O.M.; Choate, B.L.; Akbarzada, S.; Wyllie, A.L. Saliva as a sample type for SARS-CoV-2 detection: Implementation successes and opportunities around the globe. Expert. Rev. Mol. Diagn. 2022, 22, 519–535. [Google Scholar] [CrossRef]
- Sueki, A.; Matsuda, K.; Yamaguchi, A.; Uehara, M.; Sugano, M.; Uehara, T.; Honda, T. Evaluation of saliva as diagnostic materials for influenza virus infection by PCR-based assays. Clin. Chim. Acta 2016, 453, 71–74. [Google Scholar] [CrossRef]
- Kim, Y.G.; Yun, S.G.; Kim, M.Y.; Park, K.; Cho, C.H.; Yoon, S.Y.; Nam, M.H.; Lee, C.K.; Cho, Y.J.; Lim, C.S. Comparison between Saliva and Nasopharyngeal Swab Specimens for Detection of Respiratory Viruses by Multiplex Reverse Transcription-PCR. J. Clin. Microbiol. 2016, 55, 226–233. [Google Scholar] [CrossRef]
- Aitken, J.P.; Ortiz, C.; Morales-Bozo, I.; Rojas-Alcayaga, G.; Baeza, M.; Beltran, C.; Escobar, A. α-2-macroglobulin in saliva is associated with glycemic control in patients with type 2 diabetes mellitus. Dis. Markers 2015, 2015, 128653. [Google Scholar] [CrossRef]
- Goriuc, A.; Cojocaru, K.-A.; Luchian, I.; Ursu, R.-G.; Butnaru, O.; Foia, L. Using 8-Hydroxy-2′-Deoxiguanosine (8-OHdG) as a Reliable Biomarker for Assessing Periodontal Disease Associated with Diabetes. Int. J. Mol. Sci. 2024, 25, 1425. [Google Scholar] [CrossRef]
- Barnes, V.M.; Kennedy, A.D.; Panagakos, F.; Devizio, W.; Trivedi, H.M.; Jönsson, T.; Guo, L.; Cervi, S.; Scannapieco, F.A. Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PLoS ONE 2014, 9, e105181. [Google Scholar] [CrossRef]
- Cenzato, N.; Cazzaniga, F.; Maspero, C.; Tartaglia, G.M.; Del Fabbro, M. SALIVA-based diagnostic approach for diabetes mellitus: A step towards non-invasive detection—A scoping review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 12080–12087. [Google Scholar]
- Aitken-Saavedra, J.; Rojas-Alcayaga, G.; Maturana-Ramirez, A.; Escobar-Alvarez, A.; Cortes-Coloma, A.; Reyes-Rojas, M.; Viera-Sapiain, V.; Villablanca-Martínez, C.; Morales-Bozo, I. Salivary gland dysfunction markers in type 2 diabetes mellitus patients. J. Clin. Exp. Dent. 2015, 7, 501–505. [Google Scholar] [CrossRef]
- Rzepka-Migut, B.; Paprocka, J. Melatonin-Measurement Methods and the Factors Modifying the Results. A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 1916. [Google Scholar] [CrossRef]
- Amatoury, M.; Lee, J.W.; Maguire, A.M.; Ambler, G.R.; Steinbeck, K.S. Utility of salivary enzyme immunoassays for measuring estradiol and testosterone in adolescents: A pilot study. Int. J. Adolesc. Med. Health 2016, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Venkatapathy, R.; Govindarajan, V.; Oza, N.; Parameswaran, S.; Pennagaram Dhanasekaran, B.; Prashad, K.V. Salivary creatinine estimation as an alternative to serum creatinine in chronic kidney disease patients. Int. J. Nephrol. 2014, 2014, 742724. [Google Scholar] [CrossRef]
- Giacomello, G.; Scholten, A.; Parr, M.K. Current methods for stress marker detection in saliva. J. Pharm. Biomed. Anal. 2020, 191, 113604. [Google Scholar] [CrossRef]
- Coulon, N.; Brailly-Tabard, S.; Walter, M.; Tordjman, S. Altered circadian patterns of salivary cortisol in individuals with schizophrenia: A critical literature review. J. Physiol. Paris 2016, 110, 439–447. [Google Scholar] [CrossRef]
- Muraoka, M.Y.; Justino, A.B.; Caixeta, D.C.; Queiroz, J.S.; Sabino-Silva, R.; Salmen Espindola, F. Fructose and methylglyoxal-induced glycation alters structural and functional properties of salivary proteins, albumin and lysozyme. PLoS ONE 2022, 17, e0262369. [Google Scholar] [CrossRef]
- Agha, N.H.; Baker, F.L.; Kunz, H.E.; Spielmann, G.; Mylabathula, P.L.; Rooney, B.V.; Mehta, S.K.; Pierson, D.L.; Laughlin, M.S.; Markofski, M.M.; et al. Salivary antimicrobial proteins and stress biomarkers are elevated during a 6-month mission to the International Space Station. J. Appl. Physiol. 1985 2020, 128, 264–275. [Google Scholar] [CrossRef]
- Ito, Y.; Iida, T.; Yamamura, Y.; Teramura, M.; Nakagami, Y.; Kawai, K.; Nagamura, Y.; Teradaira, R. Relationships between Salivary Melatonin Levels, Quality of Sleep, and Stress in Young Japanese Females. Int. J. Tryptophan Res. 2013, 6, 75–85. [Google Scholar] [CrossRef]
- Vora, K.M.; Shah, P.P.; Patil, K.V.; Kunte, S.S.; Jagtap, C.M.; Davalbhakta, R.N. Quantification of Salivary Chromogranin A Levels during Routine Dental Procedures in Children: An In Vivo Study. Int. J. Clin. Pediatr. Dent. 2024, 17, 585–590. [Google Scholar]
- Tammayan, M.; Jantaratnotai, N.; Pachimsawat, P. Differential responses of salivary cortisol, amylase, and chromogranin A to academic stress. PLoS ONE 2021, 16, e0256172. [Google Scholar] [CrossRef] [PubMed]
- Dia, M.M.; Bocanegra, O.L.; Teixeira, R.R.; Soares, S.S.; Espindola, F.S. Response of salivary markers of autonomicactivity to elite competition. Int. J. Sports Med. 2012, 33, 763–768. [Google Scholar]
- Takatsuji, K.; Sugimoto, Y.; Ishizaki, S.; Ozaki, Y.; Matsuyama, E.; Yamaguchi, Y. The effects of examination stresson salivary cortisol, immunoglobulin A, and chromogranin A in nursing students. Biomed. Res. 2008, 29, 221–224. [Google Scholar] [CrossRef]
- Motahari, P.; Pourzare Mehrbani, S.; Jabbarvand, H. Evaluation of Salivary Level of Heat Shock Protein 70 in Patients with Chronic Periodontitis. J. Dent. 2021, 22, 175–179. [Google Scholar]
- Chojnowska, S.; Ptaszyńska-Sarosiek, I.; Kępka, A.; Knaś, M.; Waszkiewicz, N. Salivary Biomarkers of Stress, Anxiety and Depression. J. Clin. Med. 2021, 10, 517. [Google Scholar] [CrossRef]
- Manera, V.; Rovini, E.; Wais, P. Editorial: Early detection of neurodegenerative disorders using behavioral markers and new technologies: New methods and perspectives. Front. Aging Neurosci. 2023, 15, 1149886. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T. Lumbar puncture: Considerations, procedure, and complications. Encephalitis 2022, 2, 93–97. [Google Scholar] [CrossRef]
- Khanagar, S.B.; Al-Ehaideb, A.; Maganur, P.C.; Vishwanathaiah, S.; Patil, S.; Baeshen, H.A.; Sarode, S.C.; Bhandi, S. Developments, application, and performance of artificial intelligence in dentistry—A systematic review. J. Dent. Sci. 2021, 16, 508–522. [Google Scholar] [CrossRef]
- Boroumand, M.; Olianas, A.; Cabras, T.; Manconi, B.; Fanni, D.; Faa, G.; Desiderio, C.; Messana, I.; Castagnola, M. Saliva, a bodily fluid with recognized and potential diagnostic applications. J. Sep. Sci. 2021, 44, 3677–3690. [Google Scholar] [CrossRef]
- Lau, H.C.; Lee, I.K.; Ko, P.W.; Lee, H.W.; Huh, J.S.; Cho, W.J.; Lim, J.O. Non-invasive screening for Alzheimer’s disease by sensing salivary sugar using Drosophila cells expressing gustatory receptor (Gr5a) immobilized on an extended gate ion-sensitive field-effect transistor (EG-ISFET) biosensor. PLoS ONE 2015, 10, e0117810. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sui, Y.T.; Peskind, E.R.; Li, G.; Hwang, H.; Devic, I.; Ginghina, C.; Edgar, J.S.; Pan, C.; Goodlett, D.R.; et al. Salivary tau species are potential biomarkers of Alzheimer’s disease. J. Alzheimers Dis. 2011, 27, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Kayser, M.; Branicki, W.; Parson, W.; Phillips, C. Recent advances in Forensic DNA Phenotyping of appearance, ancestry and age. Forensic Sci. Int. Genet. 2023, 65, 102870. [Google Scholar] [CrossRef] [PubMed]
- Neis, M.; Groß, T.; Schneider, H.; Schneider, P.M.; Courts, C. Comprehensive body fluid identification and contributor assignment by combining targeted sequencing of mRNA and coding region SNPs. Forensic Sci. Int. Genet. 2024, 73, 103125. [Google Scholar] [CrossRef]
- Upadhyay, M.; Shrivastava, P.; Verma, K.; Joshi, B. Recent advancements in identification and detection of saliva as forensic evidence: A review. Egypt J. Forensic Sci. 2023, 13, 17. [Google Scholar] [CrossRef]
- Nijakowski, K.; Surdacka, A. Salivary Biomarkers for Diagnosis of Inflammatory Bowel Diseases: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 7477. [Google Scholar] [CrossRef] [PubMed]
- Nijakowski, K.; Rutkowski, R.; Eder, P.; Korybalska, K.; Witowski, J.; Surdacka, A. Changes in Salivary Parameters of Oral Immunity after Biologic Therapy for Inflammatory Bowel Disease. Life 2021, 11, 1409. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, L.; Agrawal, N.; Mathew, B.; Kääriäinen, S.; Kolho, K.-L.; Viljakainen, H. Pre-Diagnostic Saliva Microbiota of School-Aged Children Who Developed Type 1 Diabetes or Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2023, 24, 8279. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-B.; Kim, H.; Kim, S.; Kim, J.; Park, S.-K.; Lee, C.-W.; Kim, K.O.; Seo, G.-S.; Kim, M.S.; Cha, J.M.; et al. Potential Oral Microbial Markers for Differential Diagnosis of Crohn’s Disease and Ulcerative Colitis Using Machine Learning Models. Microorganisms 2023, 11, 1665. [Google Scholar] [CrossRef] [PubMed]
- Gürsoy, M.; Rautava, J.; Pussinen, P.; Kristoffersen, A.K.; Enersen, M.; Loimaranta, V.; Gürsoy, U.K. Salivary IgA and IgG Antibody Responses against Periodontitis-Associated Bacteria in Crohn’s Disease. Int. J. Mol. Sci. 2023, 24, 2385. [Google Scholar] [CrossRef]
- Nijakowski, K.; Motylewska, B.; Banasik, E.; Rutkowski, R.; Tsaryk, V.; Łuczak, J.; Korybalska, K.; Witowski, J.; Surdacka, A.; Eder, P. Treatment regimens and disease activity could alter salivary myeloperoxidase levels in patients with inflammatory bowel diseases. Pol. Arch Intern. Med. 2024, 134, 16596. [Google Scholar] [CrossRef]
- Nijakowski, K.; Jankowski, J.; Gruszczyński, D.; Surdacka, A. Salivary Alterations of Myeloperoxidase in Patients with Systemic Diseases: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 12078. [Google Scholar] [CrossRef] [PubMed]
- Nijakowski, K.; Rutkowski, R.; Eder, P.; Simon, M.; Korybalska, K.; Witowski, J.; Surdacka, A. Potential Salivary Markers for Differential Diagnosis of Crohn’s Disease and Ulcerative Colitis. Life 2021, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.P.; Nijakowski, K.; Swora-Cwynar, E.; Łuczak, J.; Czepulis, N.; Surdacka, A. Characteristics of salivary inflammation in obesity. Pol. Arch Intern. Med. 2020, 130, 297–303. [Google Scholar] [PubMed]
- Amar, S.; Zhou, Q.; Shaik-Dasthagirisaheb, Y.; Leeman, S. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc. Natl. Acad. Sci. USA 2007, 104, 20466–20471. [Google Scholar] [CrossRef]
- Goodson, J.M.; Kantarci, A.; Hartman, M.L.; Denis, G.V.; Stephens, D.; Hasturk, H.; Yaskell, T.; Vargas, J.; Wang, X.; Cugini, M.; et al. Metabolic disease risk in children by salivary biomarker analysis. PLoS ONE 2014, 9, e98799. [Google Scholar] [CrossRef]
- Hancox, T.P.M.; Skene, D.J.; Dallmann, R.; Dunn, W.B. Tick-Tock Consider the Clock: The Influence of Circadian and External Cycles on Time of Day Variation in the Human Metabolome—A Review. Metabolites 2021, 11, 328. [Google Scholar] [CrossRef]
- Feng, G.; Zhao, J.; Peng, J.; Luo, B.; Zhang, J.; Chen, L.; Xu, Z. Circadian clock-A promising scientific target in oral science. Front. Physiol. 2022, 13, 1031519. [Google Scholar] [CrossRef]
- Yennurajalingam, S.; Kang, D.-H.; Hwu, W.-J.; Padhye, N.S.; Masino, C.; Dibaj, S.S.; Liu, D.D.; Williams, J.L.; Lu, Z.; Bruera, E. Cranial Electrotherapy Stimulation for the Management of Depression, Anxiety, Sleep Disturbance, and Pain in Patients with Advanced Cancer: A Preliminary Study. J. Pain Symptom Manag. 2018, 55, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Boström, A.; Scheele, D.; Stoffel-Wagner, B.; Hönig, F.; Chaudhry, S.R.; Muhammad, S.; Hurlemann, R.; Krauss, J.K.; Lendvai, I.S.; Chakravarthy, K.; et al. Saliva molecular inflammatory profiling in female migraine patients responsive to adjunctive cervical non-invasive vagus nerve stimulation: The MOXY study. J. Transl. Med. 2019, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- LaVoy, E.C.; Palmer, C.A.; So, C.; Alfano, C.A. Bidirectional relationships between sleep and biomarkers of stress and immunity in youth. Int. J. Psychophysiol. 2020, 158, 331–339. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Kuehl, M.N.; Alman, A.C.; Burkhardt, B.R. Linking the oral microbiome and salivary cytokine abundance to circadian oscillations. Sci. Rep. 2021, 11, 2658. [Google Scholar] [CrossRef] [PubMed]
- Chai-Coetzer, C.L.; Antic, N.A.; Rowland, L.S.; Catcheside, P.G.; Esterman, A.; Reed, R.L.; Williams, H.; Dunn, S.V.; McEvoy, R.D. A simplified model of screening questionnaire and home monitoring for obstructive sleep apnoea in primary care. Thorax 2011, 66, 213–219. [Google Scholar] [CrossRef]
- Surdu, A.; Budala, D.G.; Luchian, I.; Foia, L.G.; Botnariu, G.E.; Scutariu, M.M. Using AI in Optimizing Oral and Dental Diagnoses—A Narrative Review. Diagnostics 2024, 14, 2804. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.E.; Maranian, M.J.; Spiteri, I.; Russell, R.; Ingle, S.; Luccarini, C.; Earl, H.M.; Pharoah, P.P.; Dunning, A.M.; Caldas, C. Saliva samples are a viable alternative to blood samples as a source of DNA for high throughput genotyping. BMC Med. Genom. 2012, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Iitani, K.; Kawase, G.; Toma, K.; Arakawa, T.; Dao, D.V.; Mitsubayashi, K. Mouthguard-Type Wearable Sensor for Monitoring Salivary Turbidity to Assess Oral Hygiene. Sensors 2024, 24, 1436. [Google Scholar] [CrossRef]
- Surlari, Z.; Budală, D.G.; Lupu, C.I.; Stelea, C.G.; Butnaru, O.M.; Luchian, I. Current Progress and Challenges of Using Artificial Intelligence in Clinical Dentistry—A Narrative Review. J. Clin. Med. 2023, 12, 7378. [Google Scholar] [CrossRef]
- Puchakayala, S.; Umamahesh, B.; Shien-Ping, F. Wireless accessing of salivary biomarkers based wearable electrochemical sensors: A mini-review. Electrochem. Commun. 2022, 140, 107314. [Google Scholar]
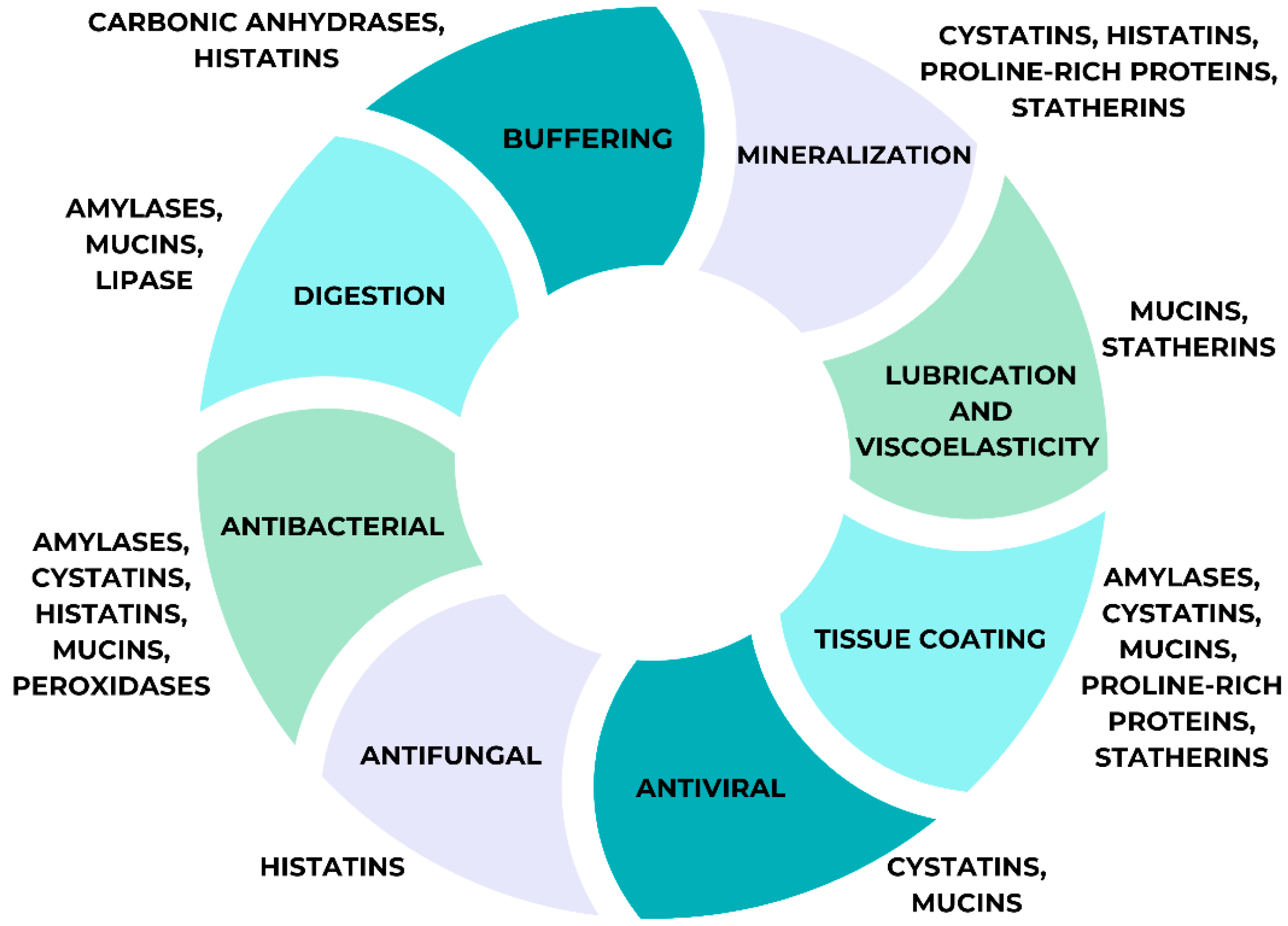
| Salivary Biomarkers | Potential Applications |
|---|---|
| DNA [1] | Standard genotyping Bacterial infection Diagnosing carcinomas of the head and neck Forensics |
| RNA [2,3] | Viral/bacterial identification carcinomas of the head and neck |
| Proteins [50] | Diagnosing carcinomas of the head and neck |
| Mucins/glycoproteins [46] | Diagnosing carcinomas of the head and neck |
| Immunoglobulins [43] | Diagnosing viruses (HIV, hepatitis B and C) |
| Drugs and their metabolites [3] | Monitoring drug abuse Detecting drugs in the body |
| Viruses, bacteria [7,8] | Epstein–Barr virus reactivation (mononucleosis) |
| Cellular material [65] | Diagnosing carcinomas of the head and neck |
| Medical Field | Role of Saliva | Applications |
|---|---|---|
| Oncology | Diagnostic tool for cancer detection and monitoring | Detection of oral, pancreatic, and gastric cancers through biomarkers like miR-31 and proteins such as CA15-3 |
| Cardiology | Risk assessment for cardiovascular diseases | Use of salivary alpha-amylase and C-reactive protein for detecting myocardial infarctions |
| Endocrinology | Monitoring hormone levels and endocrine function | Assessment of estradiol, testosterone, and melatonin levels |
| Diabetes | Biomarker source for glycemic control and disease monitoring | Salivary glucose and α-2-macroglobulin linked to diabetes and HbA1c levels |
| Neurology | Non-invasive detection of neurodegenerative disorders | Salivary trehalose and phosphorylated proteins for Alzheimer’s disease screening |
| Infectious Diseases | Identification of pathogens and immune responses | Detection of HIV, hepatitis, and COVID-19 using salivary antibodies and PCR techniques |
| Forensic Medicine | Source of biological evidence | DNA profiling, drug detection, and toxicological analyses |
| Gastroenterology | Non-invasive monitoring of gastrointestinal conditions | Use of calprotectin and lactoferrin for tracking inflammatory bowel disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surdu, A.; Foia, L.G.; Luchian, I.; Trifan, D.; Tatarciuc, M.S.; Scutariu, M.M.; Ciupilan, C.; Budala, D.G. Saliva as a Diagnostic Tool for Systemic Diseases—A Narrative Review. Medicina 2025, 61, 243. https://doi.org/10.3390/medicina61020243
Surdu A, Foia LG, Luchian I, Trifan D, Tatarciuc MS, Scutariu MM, Ciupilan C, Budala DG. Saliva as a Diagnostic Tool for Systemic Diseases—A Narrative Review. Medicina. 2025; 61(2):243. https://doi.org/10.3390/medicina61020243
Chicago/Turabian StyleSurdu, Amelia, Liliana Georgeta Foia, Ionut Luchian, Daniela Trifan, Monica Silvia Tatarciuc, Monica Mihaela Scutariu, Corina Ciupilan, and Dana Gabriela Budala. 2025. "Saliva as a Diagnostic Tool for Systemic Diseases—A Narrative Review" Medicina 61, no. 2: 243. https://doi.org/10.3390/medicina61020243
APA StyleSurdu, A., Foia, L. G., Luchian, I., Trifan, D., Tatarciuc, M. S., Scutariu, M. M., Ciupilan, C., & Budala, D. G. (2025). Saliva as a Diagnostic Tool for Systemic Diseases—A Narrative Review. Medicina, 61(2), 243. https://doi.org/10.3390/medicina61020243










