Comparing Early Intervention to Watchful Waiting: A Review on Risk Stratification and Management in Asymptomatic Aortic Stenosis
Abstract
1. Introduction
2. The Critical Role of Timely Intervention
3. Risk Stratification and Diagnosis
3.1. Echocardiography
3.2. Cardiac Computed Tomography
3.3. Cardiac Magnetic Resonance Imaging
3.4. Cardiopulmonary Stress Testing
3.5. Exercise Testing
3.6. Biomarkers
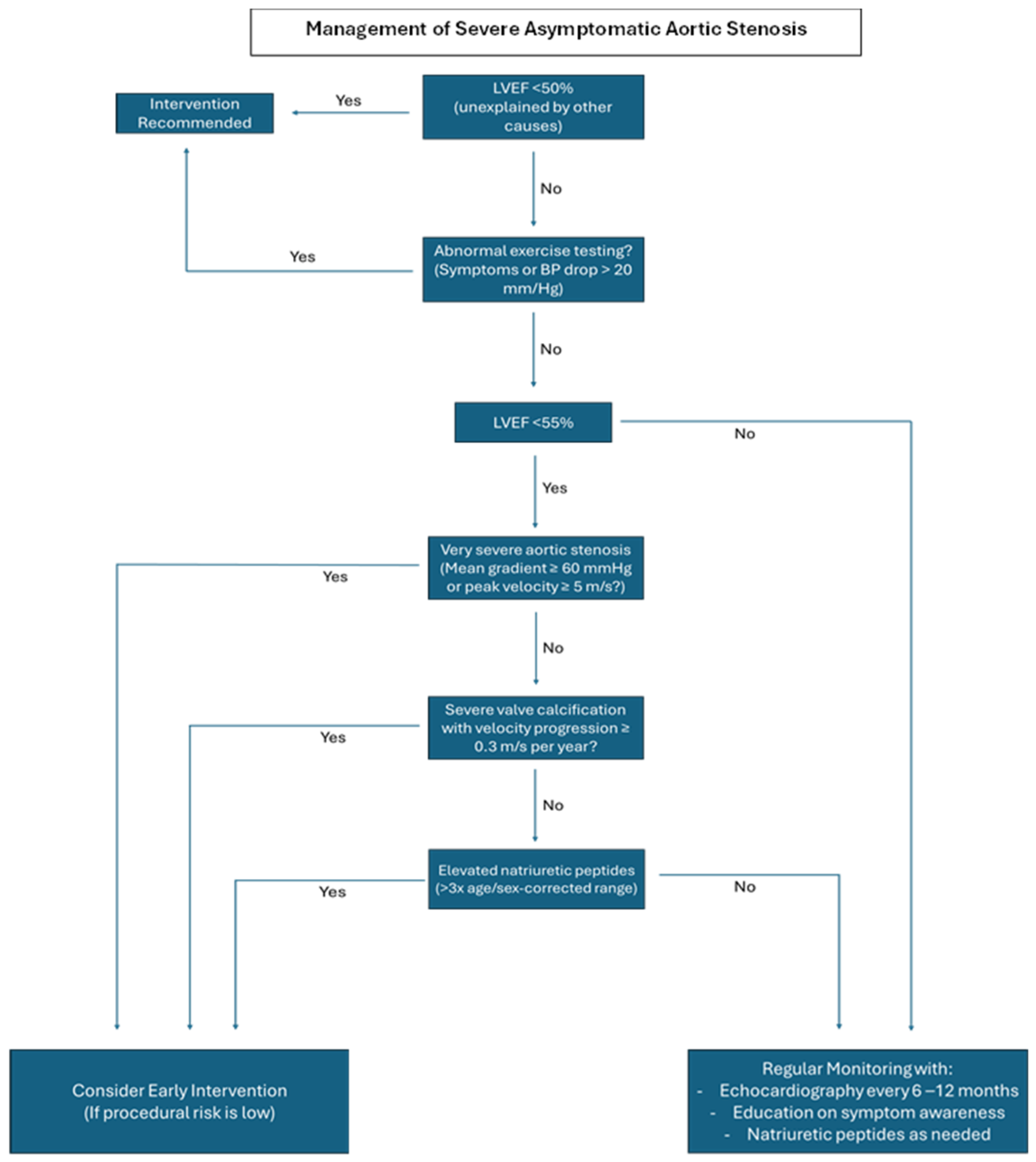
4. Watchful Waiting: Is It Sufficient?
5. Early Intervention: A Lifesaving Approach?
6. Recent Clinical Trials
6.1. The EVOLVED Randomized Clinical Trial
6.2. The EARLY TAVR Trial
7. Future Perspectives
8. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Joseph, J.; Naqvi, S.Y.; Giri, J.; Goldberg, S. Aortic Stenosis: Pathophysiology, Diagnosis, and Therapy. Am. J. Med. 2017, 130, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Carabello, B.A.; Paulus, W.J. Aortic stenosis. Lancet 2009, 373, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Massera, D.; Bartz, T.M.; Biggs, M.L.; Sotoodehnia, N.; Reiner, A.P.; Semba, R.D.; Gottdiener, J.S.; Psaty, B.M.; Owens, D.S.; Kizer, J.R. Traditional and novel risk factors for incident aortic stenosis in community-dwelling older adults. Heart 2023, 110, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.T.A.; He, G.S.; Chia, J.L.L.; Tan, G.Q.X.; Teo, Y.N.; Teo, Y.H.; Syn, N.L.; Chai, P.; Wong, R.C.C.; Yeo, T.C.; et al. Natural history of initially asymptomatic severe aortic stenosis: A one-stage meta-analysis. Clin. Res. Cardiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Bonow, R.O.; Otto, C.M. Current management of calcific aortic stenosis. Circ. Res. 2013, 113, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Grimard, B.H.; Safford, R.E.; Burns, E.L. Aortic Stenosis: Diagnosis and Treatment. Am. Fam. Physician 2016, 93, 371–378. [Google Scholar]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Banovic, M.; Putnik, S.; Da Costa, B.R.; Penicka, M.; Deja, M.A.; Kotrc, M.; Kockova, R.; Glaveckaite, S.; Gasparovic, H.; Pavlovic, N.; et al. Aortic valve replacement vs. conservative treatment in asymptomatic severe aortic stenosis: Long-term follow-up of the AVATAR trial. Eur. Heart J. 2024, 45, 4526–4535. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Sarano, M.E.; Nishimura, R.A.; Malouf, J.F.; Bailey, K.R.; Scott, C.G.; Barnes, M.E.; Tajik, A.J. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005, 111, 3290–3295. [Google Scholar] [CrossRef]
- Galper, B.Z.; Chinnakondepalli, K.M.; Wang, K.; Magnuson, E.A.; Lu, M.; Thourani, V.H.; Kodali, S.; Makkar, R.; Herrmann, H.C.; Kapadia, S.; et al. Economic Outcomes of Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Stenosis and Low Surgical Risk: Results from the PARTNER 3 Trial. Circulation 2023, 147, 1594–1605. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.J.; Wang, K.; House, J.A.; Magnuson, E.A.; Reynolds, M.R.; Makkar, R.; Herrmann, H.C.; Kodali, S.; Thourani, V.H.; Kapadia, S.; et al. Cost-Effectiveness of Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Stenosis at Intermediate Risk. Circulation 2019, 139, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Ternacle, J.; Jaber, W.A.; Salaun, E.; Dahou, A.; Asch, F.M.; Weissman, N.J.; Rodriguez, L.; Xu, K.; Annabi, M.S.; et al. Structural Deterioration of Transcatheter Versus Surgical Aortic Valve Bioprostheses in the PARTNER-2 Trial. J. Am. Coll. Cardiol. 2020, 76, 1830–1843. [Google Scholar] [CrossRef] [PubMed]
- Bismee, N.N.; Javadi, N.; Khedr, A.; Omar, F.; Awad, K.; Abbas, M.T.; Scalia, I.G.; Pereyra, M.; Bcharah, G.; Farina, J.M.; et al. Bioprosthetic Aortic Valve Degeneration After TAVR and SAVR: Incidence, Diagnosis, Predictors, and Management. J. Cardiovasc. Dev. Dis. 2024, 11, 384. [Google Scholar] [CrossRef]
- Saucke, M.C.; Jacobson, N.; Chow, S.; McKinney, G.; Neuman, H.B. Defer, Share, or Drive the Decision: Empowering Patients with Varied Preferences to Engage in Decision-making (an Analysis from Alliance A231701CD). Ann. Surg. 2025. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.; Hawken, N.; Brookes, E.; Kuehn, C.; Liden, B. Patient-centered benefit-risk analysis of transcatheter aortic valve replacement. F1000Research 2019, 8, 394. [Google Scholar] [CrossRef]
- Heen, A.F.; Lytvyn, L.; Shapiro, M.; Guyatt, G.H.; Siemieniuk, R.A.C.; Zhang, Y.; Manja, V.; Vandvik, P.O.; Agoritsas, T. Patient values and preferences on valve replacement for aortic stenosis: A systematic review. Heart 2021, 107, 1289–1295. [Google Scholar] [CrossRef]
- Ito, S.; Miranda, W.R.; Nkomo, V.T.; Boler, A.N.; Pislaru, S.V.; Pellikka, P.A.; Crusan, D.J.; Lewis, B.R.; Nishimura, R.A.; Oh, J.K. Prognostic Risk Stratification of Patients with Moderate Aortic Stenosis. J. Am. Soc. Echocardiogr. 2021, 34, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 18, 254–275. [Google Scholar] [CrossRef]
- Guzzetti, E.; Clavel, M.-A.; Pibarot, P. Importance of Flow in Risk Stratification of Aortic Stenosis. Can. J. Cardiol. 2020, 36, 27–29. [Google Scholar] [CrossRef]
- Banovic, M.; Iung, B.; Putnik, S.; Mahendiran, T.; Vanderheyden, M.; Barbato, E.; Bartunek, J. Asymptomatic Aortic Stenosis: From Risk Stratification to Treatment. Am. J. Cardiol. 2024, 218, 51–62. [Google Scholar] [CrossRef]
- Thellier, N.; Altes, A.; Appert, L.; Binda, C.; Leman, B.; Marsou, W.; Debry, N.; Joly, C.; Ennezat, P.V.; Tribouilloy, C.; et al. Prognostic Importance of Left Ventricular Global Longitudinal Strain in Patients with Severe Aortic Stenosis and Preserved Ejection Fraction. J. Am. Soc. Echocardiogr. 2020, 33, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Vollema, E.M.; Sugimoto, T.; Shen, M.; Tastet, L.; Ng, A.C.T.; Abou, R.; Marsan, N.A.; Mertens, B.; Dulgheru, R.; Lancellotti, P.; et al. Association of Left Ventricular Global Longitudinal Strain with Asymptomatic Severe Aortic Stenosis: Natural Course and Prognostic Value. JAMA Cardiol. 2018, 3, 839–847. [Google Scholar] [CrossRef]
- Chin, C.W.; Pawade, T.A.; Newby, D.E.; Dweck, M.R. Risk Stratification in Patients with Aortic Stenosis Using Novel Imaging Approaches. Circ. Cardiovasc. Imaging 2015, 8, e003421. [Google Scholar] [CrossRef]
- Pawade, T.; Sheth, T.; Guzzetti, E.; Dweck, M.R.; Clavel, M.A. Why and How to Measure Aortic Valve Calcification in Patients with Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 1835–1848. [Google Scholar] [CrossRef]
- Grodecki, K.; Warniello, M.; Spiewak, M.; Kwiecinski, J. Advanced Cardiac Imaging in the Assessment of Aortic Stenosis. J. Cardiovasc. Dev. Dis. 2023, 10, 216. [Google Scholar] [CrossRef]
- Scalia, I.G.; Farina, J.M.; Padang, R.; Jokerst, C.E.; Pereyra, M.; Mahmoud, A.K.; Naqvi, T.Z.; Chao, C.J.; Oh, J.K.; Arsanjani, R.; et al. Aortic Valve Calcium Score by Computed tomography as an Adjunct to Echocardiographic Assessment-A Review of Clinical Utility and Applications. J. Imaging 2023, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.A.; Pibarot, P.; Messika-Zeitoun, D.; Capoulade, R.; Malouf, J.; Aggarval, S.; Araoz, P.A.; Michelena, H.I.; Cueff, C.; Larose, E.; et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: Results of an international registry study. J. Am. Coll. Cardiol. 2014, 64, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Lembo, M.; Joshi, S.S.; Geers, J.; Bing, R.; Carnevale, L.; Pawade, T.A.; Doris, M.K.; Tzolos, E.; Grodecki, K.; Cadet, S.; et al. Quantitative Computed tomography Angiography for the Evaluation of Valvular Fibrocalcific Volume in Aortic Stenosis. JACC Cardiovasc. Imaging 2024, 17, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Khanal, K.; Singh, A.; Ashfaq, F.; Singh, S. Advancements in Aortic Stenosis Imaging: The Emerging Role of PET/CT and PET/MRI. J. Nucl. Med. 2024, 65, 241356. [Google Scholar]
- Barone-Rochette, G.; Piérard, S.; De Meester de Ravenstein, C.; Seldrum, S.; Melchior, J.; Maes, F.; Pouleur, A.C.; Vancraeynest, D.; Pasquet, A.; Vanoverschelde, J.L.; et al. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J. Am. Coll. Cardiol. 2014, 64, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.W.L.; Everett, R.J.; Kwiecinski, J.; Vesey, A.T.; Yeung, E.; Esson, G.; Jenkins, W.; Koo, M.; Mirsadraee, S.; White, A.C.; et al. Myocardial Fibrosis and Cardiac Decompensation in Aortic Stenosis. JACC Cardiovasc. Imaging 2017, 10, 1320–1333. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Chan, D.C.S.; Kanagala, P.; Hogrefe, K.; Kelly, D.J.; Khoo, J.P.; Sprigings, D.; Greenwood, J.P.; Abdelaty, A.M.S.E.K.; Jerosch-Herold, M.; et al. Short-term adverse remodeling progression in asymptomatic aortic stenosis. Eur. Radiol. 2021, 31, 3923–3930. [Google Scholar] [CrossRef]
- Singh, A.; Greenwood, J.P.; Berry, C.; Dawson, D.K.; Hogrefe, K.; Kelly, D.J.; Dhakshinamurthy, V.; Lang, C.C.; Khoo, J.P.; Sprigings, D.; et al. Comparison of exercise testing and CMR measured myocardial perfusion reserve for predicting outcome in asymptomatic aortic stenosis: The PRognostic Importance of MIcrovascular Dysfunction in Aortic Stenosis (PRIMID AS) Study. Eur. Heart J. 2017, 38, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ford, I.; Greenwood, J.P.; Khan, J.N.; Uddin, A.; Berry, C.; Neubauer, S.; Prendergast, B.; Jerosch-Herold, M.; Williams, B.; et al. Rationale and design of the PRognostic Importance of MIcrovascular Dysfunction in asymptomatic patients with Aortic Stenosis (PRIMID-AS): A multicentre observational study with blinded investigations. BMJ Open 2013, 3, e004348. [Google Scholar] [CrossRef]
- R Santos, R.; Paiva, M.; Gomes, D.; Presume, J.; Custodio, P.; Andrade, M.J.; Raposo, L.; Durazzo, A.; Moreno, L.; Mendes, M. Value of cardiopulmonary exercise test submaximal parameters in the assessment of aortic stenosis patients. Eur. Heart J. 2022, 43, ehac544.2457. [Google Scholar] [CrossRef]
- Le, V.D.; Jensen, G.V.; Kjøller-Hansen, L. Prognostic Usefulness of Cardiopulmonary Exercise Testing for Managing Patients with Severe Aortic Stenosis. Am. J. Cardiol. 2017, 120, 844–849. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huded, C.P.; Masri, A.; Kusunose, K.; Goodman, A.L.; Grimm, R.A.; Gillinov, A.M.; Johnston, D.R.; Rodriguez, L.L.; Popovic, Z.B.; Svensson, L.G.; et al. Outcomes in Asymptomatic Severe Aortic Stenosis with Preserved Ejection Fraction Undergoing Rest and Treadmill Stress Echocardiography. J. Am. Heart Assoc. 2018, 7, e007880. [Google Scholar] [CrossRef] [PubMed]
- Manning, W.J. Asymptomatic aortic stenosis in the elderly: A clinical review. JAMA 2013, 310, 1490–1497. [Google Scholar] [CrossRef]
- Pineda, A.M.; Kiefer, T.L. Asymptomatic Severe Aortic Valve Stenosis—When to Intervene: A Review of the Literature, Current Trials, and Guidelines. Curr. Cardiol. Rep. 2018, 20, 129. [Google Scholar] [CrossRef]
- Saeed, S.; Rajani, R.; Seifert, R.; Parkin, D.; Chambers, J.B. Exercise testing in patients with asymptomatic moderate or severe aortic stenosis. Heart 2018, 104, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.M.; Moffa, P.J.; Werner, K.E.; Ramires, J.A.F. Treatment decision in asymptomatic aortic valve stenosis: Role of exercise testing. Heart 2001, 86, 381–386. [Google Scholar] [CrossRef]
- Marcoff, L.; Gillam, L.D. Aortic Stenosis: Risk Stratification and Timing of Surgery. Curr. Cardiol. Rep. 2023, 25, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Stone, G.W.; O’Gara, P.T.; Marquis-Gravel, G.; Redfors, B.; Giustino, G.; Pibarot, P.; Bax, J.J.; Bonow, R.O.; Leon, M.B. Natural History, Diagnostic Approaches, and Therapeutic Strategies for Patients with Asymptomatic Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2016, 67, 2263–2288. [Google Scholar] [CrossRef] [PubMed]
- Henri, C.; Dulgheru, R.; Magne, J.; Caballero, L.; Laaraibi, S.; Davin, L.; Kou, S.; Voilliot, D.; Nchimi, A.; Oury, C.; et al. Impact of Serial B-Type Natriuretic Peptide Changes for Predicting Outcome in Asymptomatic Patients with Aortic Stenosis. Can. J. Cardiol. 2016, 32, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Bergler-Klein, J.; Klaar, U.; Heger, M.; Rosenhek, R.; Mundigler, G.; Gabriel, H.; Binder, T.; Pacher, R.; Maurer, G.; Baumgartner, H. Natriuretic Peptides Predict Symptom-Free Survival and Postoperative Outcome in Severe Aortic Stenosis. Circulation 2004, 109, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Biner, S.; Rafique, A.M.; Goykhman, P.; Morrissey, R.P.; Naghi, J.; Siegel, R.J. Prognostic value of E/E’ ratio in patients with unoperated severe aortic stenosis. JACC Cardiovasc. Imaging 2010, 3, 899–907. [Google Scholar] [CrossRef]
- Clavel, M.A.; Malouf, J.; Michelena, H.I.; Suri, R.M.; Jaffe, A.S.; Mahoney, D.W.; Enriquez-Sarano, M. B-type natriuretic peptide clinical activation in aortic stenosis: Impact on long-term survival. J. Am. Coll. Cardiol. 2014, 63, 2016–2025. [Google Scholar] [CrossRef]
- Sarkar, A.; Chowdhury, S.; Kumar, A.; Khan, B.; Chowdhury, S.; Gupta, R.; Hajra, A.; Aronow, W.S. Biomarkers as Prognostic Markers for Aortic Stenosis: A Review. Am. J. Cardiol. 2023, 206, 53–59. [Google Scholar] [CrossRef]
- Drăgan, A.; Mateescu, A.D. Novel Biomarkers and Advanced Cardiac Imaging in Aortic Stenosis: Old and New. Biomolecules 2023, 13, 1661. [Google Scholar] [CrossRef]
- White, M.; Baral, R.; Ryding, A.; Tsampasian, V.; Ravindrarajah, T.; Garg, P.; Koskinas, K.C.; Clark, A.; Vassiliou, V.S. Biomarkers Associated with Mortality in Aortic Stenosis: A Systematic Review and Meta-Analysis. Med. Sci. 2021, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Gada, H.; Scuffham, P.A.; Griffin, B.; Marwick, T.H. Quality-of-life implications of immediate surgery and watchful waiting in asymptomatic aortic stenosis: A decision-analytic model. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 541–548. [Google Scholar] [CrossRef] [PubMed]
- San Román, J.A.; Vilacosta, I.; Antunes, M.J.; Iung, B.; Lopez, J.; Schäfers, H.J. The ‘wait for symptoms’ strategy in asymptomatic severe aortic stenosis. Heart 2020, 106, 1792–1797. [Google Scholar] [CrossRef]
- Izumi, C. Asymptomatic severe aortic stenosis: Challenges in diagnosis and management. Heart 2016, 102, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Ennezat, P.V.; Malergue, M.C.; Le Jemtel, T.H.; Abergel, E. Watchful waiting care or early intervention in asymptomatic severe aortic stenosis: Where we are. Arch. Cardiovasc. Dis. 2021, 114, 59–72. [Google Scholar] [CrossRef]
- Ledwoch, J.; Thiele, H. Treatment of asymptomatic aortic valve stenosis: Watchful waiting or early intervention? Herz 2017, 42, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Gowda, S.N.; Khan, M.S.; Sattar, Y.; Al-Khadra, Y.; Rashid, M.; Mohamed, M.O.; Alkhouli, M.; Kapadia, S.; Bagur, R.; et al. Early intervention or watchful waiting for asymptomatic severe aortic valve stenosis: A systematic review and meta-analysis. J. Cardiovasc. Med. 2020, 21, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Takagi, H.; Kuno, T. Early surgery versus conservative management of asymptomatic severe aortic stenosis: A meta-analysis. J. Thorac. Cardiovasc. Surg. 2022, 163, 1778–1785.e1775. [Google Scholar] [CrossRef]
- Costa, G.N.F.; Cardoso, J.F.L.; Oliveiros, B.; Gonçalves, L.; Teixeira, R. Early surgical intervention versus conservative management of asymptomatic severe aortic stenosis: A systematic review and meta-analysis. Heart 2023, 109, 314–321. [Google Scholar] [CrossRef]
- Ahmad, Y.; Howard, J.P.; Seligman, H.; Arnold, A.D.; Madhavan, M.V.; Forrest, J.K.; Geirsson, A.; Mack, M.J.; Lansky, A.J.; Leon, M.B. Early Surgery for Patients with Asymptomatic Severe Aortic Stenosis: A Meta-Analysis of Randomized Controlled Trials. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100383. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Park, S.J.; Lee, S.A.; Lee, S.; Kim, D.H.; Kim, H.K.; Yun, S.C.; Hong, G.R.; Song, J.M.; Chung, C.H.; et al. Early Surgery or Conservative Care for Asymptomatic Aortic Stenosis. N. Engl. J. Med. 2020, 382, 111–119. [Google Scholar] [CrossRef]
- Loganath, K.; Craig, N.J.; Everett, R.J.; Bing, R.; Tsampasian, V.; Molek, P.; Botezatu, S.; Aslam, S.; Lewis, S.; Graham, C.; et al. Early Intervention in Patients with Asymptomatic Severe Aortic Stenosis and Myocardial Fibrosis: The EVOLVED Randomized Clinical Trial. JAMA 2024, 333, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Schwartz, A.; Oldemeyer, J.B.; Pibarot, P.; Cohen, D.J.; Blanke, P.; Lindman, B.R.; Babaliaros, V.; Fearon, W.F.; Daniels, D.V.; et al. Transcatheter Aortic-Valve Replacement for Asymptomatic Severe Aortic Stenosis. N. Engl. J. Med. 2024, 392, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.; Gilbert, T.; Aslam, S.; Brookes, C.L.; Singh, A.; Newby, D.E.; Dweck, M.R.; Stewart, R.A.H.; Myles, P.S.; Briffa, T.; et al. Rationale and design of the early valve replacement in severe asymptomatic aortic stenosis trial. Am. Heart J. 2024, 275, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, M.; Tastet, L.; Pelletier, S.; Leclercq, M.; Ohl, L.; Hermann, L.; Mattei, P.A.; Precioso, F.; Coté, N.; Pibarot, P.; et al. AI-Enhanced Prediction of Aortic Stenosis Progression: Insights from the PROGRESSA Study. JACC Adv. 2024, 3, 101234. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khedr, A.E.; Odeh, N.B.; Bcharah, G.; Abdalla, H.M.; Senjab, A.; Zeineddine, R.M.; Ram, J.; Farina, J.M.; Crystal, O.R.; Barrus, B.; et al. Comparing Early Intervention to Watchful Waiting: A Review on Risk Stratification and Management in Asymptomatic Aortic Stenosis. Medicina 2025, 61, 448. https://doi.org/10.3390/medicina61030448
Khedr AE, Odeh NB, Bcharah G, Abdalla HM, Senjab A, Zeineddine RM, Ram J, Farina JM, Crystal OR, Barrus B, et al. Comparing Early Intervention to Watchful Waiting: A Review on Risk Stratification and Management in Asymptomatic Aortic Stenosis. Medicina. 2025; 61(3):448. https://doi.org/10.3390/medicina61030448
Chicago/Turabian StyleKhedr, Ahmed E., Nour B. Odeh, George Bcharah, Hesham M. Abdalla, Abdulrahman Senjab, Rawan M. Zeineddine, Jaikrishnan Ram, Juan M. Farina, Owen R. Crystal, Bryan Barrus, and et al. 2025. "Comparing Early Intervention to Watchful Waiting: A Review on Risk Stratification and Management in Asymptomatic Aortic Stenosis" Medicina 61, no. 3: 448. https://doi.org/10.3390/medicina61030448
APA StyleKhedr, A. E., Odeh, N. B., Bcharah, G., Abdalla, H. M., Senjab, A., Zeineddine, R. M., Ram, J., Farina, J. M., Crystal, O. R., Barrus, B., Lester, S. J., Shipman, J., Alsidawi, S., Ayoub, C., Sell-Dottin, K. A., & Arsanjani, R. (2025). Comparing Early Intervention to Watchful Waiting: A Review on Risk Stratification and Management in Asymptomatic Aortic Stenosis. Medicina, 61(3), 448. https://doi.org/10.3390/medicina61030448




