Advanced ECG Analysis to Evaluate Multimodal Exercise Effects on Cardiovascular Health
Abstract
1. Introduction
2. Materials and Methods
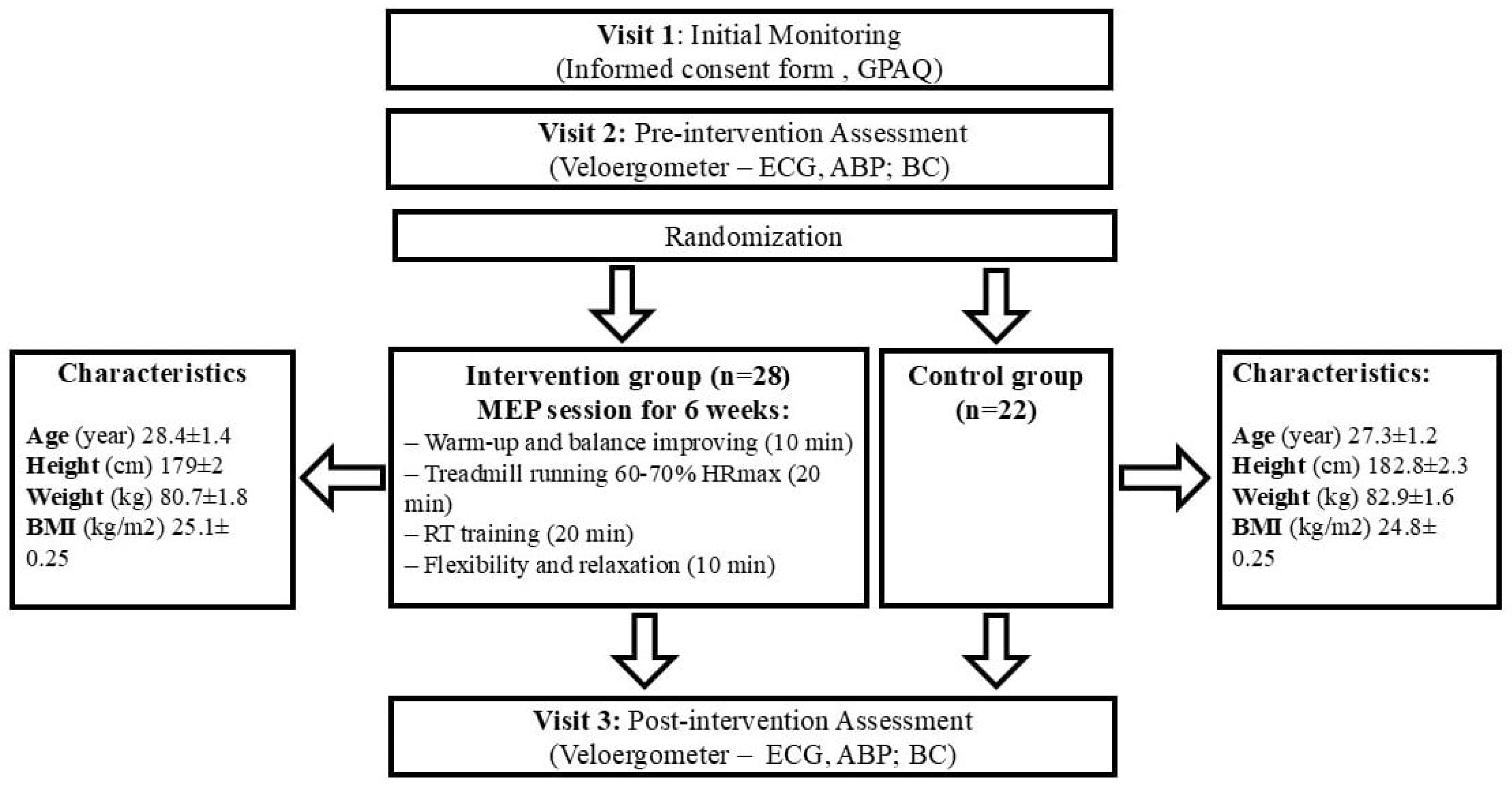
2.1. Trial Design
2.2. Participants
2.3. Training Intervention
2.4. Assessments
2.4.1. Body Composition
2.4.2. Strength
2.4.3. Measures of Physical Activity
2.4.4. ABP Measurements
2.4.5. ECG Measurements
2.5. Data Analysis
2.5.1. Statistical Analysis
2.5.2. Mathematical Methods
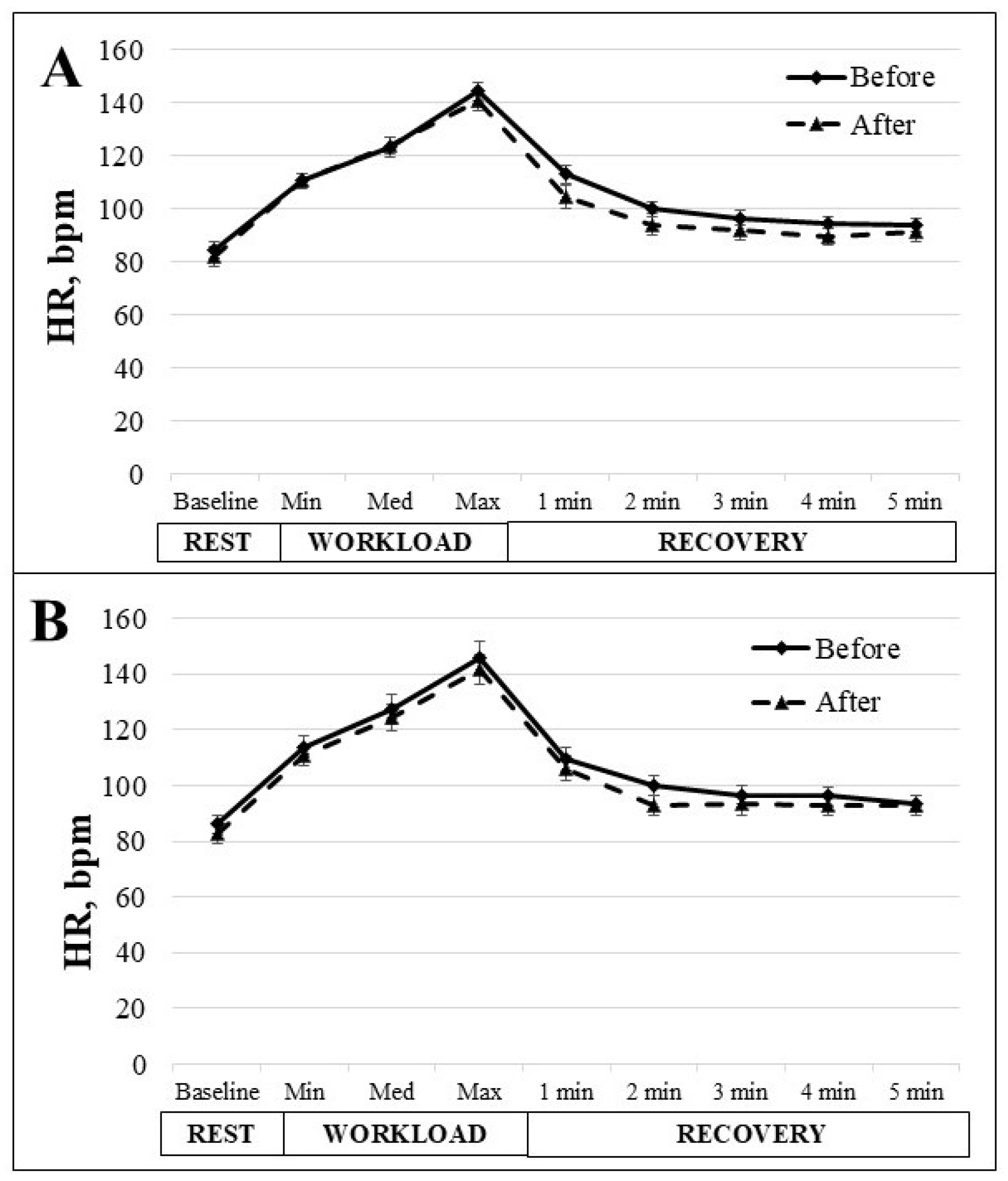
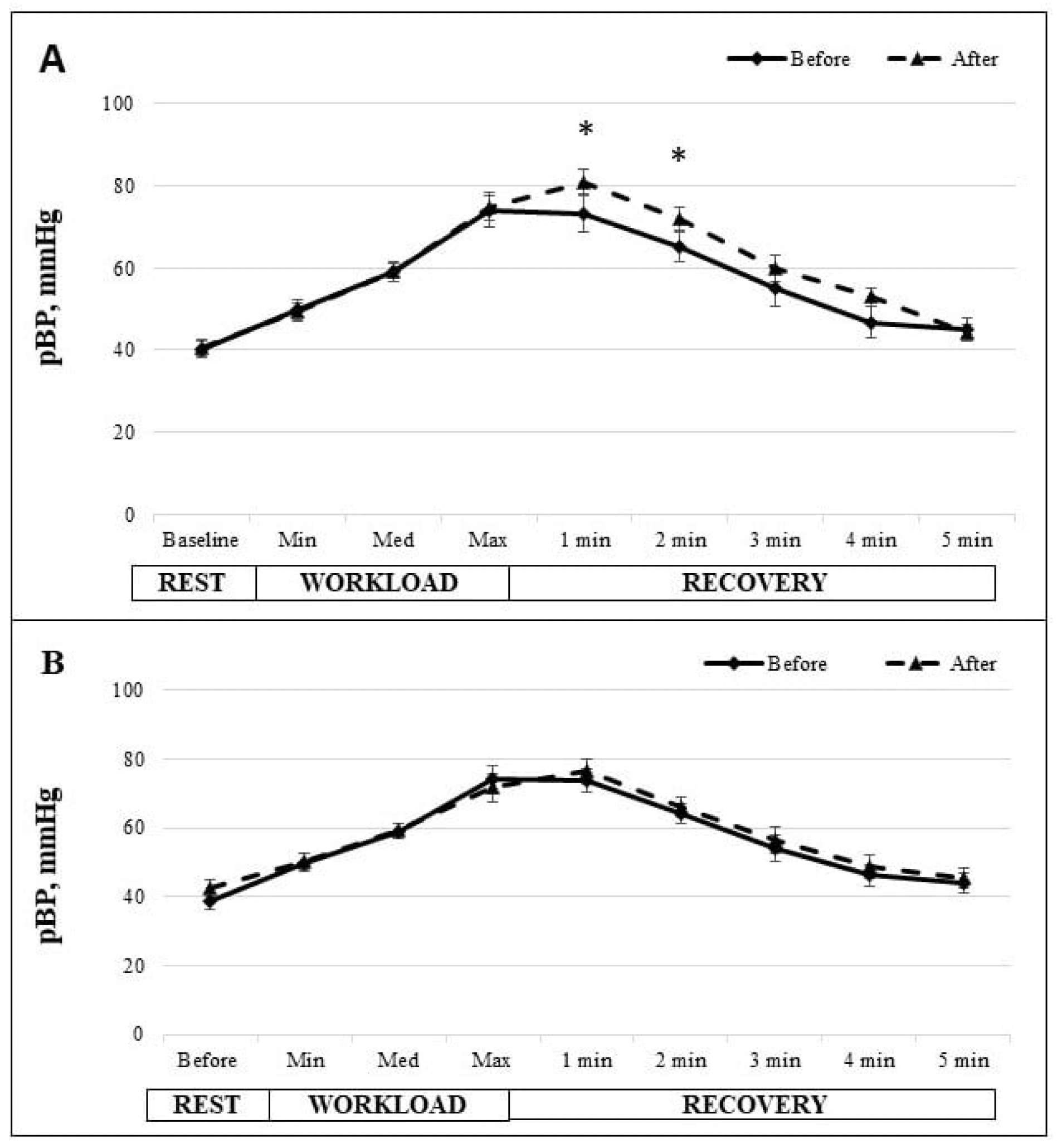
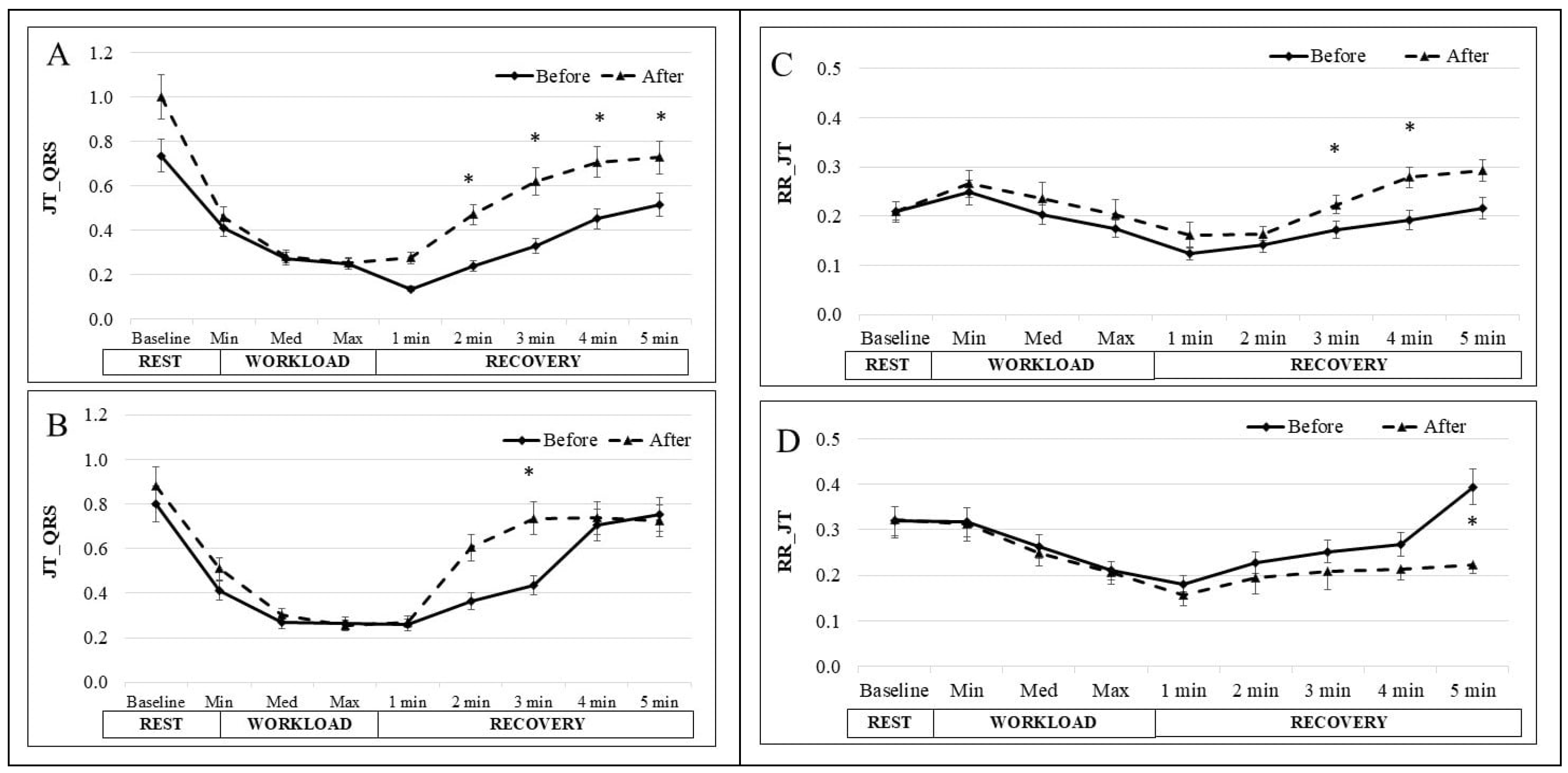
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Cesare, M.; Bixby, H.; Gaziano, T.; Hadeed, L.; Kabudula, C.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Perel, P.; Piñeiro, D.; et al. World Heart Report 2023: Confronting the World’s Number One Killer; World Heart Federation: Geneva, Switzerland, 2023. [Google Scholar]
- World Health Organization. Global Status Report on Physical Activity 2022: Country Profiles; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of exercise to improve cardiovascular health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular effects and benefits of exercise. Front. Cardiovasc. Med. 2018, 5, 408204. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.A.; Minson, C.T.; Halliwill, J.R. The cardiovascular system after exercise. J. Appl. Physiol. 2017, 122, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Ney, B.; Lanzi, S.; Calanca, L.; Mazzolai, L. Multimodal supervised exercise training is effective in improving long term walking performance in patients with symptomatic lower extremity peripheral artery disease. J. Clin. Med. 2021, 10, 2057. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Jeong, W.-M.; Rhyu, H.-S. Effects of multimodal cognitive exercise program on cognitive function, bone density, blood lipid, fitness, and depression in old women with mild cognitive impairment. J. Exerc. Rehabilitation 2023, 19, 27. [Google Scholar] [CrossRef]
- Fraga, I.; Weber, C.; Galiano, W.B.; Iraci, L.; Wohlgemuth, M.; Morales, G.; Cercato, C.; Rodriguez, J.; Pochmann, D.; Dani, C.; et al. Effects of a multimodal exercise protocol on functional outcomes, epigenetic modulation and brain-derived neurotrophic factor levels in institutionalized older adults: A quasi-experimental pilot study. Neural Regen. Res. 2021, 16, 2479–2485. [Google Scholar]
- Oreská, Ľ.; Slobodova, L.; Vajda, M.; Kaplánová, A.; Tirpáková, V.; Cvečka, J.; Buzgó, G.; Ukropec, J.; Ukropcová, B.; Sedliak, M. The effectiveness of two different multimodal training modes on physical performance in elderly. Eur. J. Transl. Myol. 2020, 30, 88–97. [Google Scholar] [CrossRef]
- Sharp, T.; Grandou, C.; Coutts, A.J.; Wallace, L. The effects of high-intensity multimodal training in apparently healthy populations: A systematic review. Sports Med. Open 2022, 8, 43. [Google Scholar] [CrossRef]
- Mafra, M.; Lenzi, O.M.W.; Silveira, F.S.; Schmitt, M.V.; de Oliveira, J.F.; de Sousa, C.A. Multimodal exercise program contributes to balance and motor functions in men and women with Parkinson’s disease differently: An intervention study. Mot. Rev. De Educ. Fis. 2022, 28, e10220015221. [Google Scholar] [CrossRef]
- De Souza, E.O.; Tricoli, V.; Rauch, J.; Alvarez, M.R.; Laurentino, G.; Aihara, A.Y.; Cardoso, F.N.; Roschel, H.; Ugrinowitsch, C.; Rauch, A. Different Patterns in Muscular Strength and Hypertrophy Adaptations in Untrained Individuals Undergoing Nonperiodized and Periodized Strength Regimens. J. Strength Cond. Res. 2018, 32, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Sylta, Ø.; Tønnessen, E.; Sandbakk, Ø.; Hammarström, D.; Danielsen, J.; Skovereng, K.; Rønnestad, B.R.; Seiler, S. Effects of High-Intensity Training on Physiological and Hormonal Adaptions in Well-Trained Cyclists. Med. Sci. Sports Exerc. 2017, 49, 1137–1146. [Google Scholar] [CrossRef]
- Bohm, S.; Mersmann, F.; Arampatzis, A. Human tendon adaptation in response to mechanical loading: A systematic review and meta-analysis of exercise intervention studies on healthy adults. Sports Med. Open 2015, 1, 7. [Google Scholar] [CrossRef]
- Przyklenk, A.; Gutmann, B.; Schiffer, T.; Hollmann, W.; Strueder, H.K.; Bloch, W.; Mierau, A.; Gehlert, S. Endurance Exercise in Hypoxia, Hyperoxia and Normoxia: Mitochondrial and Global Adaptations. Int. J. Sports Med. 2017, 38, 588–596. [Google Scholar] [CrossRef]
- Lollgen, H.; Leyk, D. Exercise Testing in Sports Medicine. Dtsch. Ärzteblatt Int. 2018, 115, 409–416. [Google Scholar] [CrossRef]
- Daanen, H.A.M.; Lamberts, R.P.; Kallen, V.L.; Jin, A.; Van Meeteren, N.L.U. A systematic review on heart-rate recovery to monitor changes in training status in athletes. Int. J. Sports Physiol. Perform. 2012, 7, 251–260. [Google Scholar] [CrossRef]
- Matabuena, M.; Vidal, J.C.; Hayes, P.R.; Huelin Trillo, F. A 6-minute sub-maximal run test to predict VO2 max. J. Sports Sci. 2018, 36, 2531–2536. [Google Scholar] [CrossRef]
- McDermott, M.; Figler, R.; Emery, M.S. The Athlete’s ECG: The Potential Utility in Screening and Prevention of Sudden Cardiac Death. Curr. Emerg. Hosp. Med. Rep. 2023, 11, 66–73. [Google Scholar] [CrossRef]
- Vasileios, E.P.; Zgekos, T. Analysis of digitalized ECG signals based on artificial intelligence and spectral analysis methods specialized in ARVC. Int. J. Numer. Methods Biomed. Eng. 2022, 38, e3644. [Google Scholar]
- Merdjanovska, E.; Rashkovska, A. Comprehensive survey of computational ECG analysis: Databases, methods and applications. Expert Syst. Appl. 2022, 203, 117206. [Google Scholar] [CrossRef]
- Saunoriene, L.; Siauciunaite, V.; Vainoras, A.; Bertasiute, V.; Navickas, Z.; Ragulskis, M. The characterization of the transit through the anaerobic threshold based on relationships between RR and QRS cardiac intervals. PLoS ONE 2019, 14, e0216938. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Carnethon, M.; Polito, K.; Kadish, A.H.; Goldberger, J.J. Autonomic effects on QT-RR interval dynamics after exercise. Am. J. Physiol. Circ. Physiol. 2008, 294, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Ziaukas, P.; Alabdulgader, A.; Vainoras, A.; Navickas, Z.; Ragulskis, M. New approach for visualization of relationships between RR and JT intervals. PLoS ONE 2017, 12, e0174279. [Google Scholar] [CrossRef]
- Brandt, T.; Heinz, E.; Klaaßen, Y.; Limbara, S.; Mörsdorf, M.; Schinköthe, T.; Schmidt, A. MedXFit—Effects of 6 months CrossFit® in sedentary and inactive employees: A prospective, controlled, longitudinal, intervention study. Health Sci. Rep. 2022, 5, e749. [Google Scholar] [CrossRef]
- Nevoit, G.; Filiunova, O.; Potyazhenko, M.; Minser, O.; Bumblyte, I.A.; Vainoras, A. Modern biophysical view of electromagnetic processes of the phenomenon of life of living biological systems as a promising basis for the development of complex medicine: Towards the concept of Bioelectronic Medicine. J. Complex. Health Sci. 2023, 6, 49–66. [Google Scholar] [CrossRef]
- Armstrong, T.; Bull, F. Development of the world health organization global physical activity questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Homan, T.D.; Cichowski, E. Physiology, Pulse Pressure; StatPearls; Stat-Pearls Publishing: Treasure Island, FL, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482408/ (accessed on 5 September 2024).
- Dahlhaus, R.; Kurths, J.; Maass, P.; Timmer, J. Mathematical Methods in Time Series Analysis and Digital Image Processing (Understanding Complex Systems Series); Springer: Berlin/Heidelberg, Germany, 2008; p. 310. [Google Scholar]
- Bikulčienė, L.; Venskaitytė, E.; Gargasas, L.; Jurkonis, V. The Measure of human vital signals complexity by matrix analysis. In Chaos and Complex Systems: Proceedings of the 4th International Interdisciplinary Chaos Symposium, Antalya, Turkey, 29 April–2 May 2012; Springer: Berlin/Heidelberg, Germany, 2013; pp. 449–458. [Google Scholar]
- Grässler, B.; Thielmann, B.; Böckelmann, I.; Hökelmann, A. Effects of different exercise interventions on heart rate variability and cardiovascular health factors in older adults: A systematic review. Eur. Rev. Aging Phys. Act. 2021, 18, 24. [Google Scholar] [CrossRef]
- Ma, D.; Li, L.; Shi, W.; Li, M.; Zhang, J.; Fan, Y.; Kang, Y.; Zhang, X.; Yu, P.; Zhang, Q.; et al. Multimodal coupling and HRV assessment characterize autonomic functional changes in congestive heart failure patients with sinus rhythm or severe arrhythmia. Biomed Signal Process Control 2024, 89, 105764. [Google Scholar] [CrossRef]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac parasympathetic reactivation following exercise: Implications for training prescription. Sports Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef]
- Travers, G.; Kippelen, P.; Trangmar, S.J.; González-Alonso, J. Physiological Function during Exercise and Environmental Stress in Humans—An Integrative View of Body Systems and Homeostasis. Cells 2022, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Suzic Lazic, J.; Dekleva, M.; Soldatovic, I.; Leischik, R.; Suzic, S.; Radovanovic, D.; Djuric, B.; Nesic, D.; Lazic, M.; Mazic, S. Heart rate recovery in elite athletes: The impact of age and exercise capacity. Clin. Physiol. Funct. Imaging 2017, 37, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, M.J.; Halliwill, J.R. Recovery from exercise: Vulnerable state, window of opportunity, or crystal ball? Front. Physiol. 2015, 6, 204. [Google Scholar] [CrossRef]
- Bartels, R.; Prodel, E.; Laterza, M.C.; de Lima, J.R.P.; Peçanha, T. Heart rate recovery fast-to-slow phase transition: Influence of physical fitness and exercise intensity. Ann. Noninvasive Electrocardiol. 2018, 23, e12521. [Google Scholar] [CrossRef]
- Green, D.J.; Hopman, M.T.E.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H.J. Vascular adaptation to exercise in humans: Role of hemodynamic stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef]
- Mason, J.W.; Ramseth, D.J.; Chanter, D.O.; Moon, T.E.; Goodman, D.B.; Mendzelevski, B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J. Electrocardiol. 2007, 40, 228–234. [Google Scholar] [CrossRef]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef]
- Joustra, R.; Boulaksil, M.; Meijburg, H.W.; Smeets, J.L. Dizziness and slow heart rate during exercise. Neth. Hear. J. 2017, 25, 461–462. [Google Scholar] [CrossRef]
- Copeland, S.R.; Mills, M.C.; Lerner, J.L.; Crizer, M.F.; Thompson, C.W.; Sullivan, J.M. Hemodynamic effects of aerobic vs resistance exercise. J. Hum. Hypertens. 1996, 10, 747–753. [Google Scholar]
- Danieli, A.; Lusa, L.; Potočnik, N.; Meglič, B.; Grad, A.; Bajrović, F.F. Resting heart rate variability and heart rate recovery after submaximal exercise. Clin. Auton. Res. 2014, 24, 53–61. [Google Scholar] [CrossRef]
- Baev, V.M.; Kudryavtseva, E.N. Adaptation to physical load and the state of the autonomic nervous system in young women with low blood pressure. Patol. Fiziol. I Eksperimental’naia Ter. 2015, 59, 97–100. [Google Scholar]
- Tang, K.S.; Medeiros, E.D.; Shah, A.D. Wide pulse pressure: A clinical review. J. Clin. Hypertens. 2020, 22, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Saltin, B.; Rådegran, G.; Koskolou, M.D.; Roach, R.C. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 1998, 162, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Venskaitytė, E.; Poderys, J.; Balagué, N.; Bikulciene, L. Assessment of dynamics of inter-parameter concatenation during exercise tests. Elektron. Ir Elektrotechnika 2009, 89–92. [Google Scholar]
- Pelchovitz, D.J.; Ng, J.; Chicos, A.B.; Bergner, D.W.; Goldberger, J.J. QT-RR hysteresis is caused by differential autonomic states during exercise and recovery. Am. J. Physiol. -Heart Circ. Physiol. 2012, 302, 2567–2573. [Google Scholar] [CrossRef]
- Torrents, C.; Balague, N. Dynamic Systems Theory and Sports Training. Educ. Phys. Train. Sport 2006, 60, 72–88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brazdzionyte, R.; Velicka, D.; Motiejunaite, K.; Poderiene, K.; Kairiukstiene, Z. Advanced ECG Analysis to Evaluate Multimodal Exercise Effects on Cardiovascular Health. Medicina 2025, 61, 473. https://doi.org/10.3390/medicina61030473
Brazdzionyte R, Velicka D, Motiejunaite K, Poderiene K, Kairiukstiene Z. Advanced ECG Analysis to Evaluate Multimodal Exercise Effects on Cardiovascular Health. Medicina. 2025; 61(3):473. https://doi.org/10.3390/medicina61030473
Chicago/Turabian StyleBrazdzionyte, Ruta, Deivydas Velicka, Kristina Motiejunaite, Kristina Poderiene, and Zivile Kairiukstiene. 2025. "Advanced ECG Analysis to Evaluate Multimodal Exercise Effects on Cardiovascular Health" Medicina 61, no. 3: 473. https://doi.org/10.3390/medicina61030473
APA StyleBrazdzionyte, R., Velicka, D., Motiejunaite, K., Poderiene, K., & Kairiukstiene, Z. (2025). Advanced ECG Analysis to Evaluate Multimodal Exercise Effects on Cardiovascular Health. Medicina, 61(3), 473. https://doi.org/10.3390/medicina61030473





