Comprehensive Insights into Sarcopenia in Dialysis Patients: Mechanisms, Assessment, and Therapeutic Approaches
Abstract
:1. Introduction
2. Epidemiology of Sarcopenia in Dialysis Patients
2.1. General Epidemiology
2.2. Dialysis Modality
2.3. Impact of Gender
2.4. Regional and Demographic Differences
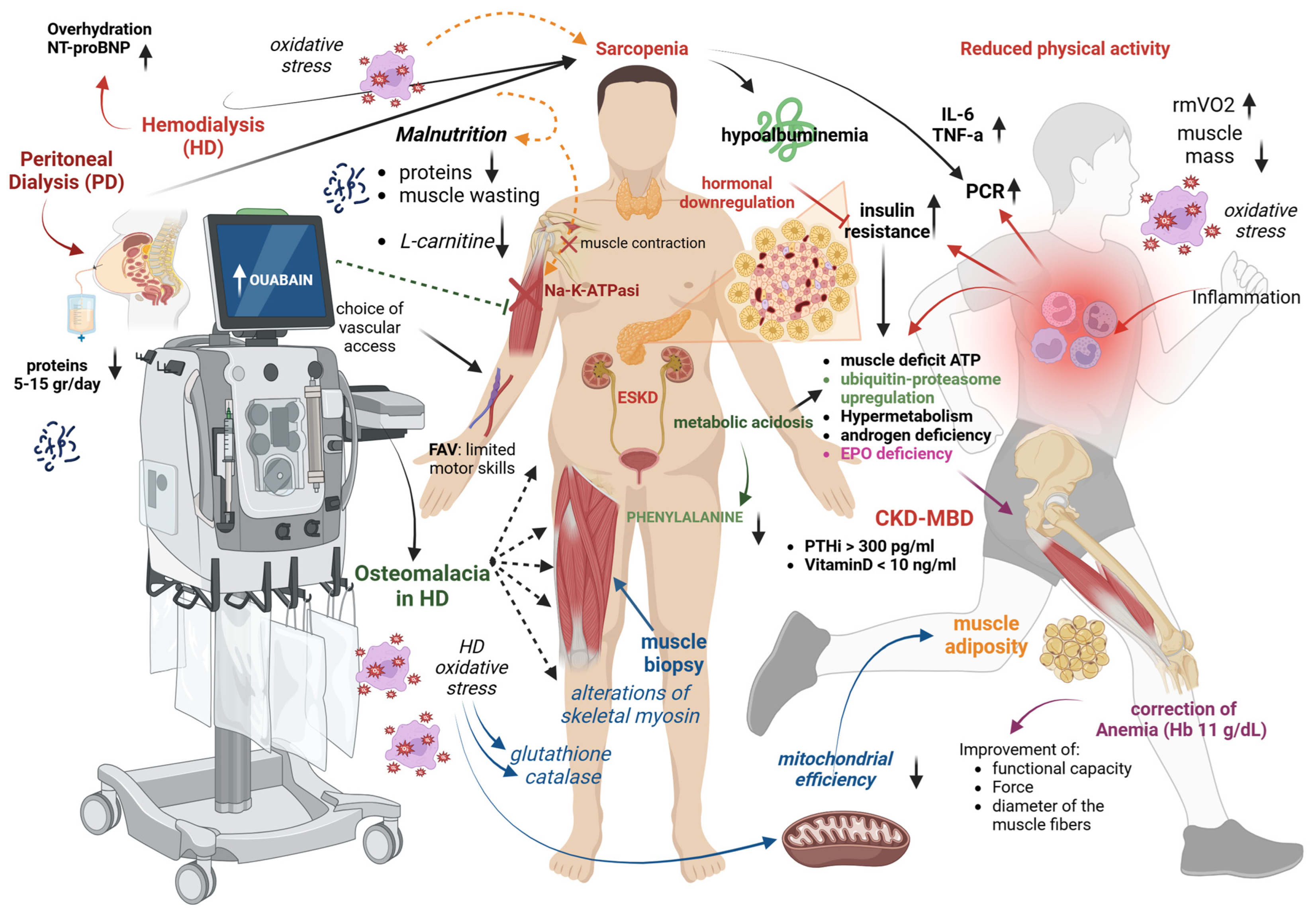
3. Risk Factors and Pathogenesis of Sarcopenia in Dialysis Patients
3.1. Reduced Physical Activity
3.2. Inflammation
3.3. Fluid Overload and Dialysis Prescription
3.4. Malnutrition and Protein Loss
3.5. L-Carnitine Deficit
3.6. Altered Mineral Metabolism
3.7. Uremic Metabolic Alterations
3.8. Metabolic Acidosis
3.9. Oxidative Stress
3.10. Mitochondrial Dysfunction
3.11. Endocrine Alterations
3.12. Other Factors
4. Clinical Consequences of Sarcopenia in Dialysis Patients
4.1. Physical Complications
4.2. Reduced Quality of Life
4.3. Frailty and Risk of Falls
4.4. Increased Hospitalization Rate
4.5. Mortality and Cardiovascular Risk
5. Methods for Assessing Sarcopenia in Dialysis Patients
5.1. Anthropometric Measurements
5.2. Measurement of Skeletal Muscle Mass
5.3. Measurement of Muscle Strength and Physical Performance
5.4. Sarcopenia Screening Tools
5.5. Surrogate Biomarkers of Sarcopenia
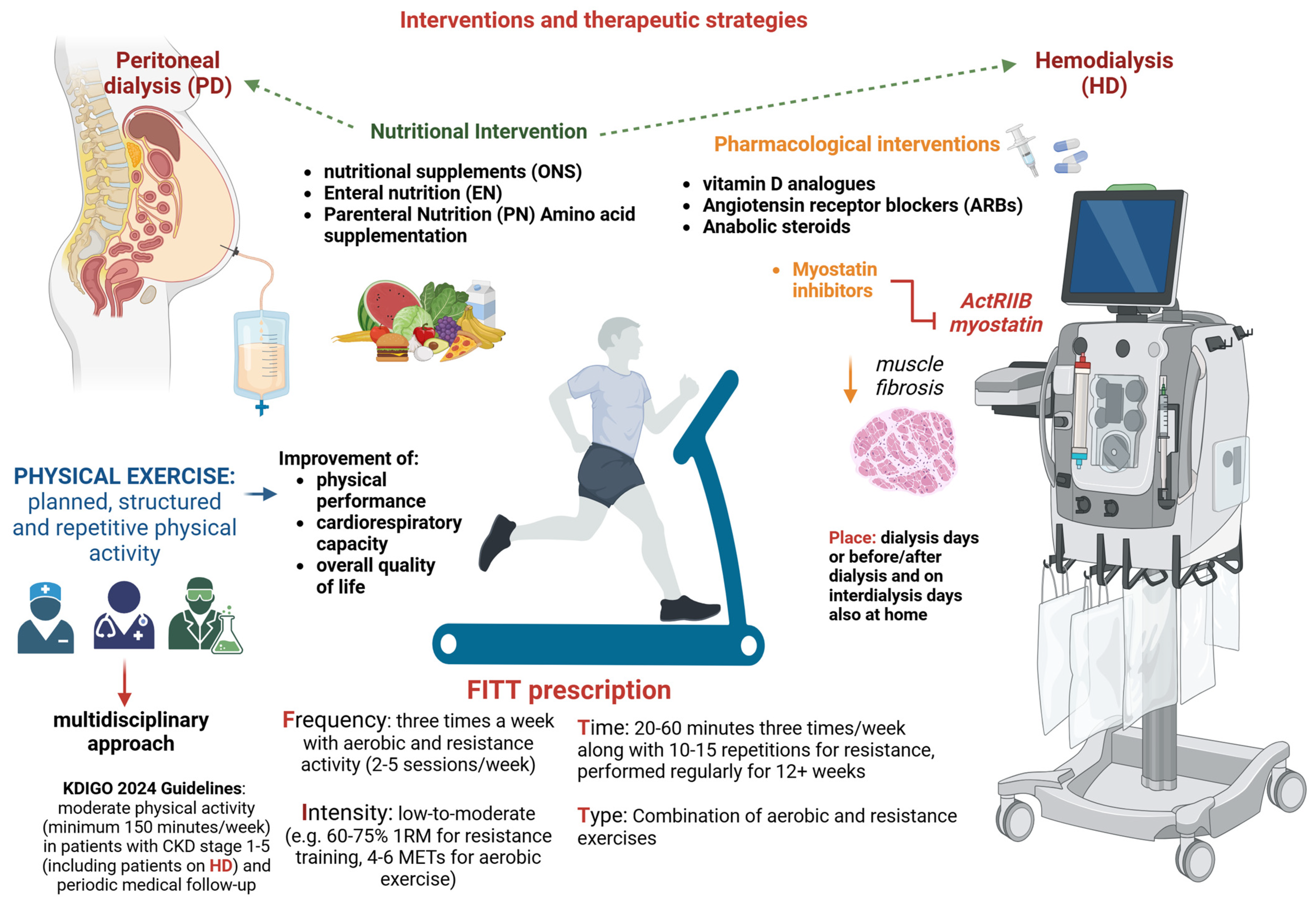
6. Interventions and Therapeutic Strategies
6.1. Physical Exercise
6.2. Nutritional Intervention
6.3. Pharmacological Interventions
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jadoul, M.; Aoun, M.; Masimango Imani, M. The major global burden of chronic kidney disease. Lancet Glob. Health 2024, 12, e342–e343. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Bohm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- van Oevelen, M.; Bonenkamp, A.A.; van Eck van der Sluijs, A.; Bos, W.J.W.; Douma, C.E.; van Buren, M.; Meuleman, Y.; Dekker, F.W.; van Jaarsveld, B.C.; Abrahams, A.C.; et al. Health-related quality of life and symptom burden in patients on haemodialysis. Nephrol. Dial. Transplant. 2024, 39, 436–444. [Google Scholar] [CrossRef]
- Vanden Wyngaert, K.; Van Craenenbroeck, A.H.; Eloot, S.; Calders, P.; Celie, B.; Holvoet, E.; Van Biesen, W. Associations between the measures of physical function, risk of falls and the quality of life in haemodialysis patients: A cross-sectional study. BMC Nephrol. 2020, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Cawthon, P.M.; Arai, H.; Avila-Funes, J.A.; Barazzoni, R.; Bhasin, S.; Binder, E.F.; Bruyere, O.; Cederholm, T.; Chen, L.K.; et al. The Conceptual Definition of Sarcopenia: Delphi Consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing 2024, 53, afae052. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Cooper, R.; Arai, H.; Cawthon, P.M.; Ntsama Essomba, M.J.; Fielding, R.A.; Grounds, M.D.; Witham, M.D.; Cruz-Jentoft, A.J. Sarcopenia. Nat. Rev. Dis. Primers 2024, 10, 68. [Google Scholar] [CrossRef]
- Shu, X.; Lin, T.; Wang, H.; Zhao, Y.; Jiang, T.; Peng, X.; Yue, J. Diagnosis, prevalence, and mortality of sarcopenia in dialysis patients: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 145–158. [Google Scholar] [CrossRef]
- Ertuglu, L.; Ikizler, T.A. Nutrition Management in Geriatric Patients with CKD. Kidney360 2024, 5, 310–319. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Isoyama, N.; Qureshi, A.R.; Avesani, C.M.; Lindholm, B.; Bàràny, P.; Heimbürger, O.; Cederholm, T.; Stenvinkel, P.; Carrero, J.J. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1720–1728. [Google Scholar] [CrossRef]
- Lamarca, F.; Carrero, J.J.; Rodrigues, J.C.; Bigogno, F.G.; Fetter, R.L.; Avesani, C.M. Prevalence of sarcopenia in elderly maintenance hemodialysis patients: The impact of different diagnostic criteria. J. Nutr. Health Aging 2014, 18, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Ter Beek, L.; Vanhauwaert, E.; Slinde, F.; Orrevall, Y.; Henriksen, C.; Johansson, M.; Vereecken, C.; Rothenberg, E.; Jager-Wittenaar, H. Unsatisfactory knowledge and use of terminology regarding malnutrition, starvation, cachexia and sarcopenia among dietitians. Clin. Nutr. 2016, 35, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Johansen, K.L.; Lindholm, B.; Stenvinkel, P.; Cuppari, L.; Avesani, C.M. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016, 90, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Wathanavasin, W.; Banjongjit, A.; Avihingsanon, Y.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S.; Susantitaphong, P. Prevalence of Sarcopenia and Its Impact on Cardiovascular Events and Mortality among Dialysis Patients: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4077. [Google Scholar] [CrossRef]
- Duarte, M.P.; Almeida, L.S.; Neri, S.G.R.; Oliveira, J.S.; Wilkinson, T.J.; Ribeiro, H.S.; Lima, R.M. Prevalence of sarcopenia in patients with chronic kidney disease: A global systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 501–512. [Google Scholar] [CrossRef]
- Duarte, M.P.; Nobrega, O.T.; Baiao, V.M.; Vieira, F.A.; Monteiro, J.S.; Pereira, M.S.; Pires, L.F.; Queiroz, G.G.; Silva, M.J.; Silva, M.Z.C.; et al. Agreement between the EWGSOP2 and SDOC consensuses for sarcopenia in patients receiving hemodialysis: Findings of a cross sectional analysis from the SARC-HD study. Nutr. Clin. Pract. 2024, 39, 1441–1451. [Google Scholar] [CrossRef]
- Hung, R.; Wong, B.; Goldet, G.; Davenport, A. Differences in Prevalence of Muscle Wasting in Patients Receiving Peritoneal Dialysis per Dual-Energy X-Ray Absorptiometry Due to Variation in Guideline Definitions of Sarcopenia. Nutr. Clin. Pract. 2017, 32, 539–544. [Google Scholar] [CrossRef]
- Yoowannakul, S.; Tangvoraphonkchai, K.; Vongsanim, S.; Mohamed, A.; Davenport, A. Differences in the prevalence of sarcopenia in haemodialysis patients: The effects of gender and ethnicity. J. Hum. Nutr. Diet. 2018, 31, 689–696. [Google Scholar] [CrossRef]
- Yoowannakul, S.; Tangvoraphonkchai, K.; Davenport, A. The prevalence of muscle wasting (sarcopenia) in peritoneal dialysis patients varies with ethnicity due to differences in muscle mass measured by bioimpedance. Eur. J. Clin. Nutr. 2018, 72, 381–387. [Google Scholar] [CrossRef]
- Ren, H.; Gong, D.; Jia, F.; Xu, B.; Liu, Z. Sarcopenia in patients undergoing maintenance hemodialysis: Incidence rate, risk factors and its effect on survival risk. Ren. Fail. 2016, 38, 364–371. [Google Scholar] [CrossRef]
- Kelly, T.L.; Wilson, K.E.; Heymsfield, S.B. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009, 4, e7038. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.; Hussain Sayed, R.; Fan, S. The effect of racial origin on total body water volume in peritoneal dialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2492–2498. [Google Scholar] [CrossRef]
- Lee, S.W.; Song, J.H.; Kim, G.A.; Lee, K.J.; Kim, M.J. Assessment of total body water from anthropometry-based equations using bioelectrical impedance as reference in Korean adult control and haemodialysis subjects. Nephrol. Dial. Transplant. 2001, 16, 91–97. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Crystal, F.; Fulai, R.; Kaonga, P.; Davenport, A. Malnutrition, protein energy wasting and sarcopenia in patients attending a haemodialysis centre in sub-Saharan Africa. Eur. J. Clin. Nutr. 2024, 78, 818–822. [Google Scholar] [CrossRef]
- Aucella, F.; Battaglia, Y.; Bellizzi, V.; Bolignano, D.; Capitanini, A.; Cupisti, A. Physical exercise programs in CKD: Lights, shades and perspectives. J. Nephrol. 2015, 28, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Torino, C.; Manfredini, F.; Bolignano, D.; Aucella, F.; Baggetta, R.; Barilla, A.; Battaglia, Y.; Bertoli, S.; Bonanno, G.; Castellino, P.; et al. Physical performance and clinical outcomes in dialysis patients: A secondary analysis of the EXCITE trial. Kidney Blood Press. Res. 2014, 39, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Musolino, M.; Presta, P.; Cianfrone, P.; Errante, M.A.; Andreucci, M.; Coppolino, G.; Bolignano, D. Self-Reported Physical Inactivity and Mood Disturbances in End-Stage Kidney Disease (ESKD) Patients on Chronic Dialysis Treatment. J. Clin. Med. 2023, 12, 7160. [Google Scholar] [CrossRef]
- Manfredini, F.; Lamberti, N.; Malagoni, A.M.; Felisatti, M.; Zuccala, A.; Torino, C.; Tripepi, G.; Catizone, L.; Mallamaci, F.; Zoccali, C. The role of deconditioning in the end-stage renal disease myopathy: Physical exercise improves altered resting muscle oxygen consumption. Am. J. Nephrol. 2015, 41, 329–336. [Google Scholar] [CrossRef]
- Deger, S.M.; Hung, A.M.; Gamboa, J.L.; Siew, E.D.; Ellis, C.D.; Booker, C.; Sha, F.; Li, H.; Bian, A.; Stewart, T.G.; et al. Systemic inflammation is associated with exaggerated skeletal muscle protein catabolism in maintenance hemodialysis patients. JCI Insight 2017, 2, e95185. [Google Scholar] [CrossRef]
- Visser, W.J.; Egmond, A.; Timman, R.; Severs, D.; Hoorn, E.J. Risk Factors for Muscle Loss in Hemodialysis Patients with High Comorbidity. Nutrients 2020, 12, 2494. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kopple, J.D. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am. J. Kidney Dis. 2001, 38, 1343–1350. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Dubin, J.A.; Muller, H.G.; Mitch, W.E.; Rosales, L.M.; Levin, N.W. Relationships among inflammation nutrition and physiologic mechanisms establishing albumin levels in hemodialysis patients. Kidney Int. 2002, 61, 2240–2249. [Google Scholar] [CrossRef]
- Kaizu, Y.; Kimura, M.; Yoneyama, T.; Miyaji, K.; Hibi, I.; Kumagai, H. Interleukin-6 may mediate malnutrition in chronic hemodialysis patients. Am. J. Kidney Dis. 1998, 31, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Hortegal, E.V.F.; Alves, J.; Santos, E.J.F.; Nunes, L.C.R.; Galvao, J.C.; Nunes, R.F.; Lula, D.A.; Carvalho, S.C.R.; Franca, A.; Santos, E.M.D.; et al. Sarcopenia and inflammation in patients undergoing hemodialysis. Nutr. Hosp. 2020, 37, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.G.; Wang, C.H.; Tsai, J.P.; Chen, Y.H.; Hung, S.C.; Lin, Y.L. Association of serum intact parathyroid hormone levels with sarcopenia in patients undergoing peritoneal dialysis. Front. Med. 2024, 11, 1487449. [Google Scholar] [CrossRef]
- Kang, S.H.; Do, J.Y. Effects of volume status on body composition in incident peritoneal dialysis patients. Eur. J. Clin. Nutr. 2020, 74, 633–641. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Q.; Ni, L.; Zhang, M.; Wang, M.; Zhang, W.; Chen, J. Risk Factors Affecting Muscle Mass Decline in Maintenance Hemodialysis Patients. BioMed Res. Int. 2022, 2022, 2925216. [Google Scholar] [CrossRef]
- Ikeda, M.; Honda, H.; Takahashi, K.; Shishido, K.; Shibata, T. N-Terminal Pro-B-Type Natriuretic Peptide as a Biomarker for Loss of Muscle Mass in Prevalent Hemodialysis Patients. PLoS ONE 2016, 11, e0166804. [Google Scholar] [CrossRef]
- Wang, M.; Liu, L.; Shen, X.; Li, Y.; He, Q. Assessing lean tissue by bioelectrical impedance analysis pre hemodialysis underestimates the prevalence of sarcopenia in maintenance hemodialysis patients. Eur. J. Clin. Nutr. 2021, 75, 1407–1413. [Google Scholar] [CrossRef]
- Molina, P.; Vizcaino, B.; Molina, M.D.; Beltran, S.; Gonzalez-Moya, M.; Mora, A.; Castro-Alonso, C.; Kanter, J.; Avila, A.I.; Gorriz, J.L.; et al. The effect of high-volume online haemodiafiltration on nutritional status and body composition: The ProtEin Stores prEservaTion (PESET) study. Nephrol. Dial. Transplant. 2018, 33, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Flakoll, P.J.; Parker, R.A.; Hakim, R.M. Amino acid and albumin losses during hemodialysis. Kidney Int. 1994, 46, 830–837. [Google Scholar] [CrossRef]
- Dulaney, J.T.; Hatch, F.E., Jr. Peritoneal dialysis and loss of proteins: A review. Kidney Int. 1984, 26, 253–262. [Google Scholar] [CrossRef]
- Do, J.Y.; Kim, A.Y.; Kang, S.H. Peritoneal Protein Loss Is Not Associated with Sarcopenia in Peritoneal Dialysis Patients. Front. Med. 2021, 8, 653807. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Diao, Z.; Huang, H.; Li, Z.; Yang, R.; Liu, W. Disturbed carnitine metabolism is independently correlated with sarcopenia and prognosis in patients on hemodialysis. Clin. Nutr. 2024, 43, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Takashima, H.; Maruyama, T.; Abe, M. Significance of Levocarnitine Treatment in Dialysis Patients. Nutrients 2021, 13, 1219. [Google Scholar] [CrossRef]
- Siami, G.; Clinton, M.E.; Mrak, R.; Griffis, J.; Stone, W. Evaluation of the effect of intravenous L-carnitine therapy on function, structure and fatty acid metabolism of skeletal muscle in patients receiving chronic hemodialysis. Nephron 1991, 57, 306–313. [Google Scholar] [CrossRef]
- Vaux, E.C.; Taylor, D.J.; Altmann, P.; Rajagopalan, B.; Graham, K.; Cooper, R.; Bonomo, Y.; Styles, P. Effects of carnitine supplementation on muscle metabolism by the use of magnetic resonance spectroscopy and near-infrared spectroscopy in end-stage renal disease. Nephron Clin. Pract. 2004, 97, c41–c48. [Google Scholar] [CrossRef]
- Abboud, M.; Rybchyn, M.S.; Liu, J.; Ning, Y.; Gordon-Thomson, C.; Brennan-Speranza, T.C.; Cole, L.; Greenfield, H.; Fraser, D.R.; Mason, R.S. The effect of parathyroid hormone on the uptake and retention of 25-hydroxyvitamin D in skeletal muscle cells. J. Steroid Biochem. Mol. Biol. 2017, 173, 173–179. [Google Scholar] [CrossRef]
- Visser, M.; Deeg, D.J.; Lips, P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Q.; Zhu, B.; Zhou, F. Relation of Serum 25-Hydroxyvitamin D Status with Skeletal Muscle Mass and Grip Strength in Patients on Peritoneal Dialysis. J. Nutr. Sci. Vitaminol. 2019, 65, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Takahashi, H.; Kondo, C.; Hayashi, F.; Tokoroyama, S.; Mori, Y.; Tsujita, M.; Shirasawa, Y.; Takeda, A.; Morozumi, K.; et al. Association between Serum 25-Hydroxyvitamin D Levels and Sarcopenia in Patients Undergoing Chronic Haemodialysis. Am. J. Nephrol. 2024, 55, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.-J.; Wang, G.-R.; Xie, R.-N.; Jiang, X.; Li, C.-X.; Nie, Z.-M.; Li, T. Risk Prediction Models for Sarcopenia in Dialysis Patients: A Systematic Review. J. Ren. Nutr. 2024, 35, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Riche, K.D.; Arnall, J.; Rieser, K.; East, H.E.; Riche, D.M. Impact of vitamin D status on statin-induced myopathy. J. Clin. Transl. Endocrinol. 2016, 6, 56–59. [Google Scholar] [CrossRef]
- Prabhala, A.; Garg, R.; Dandona, P. Severe myopathy associated with vitamin D deficiency in western New York. Arch. Intern. Med. 2000, 160, 1199–1203. [Google Scholar] [CrossRef]
- Baker, L.R.; Ackrill, P.; Cattell, W.R.; Stamp, T.C.; Watson, L. Iatrogenic osteomalacia and myopathy due to phosphate depletion. Br. Med. J. 1974, 3, 150–152. [Google Scholar] [CrossRef]
- Zahed, N.; Chehrazi, S.; Falaknasi, K. The evaluation of relationship between vitamin D and muscle power by micro manual muscle tester in end-stage renal disease patients. Saudi J. Kidney Dis. Transplant. 2014, 25, 998–1003. [Google Scholar] [CrossRef]
- Schott, G.D.; Wills, M.R. Muscle weakness in osteomalacia. Lancet 1976, 1, 626–629. [Google Scholar] [CrossRef]
- Patten, B.M.; Bilezikian, J.P.; Mallette, L.E.; Prince, A.; Engel, W.K.; Aurbach, G.D. Neuromuscular disease in primary hyperparathyroidism. Ann. Intern. Med. 1974, 80, 182–193. [Google Scholar] [CrossRef]
- Mallette, L.E.; Patten, B.M.; Engel, W.K. Neuromuscular disease in secondary hyperparathyroidism. Ann. Intern. Med. 1975, 82, 474–483. [Google Scholar] [CrossRef]
- Delbridge, L.W.; Marshman, D.; Reeve, T.S.; Crummer, P.; Posen, S. Neuromuscular symptoms in elderly patients with hyperparathyroidism: Improvement with parathyroid surgery. Med. J. Aust. 1988, 149, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Smogorzewski, M.; Piskorska, G.; Borum, P.R.; Massry, S.G. Chronic renal failure, parathyroid hormone and fatty acids oxidation in skeletal muscle. Kidney Int. 1988, 33, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Campistol, J.M. Uremic myopathy. Kidney Int. 2002, 62, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.H.; Kemp, G.J.; Taylor, D.J.; Ledingham, J.G.; Radda, G.K.; Rajagopalan, B. Effect of chronic uraemia on skeletal muscle metabolism in man. Nephrol. Dial. Transplant. 1993, 8, 218–222. [Google Scholar]
- Giovannetti, S.; Biagini, M.; Balestri, P.L.; Navalesi, R.; Giagnoni, P.; De Matteis, A.; Ferro-Milone, P.; Perfetti, C. Uraemia-like syndrome in dogs chronically intoxicated with methylguanidine and creatinine. Clin. Sci. 1969, 36, 445–452. [Google Scholar]
- Bennett, S.E.; Bevington, A.; Walls, J. Regulation of intracellular creatine in erythrocytes and myoblasts: Influence of uraemia and inhibition of Na, K-ATPase. Cell Biochem. Funct. 1994, 12, 99–106. [Google Scholar] [CrossRef]
- Sohn, H.J.; Stokes, G.S.; Johnston, H. An Na, K ATPase inhibitor from ultrafiltrate obtained by hemodialysis of patients with uremia. J. Lab. Clin. Med. 1992, 120, 264–271. [Google Scholar]
- Kittiskulnam, P.; Srijaruneruang, S.; Chulakadabba, A.; Thokanit, N.S.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S. Impact of Serum Bicarbonate Levels on Muscle Mass and Kidney Function in Pre-Dialysis Chronic Kidney Disease Patients. Am. J. Nephrol. 2020, 51, 24–34. [Google Scholar] [CrossRef]
- Visser, W.J.; van de Braak, E.E.M.; de Mik-van Egmond, A.M.E.; van der Burgh, A.C.; de Roos, N.M.; Jans, I.; van der Hoef, I.; Olieman, J.F.; Hoorn, E.J.; Severs, D. Effects of correcting metabolic acidosis on muscle mass and functionality in chronic kidney disease: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 2498–2508. [Google Scholar] [CrossRef]
- Mitch, W.E.; Du, J.; Bailey, J.L.; Price, S.R. Mechanisms causing muscle proteolysis in uremia: The influence of insulin and cytokines. Miner. Electrolyte Metab. 1999, 25, 216–219. [Google Scholar] [CrossRef]
- Garibotto, G.; Sofia, A.; Russo, R.; Paoletti, E.; Bonanni, A.; Parodi, E.L.; Viazzi, F.; Verzola, D. Insulin sensitivity of muscle protein metabolism is altered in patients with chronic kidney disease and metabolic acidosis. Kidney Int. 2015, 88, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Levin, N.W.; Delmez, J.; Depner, T.A.; Ornt, D.; Owen, W.; Yan, G. Association of acidosis and nutritional parameters in hemodialysis patients. Am. J. Kidney Dis. 1999, 34, 493–499. [Google Scholar] [CrossRef]
- Lofberg, E.; Gutierrez, A.; Anderstam, B.; Wernerman, J.; Bergstrom, J.; Price, S.R.; Mitch, W.E.; Alvestrand, A. Effect of bicarbonate on muscle protein in patients receiving hemodialysis. Am. J. Kidney Dis. 2006, 48, 419–429. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Zarogiannis, S.; Eleftheriadis, T.; Mertens, P.R. Oxidative stress in hemodialysis: Causative mechanisms, clinical implications, and possible therapeutic interventions. Semin. Dial. 2019, 32, 58–71. [Google Scholar] [CrossRef]
- Kaltsatou, A.; Sakkas, G.K.; Poulianiti, K.P.; Koutedakis, Y.; Tepetes, K.; Christodoulidis, G.; Stefanidis, I.; Karatzaferi, C. Uremic myopathy: Is oxidative stress implicated in muscle dysfunction in uremia? Front. Physiol. 2015, 6, 102. [Google Scholar] [CrossRef] [PubMed]
- Moylan, J.S.; Reid, M.B. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 2007, 35, 411–429. [Google Scholar] [CrossRef]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed]
- Lamb, G.D.; Westerblad, H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J. Physiol. 2011, 589, 2119–2127. [Google Scholar] [CrossRef]
- Taes, Y.E.; Speeckaert, M.; Bauwens, E.; De Buyzere, M.R.; Libbrecht, J.; Lameire, N.H.; Delanghe, J.R. Effect of dietary creatine on skeletal muscle myosin heavy chain isoform expression in an animal model of uremia. Nephron Exp. Nephrol. 2004, 96, e103–e110. [Google Scholar] [CrossRef]
- Lim, P.S.; Cheng, Y.M.; Wei, Y.H. Increase in oxidative damage to lipids and proteins in skeletal muscle of uremic patients. Free Radic. Res. 2002, 36, 295–301. [Google Scholar] [CrossRef]
- Crowe, A.V.; McArdle, A.; McArdle, F.; Pattwell, D.M.; Bell, G.M.; Kemp, G.J.; Bone, J.M.; Griffiths, R.D.; Jackson, M.J. Markers of oxidative stress in the skeletal muscle of patients on haemodialysis. Nephrol. Dial. Transplant. 2007, 22, 1177–1183. [Google Scholar] [CrossRef]
- Ryan, A.S. Role of Skeletal Muscle Mitochondrial Dysfunction in CKD. Clin. J. Am. Soc. Nephrol. 2020, 15, 912–913. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, B.; Kestenbaum, B.; Gamboa, J.; Jubrias, S.A.; Ayers, E.; Curtin, L.; Himmelfarb, J.; de Boer, I.H.; Conley, K.E. CKD and Muscle Mitochondrial Energetics. Am. J. Kidney Dis. 2016, 68, 658–659. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Gamboa, J.L.; Billings, F.T., IV; Bojanowski, M.T.; Gilliam, L.A.; Yu, C.; Roshanravan, B.; Roberts, L.J., 2nd; Himmelfarb, J.; Ikizler, T.A.; Brown, N.J. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol. Rep. 2016, 4, e12780. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, J.L.; Roshanravan, B.; Towse, T.; Keller, C.A.; Falck, A.M.; Yu, C.; Frontera, W.R.; Brown, N.J.; Ikizler, T.A. Skeletal Muscle Mitochondrial Dysfunction Is Present in Patients with CKD Before Initiation of Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2020, 15, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Siew, E.D.; Pupim, L.B.; Majchrzak, K.M.; Shintani, A.; Flakoll, P.J.; Ikizler, T.A. Insulin resistance is associated with skeletal muscle protein breakdown in non-diabetic chronic hemodialysis patients. Kidney Int. 2007, 71, 146–152. [Google Scholar] [CrossRef]
- Johansen, K.L.; Mulligan, K.; Schambelan, M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: A randomized controlled trial. JAMA 1999, 281, 1275–1281. [Google Scholar] [CrossRef]
- Cattran, D.C.; Fenton, S.S.; Wilson, D.R.; Oreopoulos, D.; Shimizu, A.; Richardson, R.M. A controlled trial of nondrolone decanoate in the treatment of uremic anemia. Kidney Int. 1977, 12, 430–437. [Google Scholar] [CrossRef]
- Chiang, J.M.; Kaysen, G.A.; Segal, M.; Chertow, G.M.; Delgado, C.; Johansen, K.L. Low testosterone is associated with frailty, muscle wasting and physical dysfunction among men receiving hemodialysis: A longitudinal analysis. Nephrol. Dial. Transplant. 2019, 34, 802–810. [Google Scholar] [CrossRef]
- Johansen, K.L.; Painter, P.L.; Sakkas, G.K.; Gordon, P.; Doyle, J.; Shubert, T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J. Am. Soc. Nephrol. 2006, 17, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Badura, K.; Janc, J.; Wasik, J.; Gnitecki, S.; Skwira, S.; Mlynarska, E.; Rysz, J.; Franczyk, B. Anemia of Chronic Kidney Disease—A Narrative Review of Its Pathophysiology, Diagnosis, and Management. Biomedicines 2024, 12, 1191. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.; King, R.F.; Ironside, J.W.; Will, E.J.; Davison, A.M. The effect of treatment with recombinant human erythropoietin on the histological appearance and glycogen content of skeletal muscle in patients with chronic renal failure treated by regular hospital haemodialysis. Nephron 1993, 64, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A. The effect of treatment with recombinant human erythropoietin on skeletal muscle function in patients with end-stage renal failure treated with regular hospital hemodialysis. Am. J. Kidney Dis. 1993, 22, 685–690. [Google Scholar] [CrossRef]
- Organ, J.M.; Srisuwananukorn, A.; Price, P.; Joll, J.E.; Biro, K.C.; Rupert, J.E.; Chen, N.X.; Avin, K.G.; Moe, S.M.; Allen, M.R. Reduced skeletal muscle function is associated with decreased fiber cross-sectional area in the Cy/+ rat model of progressive kidney disease. Nephrol. Dial. Transplant. 2016, 31, 223–230. [Google Scholar] [CrossRef]
- Fahal, I.H.; Bell, G.M.; Bone, J.M.; Edwards, R.H. Physiological abnormalities of skeletal muscle in dialysis patients. Nephrol. Dial. Transplant. 1997, 12, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Shubert, T.; Doyle, J.; Soher, B.; Sakkas, G.K.; Kent-Braun, J.A. Muscle atrophy in patients receiving hemodialysis: Effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003, 63, 291–297. [Google Scholar] [CrossRef]
- Bakinowska, E.; Olejnik-Wojciechowska, J.; Kielbowski, K.; Skoryk, A.; Pawlik, A. Pathogenesis of Sarcopenia in Chronic Kidney Disease-The Role of Inflammation, Metabolic Dysregulation, Gut Dysbiosis, and microRNA. Int. J. Mol. Sci. 2024, 25, 8474. [Google Scholar] [CrossRef]
- Alston, H.; Burns, A.; Davenport, A. Loss of appendicular muscle mass in haemodialysis patients is associated with increased self-reported depression, anxiety and lower general health scores. Nephrology 2018, 23, 546–551. [Google Scholar] [CrossRef]
- Xue, Q.L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Kamijo, Y.; Kanda, E.; Ishibashi, Y.; Yoshida, M. Sarcopenia and Frailty in PD: Impact on Mortality, Malnutrition, and Inflammation. Perit. Dial. Int. 2018, 38, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Abdala, R.; Elena Del Valle, E.; Negri, A.L.; Bridoux, P.; Paganti, L.G.; Bravo, M.; Sintado, L.; Di Rienzo, P.; Schiavelli, O.R.; Zanchetta, M.B.; et al. Sarcopenia in hemodialysis patients from Buenos Aires, Argentina. Osteoporos. Sarcopenia 2021, 7, 75–80. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, Q.; Wang, Z.; Li, J.; Jian, G.; Wang, N. Association Between Sarcopenia and Its Components and Dependency in Activities of Daily Living in Patients on Hemodialysis. J. Ren. Nutr. 2021, 31, 397–402. [Google Scholar] [CrossRef]
- Giglio, J.; Kamimura, M.A.; Lamarca, F.; Rodrigues, J.; Santin, F.; Avesani, C.M. Association of Sarcopenia with Nutritional Parameters, Quality of Life, Hospitalization, and Mortality Rates of Elderly Patients on Hemodialysis. J. Ren. Nutr. 2018, 28, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-L.; Liou, H.-H.; Wang, C.-H.; Lai, Y.-H.; Kuo, C.-H.; Chen, S.-Y.; Hsu, B.-G. Impact of sarcopenia and its diagnostic criteria on hospitalization and mortality in chronic hemodialysis patients: A 3-year longitudinal study. J. Formos. Med. Assoc. 2020, 119, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, A.; Mannucci, I.; Verzola, D.; Sofia, A.; Saffioti, S.; Gianetta, E.; Garibotto, G. Protein-energy wasting and mortality in chronic kidney disease. Int. J. Environ. Res. Public Health 2011, 8, 1631–1654. [Google Scholar] [CrossRef]
- Hanna, R.M.; Ghobry, L.; Wassef, O.; Rhee, C.M.; Kalantar-Zadeh, K. A Practical Approach to Nutrition, Protein-Energy Wasting, Sarcopenia, and Cachexia in Patients with Chronic Kidney Disease. Blood Purif. 2020, 49, 202–211. [Google Scholar] [CrossRef]
- Baltacı, M.A.; Atmis, V.; Metin, Y.; Aktar, M.; Eren, S.A.; Sengul, S.; Ates, K.; Kutlay, S. Sarcopenia and cardiovascular risk indices: Its impact on cardiovascular events and mortality in dialysis patients. Semin. Dial. 2023, 36, 221–230. [Google Scholar] [CrossRef]
- Hotta, C.; Hiraki, K.; Wakamiya, A.; Otobe, Y.; Watanabe, S.; Izawa, K.P.; Kaneshiro, N.; Konno, Y.; Sakurada, T.; Shibagaki, Y.; et al. Relation of physical function and physical activity to sarcopenia in hemodialysis patients: A preliminary study. Int. J. Cardiol. 2015, 191, 198–200. [Google Scholar] [CrossRef]
- Kim, J.-K.; Kim, S.G.; Oh, J.-E.; Lee, Y.-K.; Noh, J.-W.; Kim, H.J.; Song, Y.R. Impact of sarcopenia on long-term mortality and cardiovascular events in patients undergoing hemodialysis. Korean J. Intern. Med. 2019, 34, 599–607. [Google Scholar] [CrossRef]
- Li, C.; Chen, L.; He, L.; Zhang, Y.; Chen, H.; Liu, Y.; Tang, S.; Zheng, H. Study on the relationship between sarcopenia and its components and anorexia in elderly maintenance haemodialysis patients. Nurs. Open 2022, 9, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Elder, M.; Moonen, A.; Crowther, S.; Aleksova, J.; Center, J.; Elder, G.J. Chronic kidney disease-related sarcopenia as a prognostic indicator in elderly haemodialysis patients. BMC Nephrol. 2023, 24, 138. [Google Scholar] [CrossRef]
- Ferreira, M.F.; Böhlke, M.; Pauletto, M.B.; Frühauf, I.R.; Gonzalez, M.C. Sarcopenia diagnosis using different criteria as a predictor of early mortality in patients undergoing hemodialysis. Nutrition 2022, 95, 111542. [Google Scholar] [CrossRef]
- Lee, H.; Kim, K.; Ahn, J.; Lee, D.R.; Lee, J.H.; Hwang, S.D. Association of nutritional status with osteoporosis, sarcopenia, and cognitive impairment in patients on hemodialysis. Asia Pac. J. Clin. Nutr. 2020, 29, 712–723. [Google Scholar] [CrossRef]
- Hayashi, H.; Izumiya, Y.; Hayashi, O.; Ichii, M.; Tsujimoto, Y.; Yoshiyama, M. Dynapenia is an independent predictor of cardio-cerebrovascular events in patients undergoing hemodialysis. Heart Vessels 2022, 37, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Son, H.E.; Ryu, J.Y.; Lee, K.; Choi, Y.I.; Kim, M.S.; Park, I.; Shin, G.T.; Kim, H.; Ahn, C.; Kim, S.; et al. The importance of muscle mass in predicting intradialytic hypotension in patients undergoing maintenance hemodialysis. Kidney Res. Clin. Pract. 2022, 41, 611–622. [Google Scholar] [CrossRef]
- Ishimura, E.; Okuno, S.; Nakatani, S.; Mori, K.; Miyawaki, J.; Okazaki, H.; Sugie, N.; Norimine, K.; Yamakawa, K.; Tsujimoto, Y.; et al. Significant Association of Diabetes with Mortality of Chronic Hemodialysis Patients, Independent of the Presence of Obesity, Sarcopenia, and Sarcopenic Obesity. J. Ren. Nutr. 2022, 32, 94–101. [Google Scholar] [CrossRef] [PubMed]
- de Luca Corrêa, H.; Gadelha, A.B.; Vainshelboim, B.; Dutra, M.T.; Ferreira-Júnior, J.B.; Deus, L.A.; Neves, R.V.P.; Reis, A.L.; de Araújo, T.B.; Tzanno-Martins, C.; et al. Could sarcopenia-related mortality in end-stage renal disease be underpinned by the number of hospitalizations and cardiovascular diseases? Int. Urol. Nephrol. 2023, 55, 157–163. [Google Scholar] [CrossRef]
- Kittiskulnam, P.; Chertow, G.M.; Carrero, J.J.; Delgado, C.; Kaysen, G.A.; Johansen, K.L. Sarcopenia and its individual criteria are associated, in part, with mortality among patients on hemodialysis. Kidney Int. 2017, 92, 238–247. [Google Scholar] [CrossRef]
- Kobayashi, H.; Takahashi, M.; Fukutomi, M.; Oba, Y.; Funayama, H.; Kario, K. The long-term prognostic factors in hemodialysis patients with acute coronary syndrome: Perspectives from sarcopenia and malnutrition. Heart Vessels 2021, 36, 1275–1282. [Google Scholar] [CrossRef]
- Kono, K.; Moriyama, Y.; Yabe, H.; Hara, A.; Ishida, T.; Yamada, T.; Nishida, Y. Relationship between malnutrition and possible sarcopenia in the AWGS 2019 consensus affecting mortality in hemodialysis patients: A prospective cohort study. BMC Nephrol. 2021, 22, 378. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Nishide, K.; Okuno, S.; Shoji, T.; Emoto, M.; Tsuda, A.; Nakatani, S.; Imanishi, Y.; Ishimura, E.; Yamakawa, T.; et al. Impact of diabetes on sarcopenia and mortality in patients undergoing hemodialysis. BMC Nephrol. 2019, 20, 105. [Google Scholar] [CrossRef]
- Sánchez-Tocino, M.L.; Miranda-Serrano, B.; López-González, A.; Villoria-González, S.; Pereira-García, M.; Gracia-Iguacel, C.; González-Ibarguren, I.; Ortíz-Arduan, A.; Mas-Fontao, S.; González-Parra, E. Sarcopenia and Mortality in Older Hemodialysis Patients. Nutrients 2022, 14, 2354. [Google Scholar] [CrossRef]
- Lin, T.Y.; Wu, M.Y.; Chen, H.S.; Hung, S.C.; Lim, P.S. Development and validation of a multifrequency bioimpedance spectroscopy equation to predict appendicular skeletal muscle mass in hemodialysis patients. Clin. Nutr. 2021, 40, 3288–3295. [Google Scholar] [CrossRef]
- Tsujimoto, N.; Matsuzawa, R.; Kakita, D.; Imai, H.; Harada, M.; Yoshikoshi, S.; Yamabe, S.; Osada, S.; Shimokado, K.; Matsunaga, A.; et al. Concomitant sarcopenia and undernutrition: Impact on clinical outcomes in patients undergoing hemodialysis. Clin. Nutr. ESPEN 2024, 63, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Ulgen, C.; Ozturk, I.; Sahin, M.; Guzel, F.B.; Oguz, A.; Altunoren, O.; Gungor, O. The amount of skeletal muscle mass is associated with arterial stiffness in hemodialysis patients. Ther. Apher. Dial. 2023, 27, 24–30. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, W.; Pu, J.; Chen, F. Interrelationships between sarcopenia, bone turnover markers and low bone mineral density in patients on hemodialysis. Ren. Fail. 2023, 45, 2200846. [Google Scholar] [CrossRef]
- Xavier, J.S.; Góes, C.R.; Borges, M.C.C.; Caramori, J.C.T.; Vogt, B.P. Handgrip Strength Thresholds are Associated with Malnutrition Inflammation Score (MIS) in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2022, 32, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Fu, P.; Zhou, L. Sarcopenia and osteosarcopenia among patients undergoing hemodialysis. Front. Endocrinol. 2023, 14, 1181139. [Google Scholar] [CrossRef]
- Yang, Y.; Da, J.; Yuan, J.; Zha, Y. One-year change in sarcopenia was associated with cognitive impairment among haemodialysis patients. J. Cachexia Sarcopenia Muscle 2023, 14, 2264–2274. [Google Scholar] [CrossRef]
- Yoshikoshi, S.; Yamamoto, S.; Suzuki, Y.; Imamura, K.; Harada, M.; Kamiya, K.; Matsunaga, A. Prevalence of osteosarcopenia and its association with mortality and fractures among patients undergoing hemodialysis. J. Bone Miner. Metab. 2024, 42, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Yoshikoshi, S.; Yamamoto, S.; Suzuki, Y.; Imamura, K.; Harada, M.; Osada, S.; Kamiya, K.; Matsunaga, A. Associations between dynapenia, cardiovascular hospitalizations, and all-cause mortality among patients on haemodialysis. J. Cachexia Sarcopenia Muscle 2022, 13, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, J.S.; Jung, S.W.; Hwang, H.S.; Moon, J.Y.; Jeong, K.H.; Lee, S.H.; Lee, S.Y.; Ko, G.J.; Lee, D.Y.; et al. Gait speed and handgrip strength as predictors of all-cause mortality and cardiovascular events in hemodialysis patients. BMC Nephrol. 2020, 21, 166. [Google Scholar] [CrossRef]
- Yuenyongchaiwat, K.; Jongritthiporn, S.; Somsamarn, K.; Sukkho, O.; Pairojkittrakul, S.; Traitanon, O. Depression and low physical activity are related to sarcopenia in hemodialysis: A single-center study. PeerJ 2021, 9, e11695. [Google Scholar] [CrossRef]
- Zhou, C.; Zhan, L.; He, P.; Yuan, J.; Zha, Y. Associations of sarcopenic obesity vs. either sarcopenia or obesity alone with cognitive impairment risk in patients requiring maintenance hemodialysis. Nutr. Clin. Pract. 2023, 38, 1115–1123. [Google Scholar] [CrossRef]
- Lin, Y.L.; Hsu, B.G. Assessment of uremic sarcopenia in dialysis patients: An update. Tzu Chi Med. J. 2022, 34, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, C.; Cavaliere, A.; Lupi, A.; Guglielmi, G.; Quaia, E. Established paths and new avenues: A review of the main radiological techniques for investigating sarcopenia. Quant. Imaging Med. Surg. 2020, 10, 1602–1613. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Chamney, P.W.; Wabel, P.; Moissl, U.M.; Muller, M.J.; Bosy-Westphal, A.; Korth, O.; Fuller, N.J. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am. J. Clin. Nutr. 2007, 85, 80–89. [Google Scholar] [CrossRef]
- Wabel, P.; Chamney, P.; Moissl, U.; Jirka, T. Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif. 2009, 27, 75–80. [Google Scholar] [CrossRef]
- Reis, N.; Vaninni, F.C.D.; Silva, M.Z.C.; de Oliveira, R.C.; Reis, F.M.; Costa, F.L.; Martin, L.C.; Barretti, P. Agreement of Single-Frequency Electrical Bioimpedance in the Evaluation of Fat Free Mass and Fat Mass in Peritoneal Dialysis Patients. Front. Nutr. 2021, 8, 686513. [Google Scholar] [CrossRef] [PubMed]
- Bellafronte, N.T.; Batistuti, M.R.; Dos Santos, N.Z.; Holland, H.; Romão, E.A.; Chiarello, P.G. Estimation of Body Composition and Water Data Depends on the Bioelectrical Impedance Device. J. Electr. Bioimpedance 2018, 9, 96–105. [Google Scholar] [CrossRef]
- Kim, C.; Kim, J.K.; Lee, H.S.; Kim, S.G.; Song, Y.R. Longitudinal changes in body composition are associated with all-cause mortality in patients on peritoneal dialysis. Clin. Nutr. 2021, 40, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Nijholt, W.; Scafoglieri, A.; Jager-Wittenaar, H.; Hobbelen, J.S.M.; van der Schans, C.P. The reliability and validity of ultrasound to quantify muscles in older adults: A systematic review. J. Cachexia Sarcopenia Muscle 2017, 8, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Perkisas, S.; Baudry, S.; Bauer, J.; Beckwee, D.; De Cock, A.M.; Hobbelen, H.; Jager-Wittenaar, H.; Kasiukiewicz, A.; Landi, F.; Marco, E.; et al. Application of ultrasound for muscle assessment in sarcopenia: Towards standardized measurements. Eur. Geriatr. Med. 2018, 9, 739–757. [Google Scholar] [CrossRef]
- Matsuzawa, R.; Yamamoto, S.; Suzuki, Y.; Imamura, K.; Harada, M.; Matsunaga, A.; Tamaki, A.; Fukui, T.; Shimokado, K. The clinical applicability of ultrasound technique for diagnosis of sarcopenia in hemodialysis patients. Clin. Nutr. 2021, 40, 1161–1167. [Google Scholar] [CrossRef]
- Sabatino, A.; Kooman, J.P.; Di Motta, T.; Cantarelli, C.; Gregorini, M.; Bianchi, S.; Regolisti, G.; Fiaccadori, E. Quadriceps muscle thickness assessed by ultrasound is independently associated with mortality in hemodialysis patients. Eur. J. Clin. Nutr. 2022, 76, 1719–1726. [Google Scholar] [CrossRef]
- Sabatino, A.; Kooman, J.; Avesani, C.M.; Gregorini, M.; Bianchi, S.; Regolisti, G.; Fiaccadori, E. Sarcopenia diagnosed by ultrasound-assessed quadriceps muscle thickness and handgrip strength predicts mortality in patients on hemodialysis. J. Nephrol. 2024, 37, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- MacRae, J.M.; Harasemiw, O.; Lightfoot, C.J.; Thompson, S.; Wytsma-Fisher, K.; Koufaki, P.; Bohm, C.; Wilkinson, T.J. Measurement properties of performance-based measures to assess physical function in chronic kidney disease: Recommendations from a COSMIN systematic review. Clin. Kidney J. 2023, 16, 2108–2128. [Google Scholar] [CrossRef]
- Pinto, A.P.; Ramos, C.I.; Meireles, M.S.; Kamimura, M.A.; Cuppari, L. Impact of hemodialysis session on handgrip strength. J. Bras. Nefrol. 2015, 37, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Fahal, I.H. Uraemic sarcopenia: Aetiology and implications. Nephrol. Dial. Transplant. 2014, 29, 1655–1665. [Google Scholar] [CrossRef]
- Bataille, S.; Serveaux, M.; Carreno, E.; Pedinielli, N.; Darmon, P.; Robert, A. The diagnosis of sarcopenia is mainly driven by muscle mass in hemodialysis patients. Clin. Nutr. 2017, 36, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, Z.; Ma, T.; Li, Z.; Chen, Y.; Zheng, Y.; Dong, J. The cut-off values of handgrip strength and lean mass index for sarcopenia among patients on peritoneal dialysis. Nutr. Metab. 2020, 17, 84. [Google Scholar] [CrossRef]
- Vogt, B.P.; Borges, M.C.C.; Goes, C.R.; Caramori, J.C.T. Handgrip strength is an independent predictor of all-cause mortality in maintenance dialysis patients. Clin. Nutr. 2016, 35, 1429–1433. [Google Scholar] [CrossRef]
- Vanden Wyngaert, K.; Van Biesen, W.; Eloot, S.; Van Craenenbroeck, A.H.; Calders, P.; Holvoet, E. The importance of physical performance in the assessment of patients on haemodialysis: A survival analysis. PLoS ONE 2022, 17, e0268115. [Google Scholar] [CrossRef]
- Duarte, M.P.; Nobrega, O.T.; Vogt, B.P.; Pereira, M.S.; Silva, M.Z.C.; Mondini, D.R.; Disessa, H.S.; Adamoli, A.N.; Bundchen, D.C.; Sant’Helena, B.R.M.; et al. Reference Values for Handgrip Strength, Five Times Sit-to-Stand, and Gait Speed in Patients on Hemodialysis. Nephrol. Dial. Transplant. 2024, gfae232. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef]
- Lin, Y.L.; Hou, J.S.; Lai, Y.H.; Wang, C.H.; Kuo, C.H.; Liou, H.H.; Hsu, B.G. Association of SARC-F Questionnaire and Mortality in Prevalent Hemodialysis Patients. Diagnostics 2020, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Silva, T.G.; Menezes, A.M.; Bielemann, R.M.; Malmstrom, T.K.; Gonzalez, M.C.; Grupo de Estudos em Composição Corporal e Nutrição (COCONUT). Enhancing SARC-F: Improving Sarcopenia Screening in the Clinical Practice. J. Am. Med. Dir. Assoc. 2016, 17, 1136–1141. [Google Scholar] [CrossRef]
- Lin, Y.L.; Wang, C.H.; Tsai, J.P.; Chen, C.T.; Chen, Y.H.; Hung, S.C.; Hsu, B.G. A Comparison of SARC-F, Calf Circumference, and Their Combination for Sarcopenia Screening Among Patients Undergoing Peritoneal Dialysis. Nutrients 2022, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Kakita, D.; Matsuzawa, R.; Yamamoto, S.; Suzuki, Y.; Harada, M.; Imamura, K.; Yoshikoshi, S.; Imai, H.; Osada, S.; Shimokado, K.; et al. Simplified discriminant parameters for sarcopenia among patients undergoing haemodialysis. J. Cachexia Sarcopenia Muscle 2022, 13, 2898–2907. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Ye, X.; Usvyat, L.; Kooman, J.; van der Sande, F.; Raimann, J.; Wang, Y.; Kotanko, P. Clinical and predictive value of simplified creatinine index used as muscle mass surrogate in end-stage kidney disease haemodialysis patients-results from the international MONitoring Dialysis Outcome initiative. Nephrol. Dial. Transplant. 2020, 35, 2161–2171. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, H.S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Verzola, D.; Barisione, C.; Picciotto, D.; Garibotto, G.; Koppe, L. Emerging role of myostatin and its inhibition in the setting of chronic kidney disease. Kidney Int. 2019, 95, 506–517. [Google Scholar] [CrossRef]
- Zhou, Y.; Hellberg, M.; Hellmark, T.; Hoglund, P.; Clyne, N. Muscle mass and plasma myostatin after exercise training: A substudy of Renal Exercise (RENEXC)-a randomized controlled trial. Nephrol. Dial. Transplant. 2021, 36, 95–103. [Google Scholar] [CrossRef]
- Sakashita, M.; Hamasaki, Y.; Oki, R.; Komaru, Y.; Miyamoto, Y.; Yoshida, T.; Matsuura, R.; Doi, K.; Nangaku, M. Serum Myostatin at Dialysis Initiation May Predict 1-Year Mortality and Hospitalization. Nephron 2024, 148, 544–552. [Google Scholar] [CrossRef]
- Qaisar, R.; Karim, A.; Muhammad, T.; Shah, I.; Khan, J. Prediction of sarcopenia using a battery of circulating biomarkers. Sci. Rep. 2021, 11, 8632. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Hwang, H.; Kim, S.K.; Choi, J.Y.; Lee, S.M.; Bang, H.; Kwon, E.S.; Lee, K.P.; Chung, S.G.; Kwon, K.S. Prediction of sarcopenia using a combination of multiple serum biomarkers. Sci. Rep. 2018, 8, 8574. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Mehrotra, R.; Rhee, C.M.; Molnar, M.Z.; Lukowsky, L.R.; Patel, S.S.; Nissenson, A.R.; Kopple, J.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in peritoneal dialysis patients. Nephrol. Dial. Transplant. 2013, 28, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Streja, E.; Kovesdy, C.P.; Oreopoulos, A.; Noori, N.; Jing, J.; Nissenson, A.R.; Krishnan, M.; Kopple, J.D.; Mehrotra, R.; et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin. Proc. 2010, 85, 991–1001. [Google Scholar] [CrossRef]
- Keshaviah, P.R.; Nolph, K.D.; Moore, H.L.; Prowant, B.; Emerson, P.F.; Meyer, M.; Twardowski, Z.J.; Khanna, R.; Ponferrada, L.; Collins, A. Lean body mass estimation by creatinine kinetics. J. Am. Soc. Nephrol. 1994, 4, 1475–1485. [Google Scholar] [CrossRef]
- Noori, N.; Kovesdy, C.P.; Bross, R.; Lee, M.; Oreopoulos, A.; Benner, D.; Mehrotra, R.; Kopple, J.D.; Kalantar-Zadeh, K. Novel equations to estimate lean body mass in maintenance hemodialysis patients. Am. J. Kidney Dis. 2011, 57, 130–139. [Google Scholar] [CrossRef]
- Zhang, F.; Yin, X.; Huang, L.; Zhang, H. The “adult inactivity triad” in patients with chronic kidney disease: A review. Front. Med. 2023, 10, 1160450. [Google Scholar] [CrossRef]
- Battaglia, Y.; Baciga, F.; Bulighin, F.; Amicone, M.; Mosconi, G.; Storari, A.; Brugnano, R.; Pozzato, M.; Motta, D.; D’Alessandro, C.; et al. Physical activity and exercise in chronic kidney disease: Consensus statements from the Physical Exercise Working Group of the Italian Society of Nephrology. J. Nephrol. 2024, 37, 1735–1765. [Google Scholar] [CrossRef] [PubMed]
- Deligiannis, A.; D’Alessandro, C.; Cupisti, A. Exercise training in dialysis patients: Impact on cardiovascular and skeletal muscle health. Clin. Kidney J. 2021, 14, ii25–ii33. [Google Scholar] [CrossRef]
- Araujo, A.M.; Orcy, R.B.; Feter, N.; Weymar, M.K.; Cardoso, R.K.; Bohlke, M.; Rombaldi, A.J. Effects of intradialytic exercise on functional capacity in patients with end-stage chronic kidney disease: A systematic review and meta-analysis. Res. Sports Med. 2024, 32, 28–48. [Google Scholar] [CrossRef]
- Sabatino, A.; Cuppari, L.; Stenvinkel, P.; Lindholm, B.; Avesani, C.M. Sarcopenia in chronic kidney disease: What have we learned so far? J. Nephrol. 2021, 34, 1347–1372. [Google Scholar] [CrossRef]
- Kopple, J.D.; Wang, H.; Casaburi, R.; Fournier, M.; Lewis, M.I.; Taylor, W.; Storer, T.W. Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J. Am. Soc. Nephrol. 2007, 18, 2975–2986. [Google Scholar] [CrossRef] [PubMed]
- Bulighin, F.; Aucella, F.; Bellizzi, V.; Cupisti, A.; Faga, T.; Gambaro, G.; Regolisti, G.; Storari, A.; Capitanini, A.; Battaglia, Y.; et al. Physical activity and exercise programs for kidney patients: An Italian survey of nephrology centres. J. Nephrol. 2024, 37, 695–705. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Regolisti, G. What is the role of exercise in chronic kidney disease? Nephrol. Dial. Transplant. 2022, 37, 258–261. [Google Scholar] [CrossRef]
- Clyne, N.; Anding-Rost, K. Exercise training in chronic kidney disease-effects, expectations and adherence. Clin. Kidney J. 2021, 14, ii3–ii14. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Lightfoot, C.J.; Jegatheesan, D.K.; Gabrys, I.; Bennett, P.N. Physical activity and exercise recommendations for people receiving dialysis: A scoping review. PLoS ONE 2022, 17, e0267290. [Google Scholar] [CrossRef]
- Kirkman, D.L.; Mullins, P.; Junglee, N.A.; Kumwenda, M.; Jibani, M.M.; Macdonald, J.H. Anabolic exercise in haemodialysis patients: A randomised controlled pilot study. J. Cachexia Sarcopenia Muscle 2014, 5, 199–207. [Google Scholar] [CrossRef]
- Koufaki, P.; Greenwood, S.; Painter, P.; Mercer, T. The BASES expert statement on exercise therapy for people with chronic kidney disease. J. Sports Sci. 2015, 33, 1902–1907. [Google Scholar] [CrossRef]
- Baggetta, R.; Bolignano, D.; Torino, C.; Manfredini, F.; Aucella, F.; Barilla, A.; Battaglia, Y.; Bertoli, S.; Bonanno, G.; Castellino, P.; et al. Fitness for entering a simple exercise program and mortality: A study corollary to the exercise introduction to enhance performance in dialysis (EXCITE) trial. Kidney Blood Press. Res. 2014, 39, 197–204. [Google Scholar] [CrossRef]
- Baggetta, R.; D’Arrigo, G.; Torino, C.; ElHafeez, S.A.; Manfredini, F.; Mallamaci, F.; Zoccali, C.; Tripepi, G.; EXCITE Working group. Effect of a home based, low intensity, physical exercise program in older adults dialysis patients: A secondary analysis of the EXCITE trial. BMC Geriatr. 2018, 18, 248. [Google Scholar] [CrossRef]
- Manfredini, F.; Mallamaci, F.; D’Arrigo, G.; Baggetta, R.; Bolignano, D.; Torino, C.; Lamberti, N.; Bertoli, S.; Ciurlino, D.; Rocca-Rey, L.; et al. Exercise in Patients on Dialysis: A Multicenter, Randomized Clinical Trial. J. Am. Soc. Nephrol. 2017, 28, 1259–1268. [Google Scholar] [CrossRef]
- Manfredini, F.; D’Arrigo, G.; Lamberti, N.; Torino, C.; Tripepi, G.; Mallamaci, F.; Zoccali, C. The legacy effect of a home walking exercise programme in kidney failure patients on dialysis. Nephrol. Dial. Transplant. 2022, 37, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.A.; March, D.S.; Wilkinson, T.J.; Billany, R.E.; Bishop, N.C.; Castle, E.M.; Chilcot, J.; Davies, M.D.; Graham-Brown, M.P.M.; Greenwood, S.A.; et al. Clinical practice guideline exercise and lifestyle in chronic kidney disease. BMC Nephrol. 2022, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, X.; Deng, S.; Chen, J.; Zhang, L.; Huang, Y.; Hu, H. Combined aerobic and resistance exercise in maintenance hemodialysis patients: A meta-analysis. Semin. Dial. 2023, 36, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhang, X.; Wang, Z.; Qu, Z.; Sun, X.; Song, Y.; Zhang, H. Application of exercise therapy in patients with chronic kidney disease-induced muscle atrophy: A scoping review. BMC Sports Sci. Med. Rehabil. 2024, 16, 100. [Google Scholar] [CrossRef]
- Battaglia, Y.; Amicone, M.; Mantovani, A.; Combe, C.; Mitra, S.; Basile, C.; EuDial Working Group of ERA. Home-based exercise in patients on maintenance dialysis: A systematic review and meta-analysis of randomized clinical trials. Nephrol. Dial. Transplant. 2023, 38, 2550–2561. [Google Scholar] [CrossRef]
- Sabatino, A.; Piotti, G.; Cosola, C.; Gandolfini, I.; Kooman, J.P.; Fiaccadori, E. Dietary protein and nutritional supplements in conventional hemodialysis. Semin. Dial. 2018, 31, 583–591. [Google Scholar] [CrossRef]
- Matsuzawa, R.; Yamamoto, S.; Suzuki, Y.; Abe, Y.; Harada, M.; Shimoda, T.; Imamura, K.; Yamabe, S.; Ito, H.; Yoshikoshi, S.; et al. The effects of amino acid/protein supplementation in patients undergoing hemodialysis: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2021, 44, 114–121. [Google Scholar] [CrossRef]
- Mori, K. Maintenance of Skeletal Muscle to Counteract Sarcopenia in Patients with Advanced Chronic Kidney Disease and Especially Those Undergoing Hemodialysis. Nutrients 2021, 13, 1538. [Google Scholar] [CrossRef]
- Molina, P.; Carrero, J.J.; Bover, J.; Chauveau, P.; Mazzaferro, S.; Torres, P.U.; European Renal Nutrition (ERN) and Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Working Groups of the European Renal Association—European Dialysis Transplant Association (ERA-EDTA). Vitamin D, a modulator of musculoskeletal health in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2017, 8, 686–701. [Google Scholar] [CrossRef]
- Marckmann, P.; Agerskov, H.; Thineshkumar, S.; Bladbjerg, E.M.; Sidelmann, J.J.; Jespersen, J.; Nybo, M.; Rasmussen, L.M.; Hansen, D.; Scholze, A. Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol. Dial. Transplant. 2012, 27, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, N.A.; O’Connor, A.A.; O’Shaughnessy, D.V.; Elder, G.J. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 1143–1149. [Google Scholar] [CrossRef]
- Gordon, P.L.; Sakkas, G.K.; Doyle, J.W.; Shubert, T.; Johansen, K.L. Relationship between vitamin D and muscle size and strength in patients on hemodialysis. J. Ren. Nutr. 2007, 17, 397–407. [Google Scholar] [CrossRef]
- Kang, S.H.; Do, J.Y.; Cho, J.H.; Jeong, H.Y.; Yang, D.H.; Kim, J.C. Association Between Vitamin D Level and Muscle Strength in Patients Undergoing Hemodialysis. Kidney Blood Press. Res. 2020, 45, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Chen, S.Y.; Lai, Y.H.; Wang, C.H.; Kuo, C.H.; Liou, H.H.; Hsu, B.G. Angiotensin II receptor blockade is associated with preserved muscle strength in chronic hemodialysis patients. BMC Nephrol. 2019, 20, 54. [Google Scholar] [CrossRef]
- Picciotto, D.; Maccio, L.; Verzola, D.; Baciga, F.; Momente, C.; Russo, E.; Viazzi, F.; Battaglia, Y.; Esposito, P. Pathophysiology of Physical Exercise in Kidney Patients: Unveiling New Players—The Role of Myokines. Kidney Blood Press. Res. 2024, 49, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Esposito, P.; Picciotto, D.; Battaglia, Y.; Costigliolo, F.; Viazzi, F.; Verzola, D. Myostatin: Basic biology to clinical application. Adv. Clin. Chem. 2022, 106, 181–234. [Google Scholar] [CrossRef]
- Esposito, P.; Battaglia, Y.; La Porta, E.; Grignano, M.A.; Caramella, E.; Avella, A.; Peressini, S.; Sessa, N.; Albertini, R.; Di Natali, G.; et al. Significance of serum Myostatin in hemodialysis patients. BMC Nephrol. 2019, 20, 462. [Google Scholar] [CrossRef]
- Esposito, P.; La Porta, E.; Calatroni, M.; Grignano, M.A.; Milanesi, S.; Verzola, D.; Battaglia, Y.; Gregorini, M.; Libetta, C.; Garibotto, G.; et al. Modulation of Myostatin/Hepatocyte Growth Factor Balance by Different Hemodialysis Modalities. BioMed Res. Int. 2017, 2017, 7635459. [Google Scholar] [CrossRef]
- Bataille, S.; Chauveau, P.; Fouque, D.; Aparicio, M.; Koppe, L. Myostatin and muscle atrophy during chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 1986–1993. [Google Scholar] [CrossRef]
- Gascon, A.; Belvis, J.J.; Berisa, F.; Iglesias, E.; Estopinan, V.; Teruel, J.L. Nandrolone decanoate is a good alternative for the treatment of anemia in elderly male patients on hemodialysis. Geriatr. Nephrol. Urol. 1999, 9, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.H.; Marcora, S.M.; Jibani, M.M.; Kumwenda, M.J.; Ahmed, W.; Lemmey, A.B. Nandrolone decanoate as anabolic therapy in chronic kidney disease: A randomized phase II dose-finding study. Nephron Clin. Pract. 2007, 106, c125–c135. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Ottaviani, E.; Guerriero, C.; Di Daniele, F.; Pietroboni Zaitseva, A.; Di Daniele, N. Uremic Sarcopenia and Its Possible Nutritional Approach. Nutrients 2021, 13, 147. [Google Scholar] [CrossRef] [PubMed]

| Author/Year | Study Type | Population | Findings | Notes |
|---|---|---|---|---|
| Isoyama et al., 2014 [10] | Post hoc cross-sectional analysis with prospective follow-up | Incident dialysis patients N = 330 Gender: 62% men Mean age: 53 ± 13 years | Sarcopenia prevalence: 20%. Low muscle mass prevalence: 24%. Low muscle strength prevalence: 15%. Multivariable analysis: increased sarcopenia risk associated with old age, low albumin, PEW. | Sarcopenia definition: DXA, MAMC, HGS, EWGS. Old age, comorbidities, PEW, physical inactivity, low albumin, and inflammation associated with low muscle strength but not with low muscle mass. |
| Lamarca et al., 2014 [11] | Multicenter observational and cross-sectional study | Maintenance HD patients (Rio de Janeiro, Brazil) N = 102 Age > 60 years old Gender: 73.5% men Mean age: 70.7 ± 7 years Median dialysis vintage: 2.25 (1; 5.3) | Wide prevalence of sarcopenia, depending on method and cut-off. Sarcopenia prevalence: 4–63%. Decreased muscle mass prevalence: 4–73.5%. Decreased muscle strength prevalence: 85%. Sarcopenia prevalence by >2 criteria: 2–15%. | Sarcopenia definition: one criterion for low muscle mass (DXA, BIA, sum of SKF, calf circumference, and MAMC) and one for low muscle strength. Comparable agreement between DXA, BIA, and SKF. No gait speed available. |
| Wathanavasin et al., 2022 [14] | Systematic review and meta-analysis | Dialysis patients N = 7576 Studies N = 41 (31 in HD, 7 in PD, 3 in HD + PD population) Mean age: 62.3 years Gender: 61.4% men Five continents: Asia 45.6%; Europe 25.9%; North America 14.3%; South America 13.7%; Australia: 0.5% Mean dialysis vintage: 52.4 months | Pooled sarcopenia prevalence: 25.6% (95% CI: 22.1% to 29.4%). Regional sarcopenia prevalence: 15.4% in the USA, 17.9% in Australia, 20.4% in South America, 27.9% in Asia, 29.1% in Europe. Sarcopenia prevalence by diagnostic criteria: 36.9% by AWGS 2019, 34.9% by IWGS, 24.4% by EWGSOP 2010, 24.1% by EWGSOP 2019, 22% by AWGS 2014, 20% by FNIH. Sarcopenia prevalence by dialysis modality: 26.8% in HD, 17.5% in PD. | Sarcopenia definition: both low muscle mass and low muscle strength. Substantial heterogeneity (I2 = 91.98%, p < 0.001). Higher risk of sarcopenia in men and diabetics. |
| Duarte et al., 2024 [15] | Systematic review and meta-analysis | Studies N = 140 (42,041 patients, 25 countries, 5 continents) Sarcopenia prevalence: CKD + dialysis patients (114 studies) N = 36,190 HD patients (63 studies) N = 18,190 PD patients (8 studies) N = 1283 HD + PD patients (4 studies) N = 662 | Sarcopenia prevalence in dialysis patients: 27.7 (95% CI: 24.7–30.9). Severe sarcopenia prevalence in dialysis patients: 26.2% (95% CI: 16.6–37.1). Similar sarcopenia prevalence between dialysis and non-dialysis patients. Significantly higher frequency of severe sarcopenia in dialysis vs. non-dialysis patients. | Sarcopenia definition: EWGSOP; EWGSOP2; IWGS; AWGS; AWGS 2019; FNIH. Sarcopenia traits prevalence in dialysis: 50% for low muscle strength, 32.2% for low muscle mass, 46.8% (HD), and 47.5% (PD) for low physical performance. Higher frequency of low muscle strength in dialysis vs. non-dialysis patients. |
| Shu et al., 2022 [7] | Systematic review and meta-analysis | Dialysis patients: N = 6162 Studies N = 30 (20 in the HD population and 10 in the PD population; 14 in Asia, 8 in Europe, and 8 in America) Mean age: 47.5 to 77.5 years; mean dialysis vintage: 3 to 91.7 months | Overall sarcopenia prevalence: 28.5% (95% CI: 22.9–34.1%). Sarcopenia prevalence by combined criteria: 25.9% (95% CI: 20.4–31.3%). Higher sarcopenia prevalence in HD (31%) vs. PD populations (23.4%). Higher sarcopenia prevalence in studies using low muscle mass only (34.6%) vs. those using combined criteria (25.9%). Lower sarcopenia prevalence by EWGSOP criteria (23.4%) vs. AWGS criteria (42.6%) and other criteria (32.2%). | Sarcopenia definition: Low muscle mass plus low muscle strength and/or low physical performance (22 studies); low muscle mass (8 studies); EWGSOP criteria (17 studies); AWGS criteria (4 studies). No effects of age and dialysis duration on prevalence. No significant differences between dialysis modalities, diagnostic criteria, and consensuses. |
| Duarte et al., 2024 [16] | Multicenter, cross-sectional | HD patients (Brazil) N = 838 Gender: 61% men Mean age: 57.8 ± 15.0 years | Sarcopenia prevalences are similar between consensuses: 15.3% (128 patients) by EWGSOP2; 12.5% (105 patients) by SDOC. Low muscle strength: 52.3% by SDOC vs. 25.9% by EWGSOP2. Agreement between consensuses: Weak (50 of 233 patients, 21.5%; κ = 0.34, 95% CI: 0.25–0.43). | Sarcopenia definition: EWGSOP2 and SDOC. Marginally better agreement for older patients. |
| Hung et al., 2017 [17] | Retrospective analysis, cross-sectional | PD patients N = 325 Gender: 57.2% men Mean age: 56.7 ± 16.5 years Ethnicity: White, Asian, African/Afro-Caribbean | Sarcopenia prevalence by gender: 2.2–31.3% for women 25.1–75.6% for men Greater sarcopenia prevalence for men by all grading systems. No effects of diabetes, ethnicity, or dialysis adequacy on prevalence. | Sarcopenia definition: DXA. No muscle strength measurements. Male patients older (58.3 ± 16) compared with women (53.4 ± 15.7 years). Increased sarcopenia prevalence in PD patients vs. age-matched subjects by ALM sex-specific cut-offs from healthy young adults. |
| Yoowannakul et al., 2018 [18] | Cross-sectional | HD patients N = 600 Gender: 62.2% men Mean age 66.3 ± 14.7 Ethnicity: White, N = 281; Asian, N = 167; Black, N = 149; Unclassified, N = 3 | Similar muscle strength between ethnicities: AWGS: 80% Asian vs. 70% White vs. 64%; Black; EWGS: 90% Asian vs. 77.5% White vs. 76% Black; FNIH: 70% Asian vs. 62.5% White vs. 40% Black. Lower muscle mass in Asian vs. White and Black: AWGS: 45% Asian vs. 25% White vs. 8%, Black; EWGS: 45% Asian vs. 25% White vs. 8% Black; FNIH: 55% Asian vs. 25% White vs. 8% Black | Sarcopenia definition: HGS, multifrequency BIA; FNIH, EWGS, AWGS. Muscle weakness more common than reduced muscle mass. Increased prevalence of low muscle mass in Asians after adjusting for height. |
| Yoowannakul et al.; 2018 [19] | Cross-sectional | PD patients N = 434 Gender: 55.1% men Mean age: 55.3 ± 16.2 years Ethnicity: White N = 235; Black N = 83; Asian N = 113; other ethnicities N = 13 | Sarcopenia prevalence: 6.5–26.3%. Greater sarcopenia prevalence in men by EWG, FHIN. Greater sarcopenia prevalence in Asians > 40% by EWG; >35% by FNIH; vs. White (2.3–18.7%), Black (3.8–15.7%) by EWG and FNIH. Sarcopenia prevalence in Asians < 11% by AWGS cut-off points. | Sarcopenia definition: BIA; ESWGOP, FHIN, AWGS. No association between sarcopenia prevalence and residual renal function, serum albumin, CRP, or co-morbidity score. |
| Ren et al.; 2016 [20] | Cross-sectional | HD patients N = 131 Gender: 81.1% men Mean age: 49.4 ± 11.7 | Sarcopenia prevalence: 13.7%. Gender-related sarcopenia prevalence: 5% in men, 11.8% in women. Age-related sarcopenia prevalence: 18.0% in patients > 50 years; 33.3% in patients > 60 years. | Sarcopenia definition: BIA, HGS, EWGSOP. Independent sarcopenia risk factors in multivariate analysis: dialysis duration, diabetes, serum phosphorus. |
| Study | Year | Study Design | Sample Size (n) | Main Findings |
|---|---|---|---|---|
| Abdala et al. [110] | 2021 | Cross-sectional | 100 | High prevalence of falls in patients with lower HGS. |
| Alston et al. [99] | 2018 | Cross-sectional | 113 | Association between appendicular lean mass (ALM) index and increased self-reported depression, anxiety, and decreased general health. |
| Baltac et al. [108] | 2022 | Prospective cohort | 106 | No association between sarcopenia and pre-atherosclerotic markers, cardiovascular events, and all-cause mortality. |
| Chao Li et al. [111] | 2021 | Cross-sectional | 112 | Severe sarcopenia was independently associated with anorexia. |
| Cheng et al. [103] | 2021 | Cross-sectional | 238 | Severe sarcopenia was significantly associated with dependency in the basic and instrumental activities of daily living (ADLs) (OR, 4.68, 95% CI: 2.11–10.40; OR, 3.24, 95% CI: 1.61–6.53, respectively). |
| Elder et al. [112] | 2023 | Prospective cohort | 77 | Sarcopenia is highly prevalent in elderly hemodialysis patients but is not an independent predictor of mortality. |
| Ferreira et al. [113] | 2022 | Cross-sectional | 127 | Patients diagnosed with sarcopenia had almost three times higher risk for mortality. |
| Giglio et al. [104] | 2018 | Cross-sectional | 170 | In the adjusted multivariate Cox analysis, low muscle strength and sarcopenia were associated with higher hospitalization rates. Sarcopenia was a predictor of mortality. |
| Heeryong Lee et al. [114] | 2020 | Cross-sectional | 131 | Inadequate nutrition was associated with the risk of osteoporosis and sarcopenia but not cognitive impairment. |
| Hiroya Hayashi et al. [115] | 2022 | Retrospective cohort | 244 | Both sarcopenia and dynapenia resulted in significantly higher CV events than non-sarco-dynapenia in patients undergoing HD (HR 8.00; 95% CI: 2.73–34.1; p < 0.0001 vs. HR 4.85; 95% CI: 1.28–23.0; p < 0.02). |
| Hyung Eun Son et al. [116] | 2022 | Cross-sectional | 177 | Low skeletal muscle mass to dry body weight ratio (SMM/WT) had a higher rate of intradialytic hypotension (40.7%). |
| Ishimura et al. [117] | 2022 | Retrospective cohort | 308 | Patients with sarcopenia and sarcopenic obesity had significantly higher rates of all-cause mortality (p = 0.0004). |
| Isoyama et al. [10] | 2014 | Cross-sectional | 330 | Low muscle strength was more strongly associated with aging, protein-energy wasting, physical inactivity, inflammation, and mortality. |
| de Luca Corrêa et al. [118] | 2023 | Prospective study | 247 | Sarcopenic patients had higher numbers of cardiovascular disease (56.9% vs. 12.6%) and hospitalizations (93.8% vs. 49.5%). Sarcopenia was associated with a significantly higher risk of mortality (HR = 3.3, 95% CI: 1.6–6.9, p = 0.001). |
| Kittiskulnam et al. [119] | 2017 | Prospective cohort | 645 | Both gait slowness and low hand-grip strength significantly improved the net reclassification index compared with models without performance measures (50.5% for slowness and 33.7% for weakness). |
| Kobayashi et al. [120] | 2021 | Cross-sectional | 58 | The Skeletal Muscle Mass Index (SMI) and Geriatric Nutritional Risk Index (GNRI) were the factors associated with all-cause mortality in all patients. |
| Kono et al. [121] | 2021 | Prospective cohort | 635 | Hand-grip strength (HR 3.61, 95% CI: 1.70–7.68, p < 0.001) and the five-times chair stand test (HR 1.71 95% CI: 1.01–2.90, p = 0.045) were significant predictors for mortality. |
| Mori et al. [122] | 2019 | Prospective cohort | 308 | Patients with sarcopenia demonstrated significantly higher rates of all-cause mortality. |
| Ren et al. [20] | 2016 | Cross-sectional | 131 | The one-year survival in sarcopenic patients (88.9%) was significantly lower than that in non-sarcopenic patients. |
| Sánchez-Tocino et al. [123] | 2022 | Prospective cohort | 60 | Appendicular skeletal muscle mass (ASM) and severity (gait speed, GS) variables were associated with mortality (HR 3.03, 95% CI: 1.14–8.08, p = 0.028). |
| Ting-Yun Lin et al. [124] | 2020 | Cross-sectional | 263 | Low appendicular skeletal muscle mass predicted by the Body Composition Monitor (BCM) equation was associated with significantly worse overall survival among CKD patients but not those on chronic hemodialysis. |
| Tsujimoto et al. [125] | 2024 | Prospective cohort | 450 | Concomitant sarcopenia and malnutrition were significantly associated with a risk of mortality (HR 2.10; 95% CI: 1.05–4.21; p = 0.037). |
| Ulgen et al. [126] | 2022 | Cross-sectional | 79 | Decreased skeletal muscle mass contributes to increased arterial stiffness in hemodialysis patients. |
| Wang et al. [127] | 2023 | Cross-sectional | 130 | Sarcopenia was associated with Low Bone Mineral Disease (BMD) (OR = 5.894, 95% CI: 1.592–21.830, p < 0.01). |
| Xavier et al. [128] | 2022 | Cross-sectional | 218 | Worse nutritional status increases the risk of lower hand-grip strength and mortality in hemodialysis patients. |
| Xiang et al. [129] | 2023 | Prospective cohort | 209 | Osteosarcopenia was independently associated with all-cause mortality (HR = 3.74, 95% CI: 1.172–11.938), while osteoporosis alone and sarcopenia alone were not. |
| Yang et al. [130] | 2023 | Prospective cohort | 1117 | Both greater changes in the appendicular skeletal mass index and hand-grip strength had a lower risk of cognitive impairment (adjusted OR = 0.857, 95% CI: 0.778–0.945, p = 0.002; adjusted OR = 0.976, 95% CI: 0.963–0.989, p < 0.001, respectively). |
| Yoshikoshi et al. [131] | 2024 | Retrospective cohort | 328 | Osteosarcopenia showed a higher risk of all-cause mortality than the robust group. |
| Yoshikoshi et al. [132] | 2022 | Retrospective cohort | 616 | Dynapenia was associated with increased risks of all-cause mortality and CV hospitalizations among patients on hemodialysis. |
| Yu Ho Lee et al. [133] | 2020 | Prospective cohort | 277 | Patients with low gait speed and hand-grip strength had the highest risks for all-cause mortality and cardiovascular events among the groups (adjusted HR 2.72, p = 0.024). |
| Yuenyongchaiwat et al. [134] | 2021 | Cross-sectional | 104 | Sarcopenic patients had low physical activity, a high depression score, and an increased mortality risk. |
| Yu-Li Lin et al. [105] | 2020 | Cross-sectional | 126 | Muscle quality (HR = 0.42, 95% CI: = 0.19–0.93, p = 0.032) was independently associated with the composite outcome of hospitalization or death. |
| Zhou et al. [135] | 2023 | Cross-sectional | 2743 | The association between sarcopenic obesity and cognitive impairment was statistically significant after adjusting for age, sex, and educational status (OR, 1.47; 95% CI: 1.11–1.96). |
| Guidelines | Hemodialysis | Peritoneal Dialysis |
|---|---|---|
| ESPEN (2006) | Protein intake: 1.2–1.4 g/kg/day Energy intake: 35 kcal/kg/day | Protein intake: 1.2–1.5 g/kg/day Energy intake: 35 kcal/kg/day |
| EBPG (2007) | Protein intake: >1.1 g/kg/day Energy intake: 30–40 kcal/kg/day | Protein intake: ≥1.2 g/kg/day Energy intake: 35 kcal/kg/day |
| ISRNM (2013) | Protein intake: >1.2 g/kg/day Energy intake: 30 kcal/kg/day (≥60 years) 35 kcal/kg/day (<60 years) | Protein intake: >1.2 g/kg/day Energy intake: 30–35 kcal/kg/day (including dialysate) |
| KDOQI (2020) | Protein intake: 1–1.2 g/kg/day Energy intake: 25–35 kcal/kg/day | Protein intake: 1–1.2 g/kg/day Energy intake: 25–35 kcal/kg/day |
| Type of Nutrition | Indication |
|---|---|
| ONS | Poor appetite and failure in spontaneous dietary intake Energy intake < 30 kcal/kg/day and protein intake < 1.2 g/kg/day * Serum albumin < 3.8 g/dL or prealbumin < 28 mg/mL Unintentional weight loss > 5% Diagnosis of PEW using SGA definition |
| EN | Patients unable to tolerate nutritional supplementation by mouth and failing on dietary intake with ONS Severe PEW Energy intake < 20 kcal/kg/day Undergoing metabolic stress |
| PN | If all other forms of nutrition failed ^ For patients with spontaneous intake of at least 20 kcal/kg/day and protein intake of 0.8 g/kg/day |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zicarelli, M.; Duni, A.; Leivaditis, K.; Lin, Y.-L.; Baciga, F.; Pugliese, S.; Fiorentino, M.; Hsu, B.-G.; Roumeliotis, S.; Battaglia, Y.; et al. Comprehensive Insights into Sarcopenia in Dialysis Patients: Mechanisms, Assessment, and Therapeutic Approaches. Medicina 2025, 61, 449. https://doi.org/10.3390/medicina61030449
Zicarelli M, Duni A, Leivaditis K, Lin Y-L, Baciga F, Pugliese S, Fiorentino M, Hsu B-G, Roumeliotis S, Battaglia Y, et al. Comprehensive Insights into Sarcopenia in Dialysis Patients: Mechanisms, Assessment, and Therapeutic Approaches. Medicina. 2025; 61(3):449. https://doi.org/10.3390/medicina61030449
Chicago/Turabian StyleZicarelli, Mariateresa, Anila Duni, Konstantinos Leivaditis, Yu-Li Lin, Federica Baciga, Sara Pugliese, Marco Fiorentino, Bang-Gee Hsu, Stefanos Roumeliotis, Yuri Battaglia, and et al. 2025. "Comprehensive Insights into Sarcopenia in Dialysis Patients: Mechanisms, Assessment, and Therapeutic Approaches" Medicina 61, no. 3: 449. https://doi.org/10.3390/medicina61030449
APA StyleZicarelli, M., Duni, A., Leivaditis, K., Lin, Y.-L., Baciga, F., Pugliese, S., Fiorentino, M., Hsu, B.-G., Roumeliotis, S., Battaglia, Y., Dounousi, E., & Bolignano, D. (2025). Comprehensive Insights into Sarcopenia in Dialysis Patients: Mechanisms, Assessment, and Therapeutic Approaches. Medicina, 61(3), 449. https://doi.org/10.3390/medicina61030449











