Imaging Diagnosis of Major Kidney and Urinary Tract Disorders in Children
Abstract
1. Introduction
2. Methodology
2.1. Study Design
2.2. Data Collection and Selection
2.3. Inclusion and Exclusion Criteria
2.4. Data Categorization and Analysis
2.5. Ethical Considerations
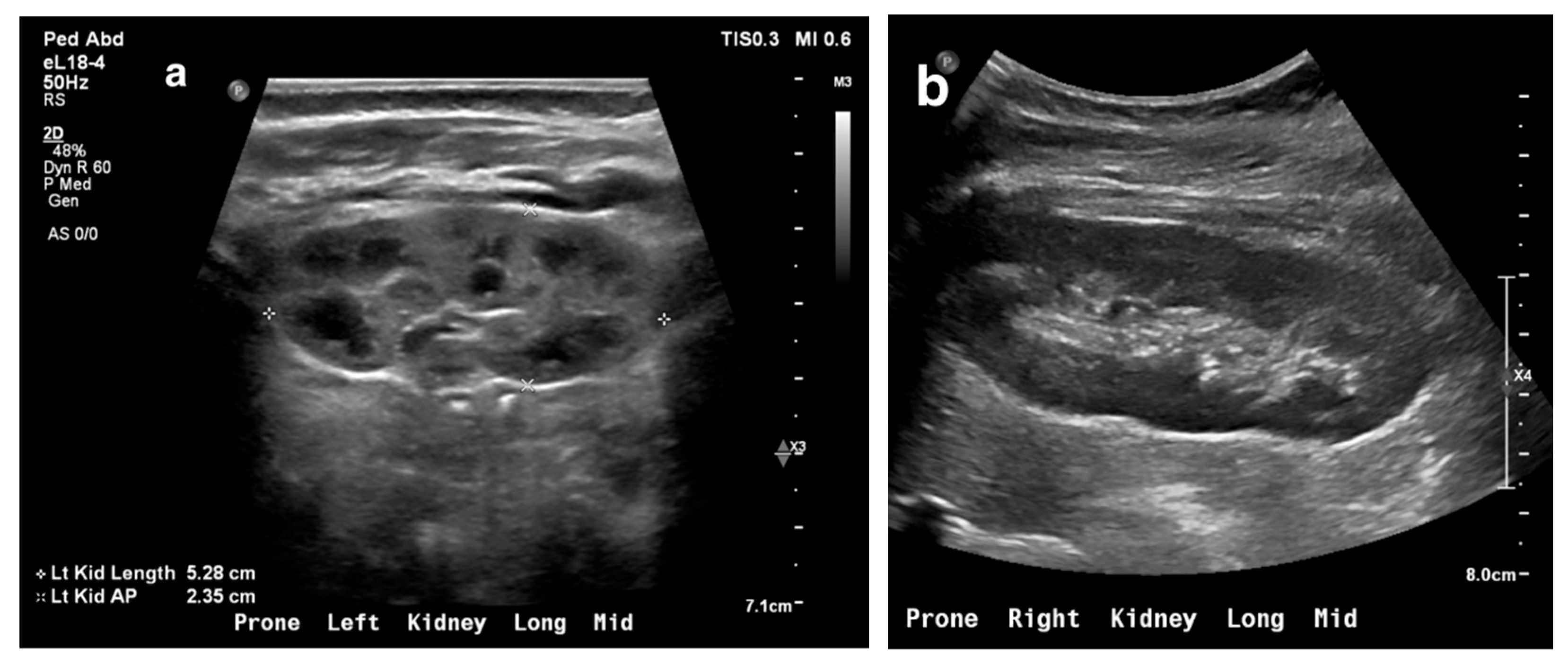
3. Ultrasonography
3.1. Procedure
- Renal shape, size, cortical thickness, and echogenicity
- Corticomedullary differentiation
- Renal pelvis, ureters, and bladder
3.2. US Applications
3.3. Limitations
4. Emerging Ultrasound Technologies
4.1. Three-Dimensional Ultrasound (3D US)
4.2. Contrast-Enhanced Ultrasound (CEUS)
4.3. Contrast-Enhanced Voiding Urosonography (CEVUS)
4.3.1. Indications
4.3.2. Advantages
4.3.3. Limitations
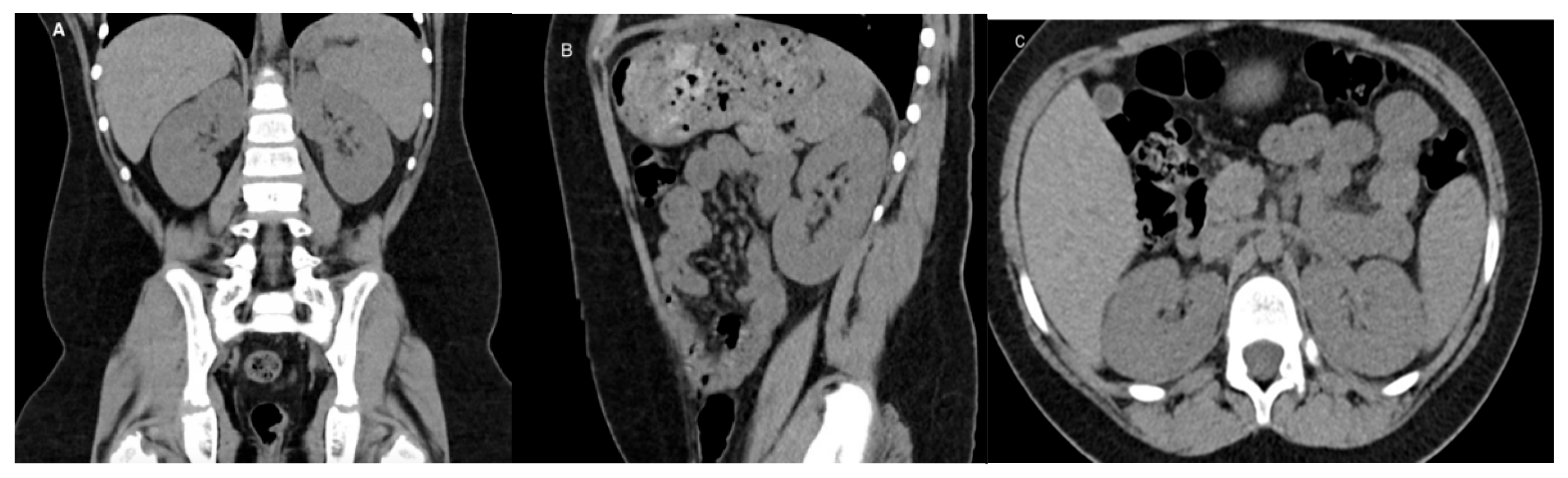
5. Computed Tomography (CT)
5.1. Technique
5.2. Application
5.3. Limitations
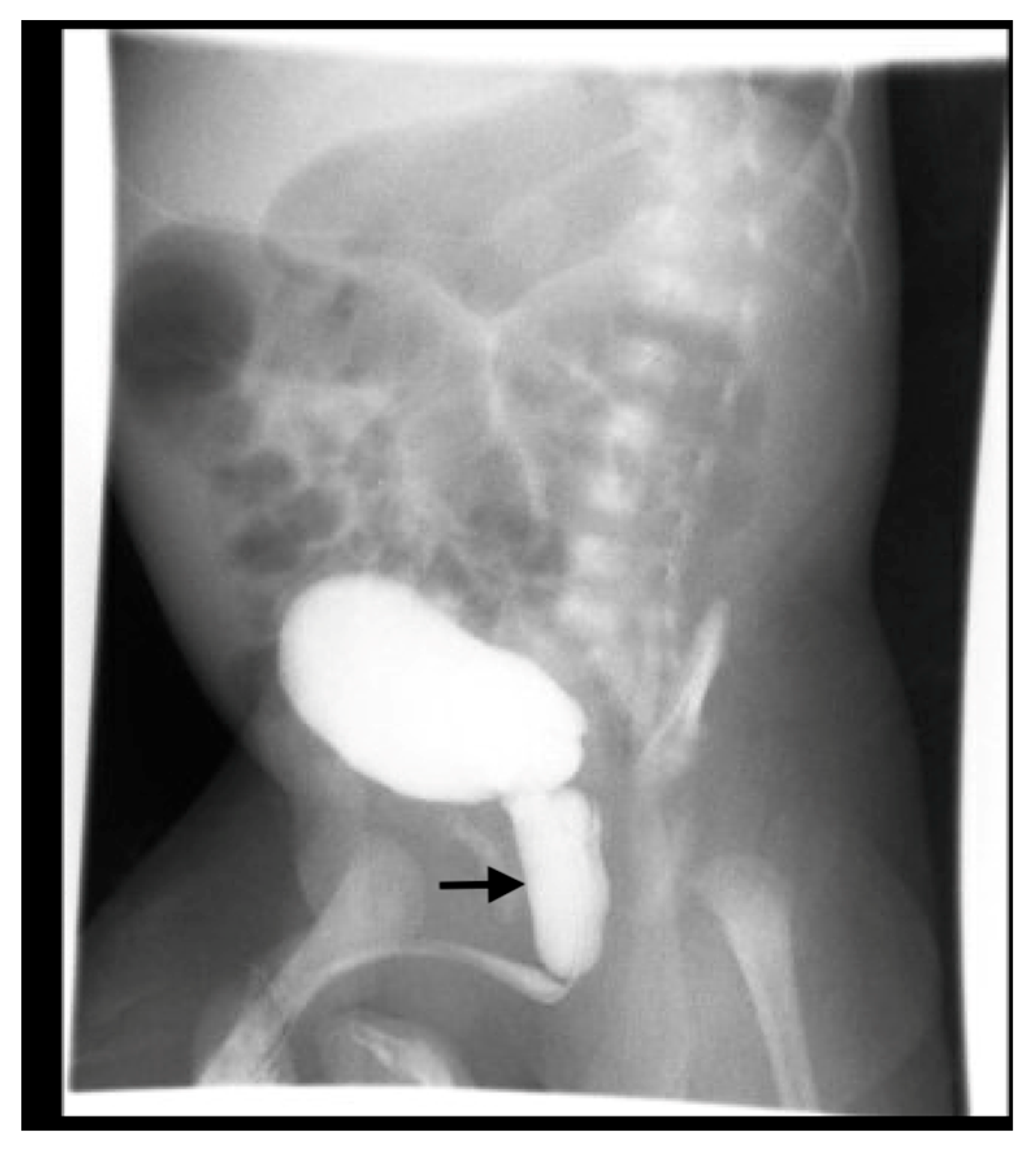
6. Voiding Cystourethrography (VCUG)
6.1. Procedure
6.2. Application
6.3. Complications
6.4. Limitations
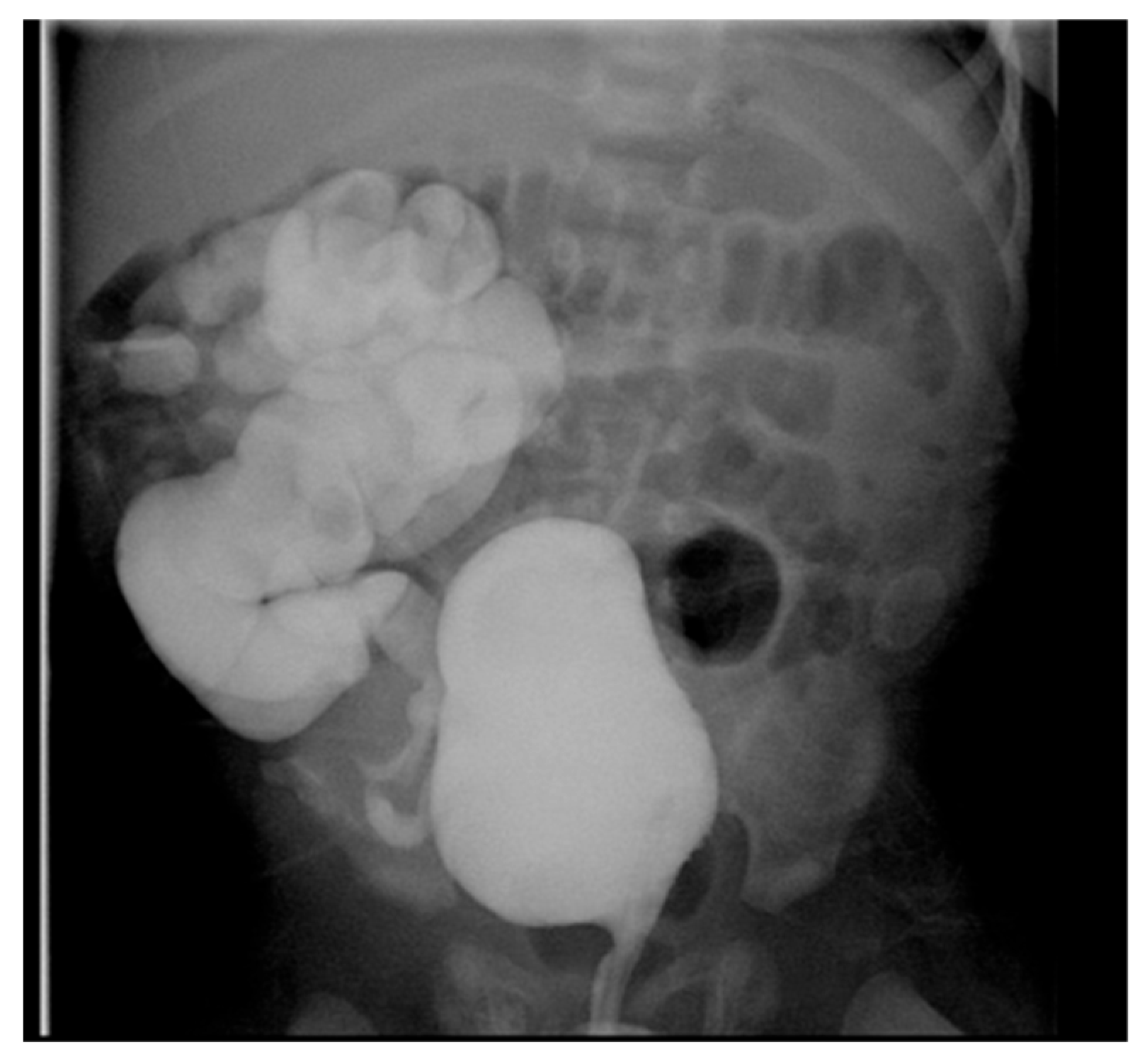
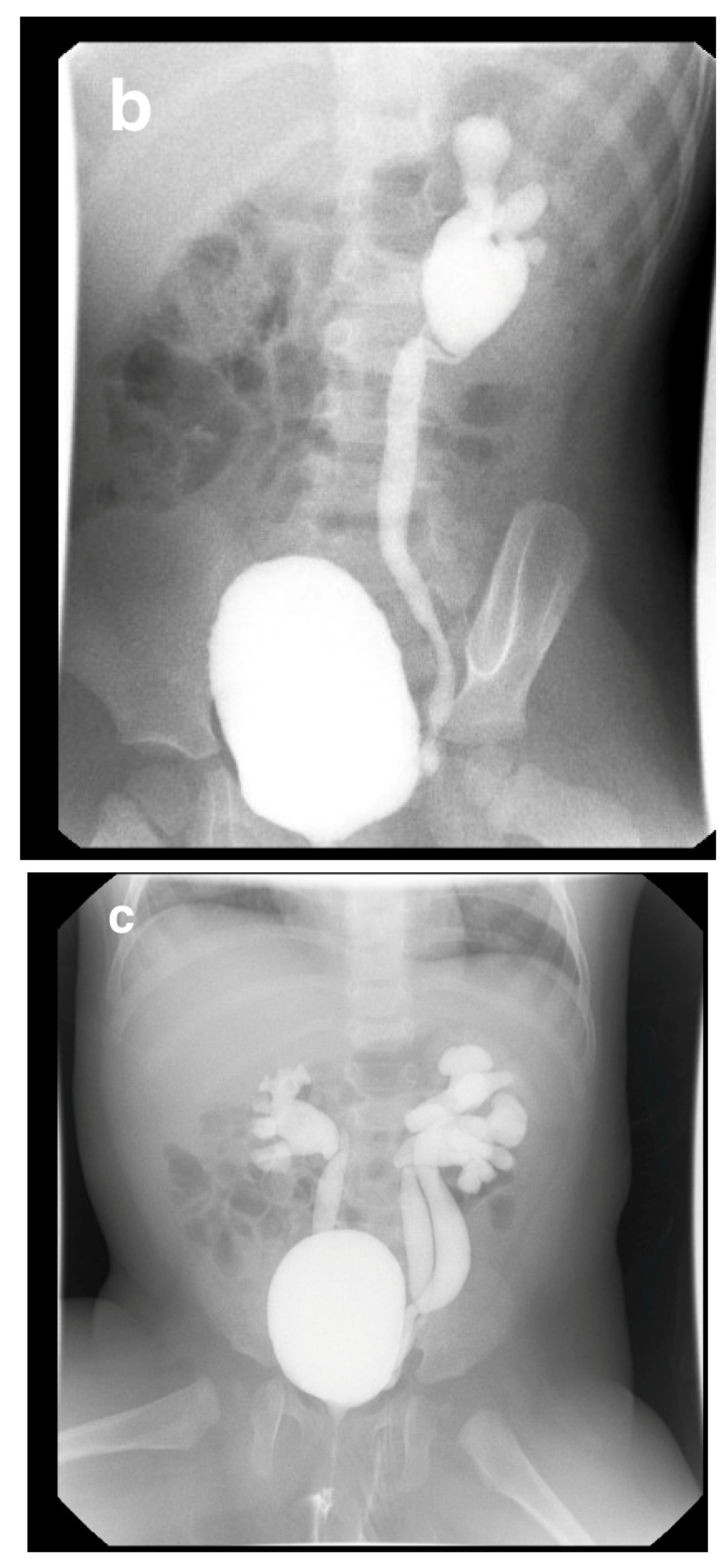
7. Intravenous Pyelography (IVP)
7.1. Technique
7.2. Indications
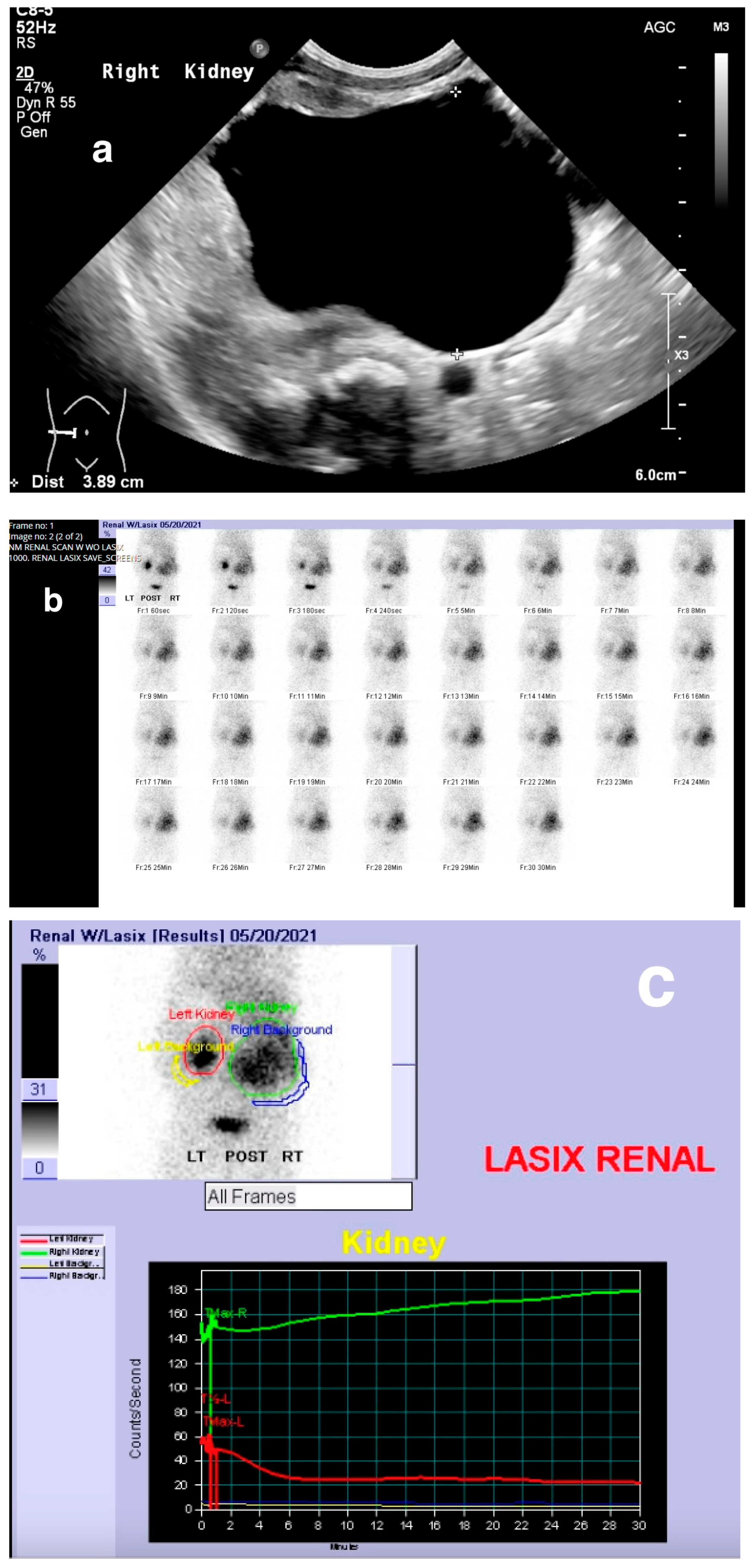
8. Nuclear Medicine
8.1. Procedure
8.1.1. Renal Cortical Imaging
Application
- Acute Pyelonephritis
- 2.
- Renal Scarring
8.1.2. Dynamic Renal Scintigraphy
8.1.3. Radionuclide Cystography (RNC)
9. Magnetic Resonance Imaging (MRI)
- MRI provides high-resolution images of the urinary tract without radiation risk. It can image the abdomen in multiple planes, though sedation is often required for infants and younger children.
- While MRI contrast agents are less nephrotoxic than iodinated contrasts, gadolinium-based contrast agents (GBCAs) can cause nephrogenic systemic fibrosis (NSF), a rare but serious condition, particularly in patients with advanced chronic kidney disease (CKD, GFR < 30 mL/min/1.73 m2). The need for GBCAs should be carefully considered in patients with CKD or acute kidney injury (AKI) [47,48].
9.1. Application
9.2. Limitations
- Expensive, requires sedation in children.
- Longer scan times compared to CT.
10. Fetal Urogenital Imaging
11. Dysplastic Kidney
12. Multicystic Dysplastic Kidney (MCDK)
13. Obstructive Uropathy
14. Duplex Collecting System
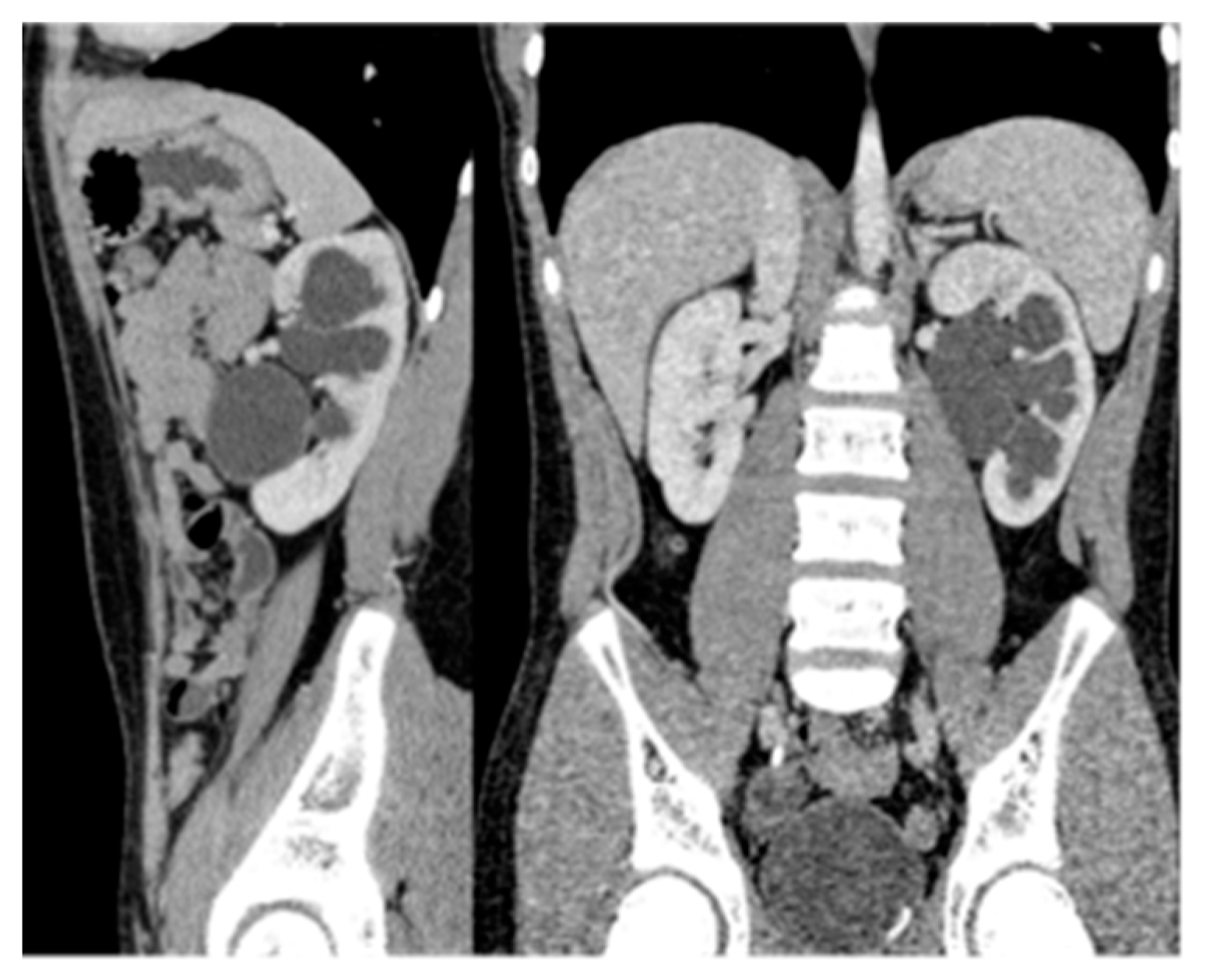
15. Horseshoe and Ectopic Kidneys
- A horseshoe kidney is the most common renal fusion anomaly, occurring in about 1 in 400 live births. In this condition, both kidneys are fused at their lower poles, forming a “horseshoe” shape (Figure 14)
- Renal ectopia occurs in approximately 1 in 900 births and is typically unilateral. In about 40% of cases, the ectopic kidney is located in the pelvis. Crossed ectopia occurs when one kidney crosses the midline and fuses with the other, often resulting in a “crossed-fused” appearance.

16. Vesicoureteral Reflux
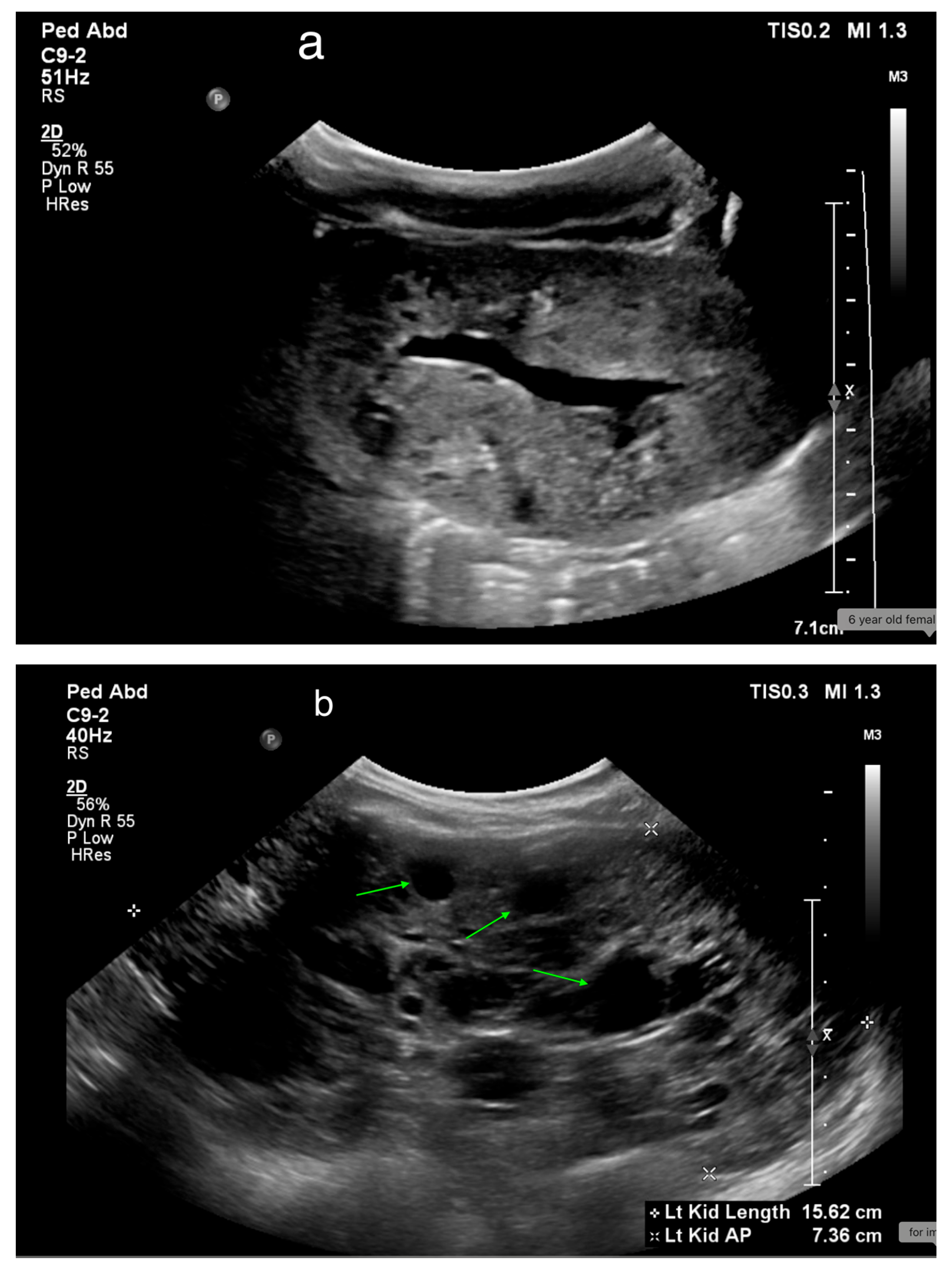
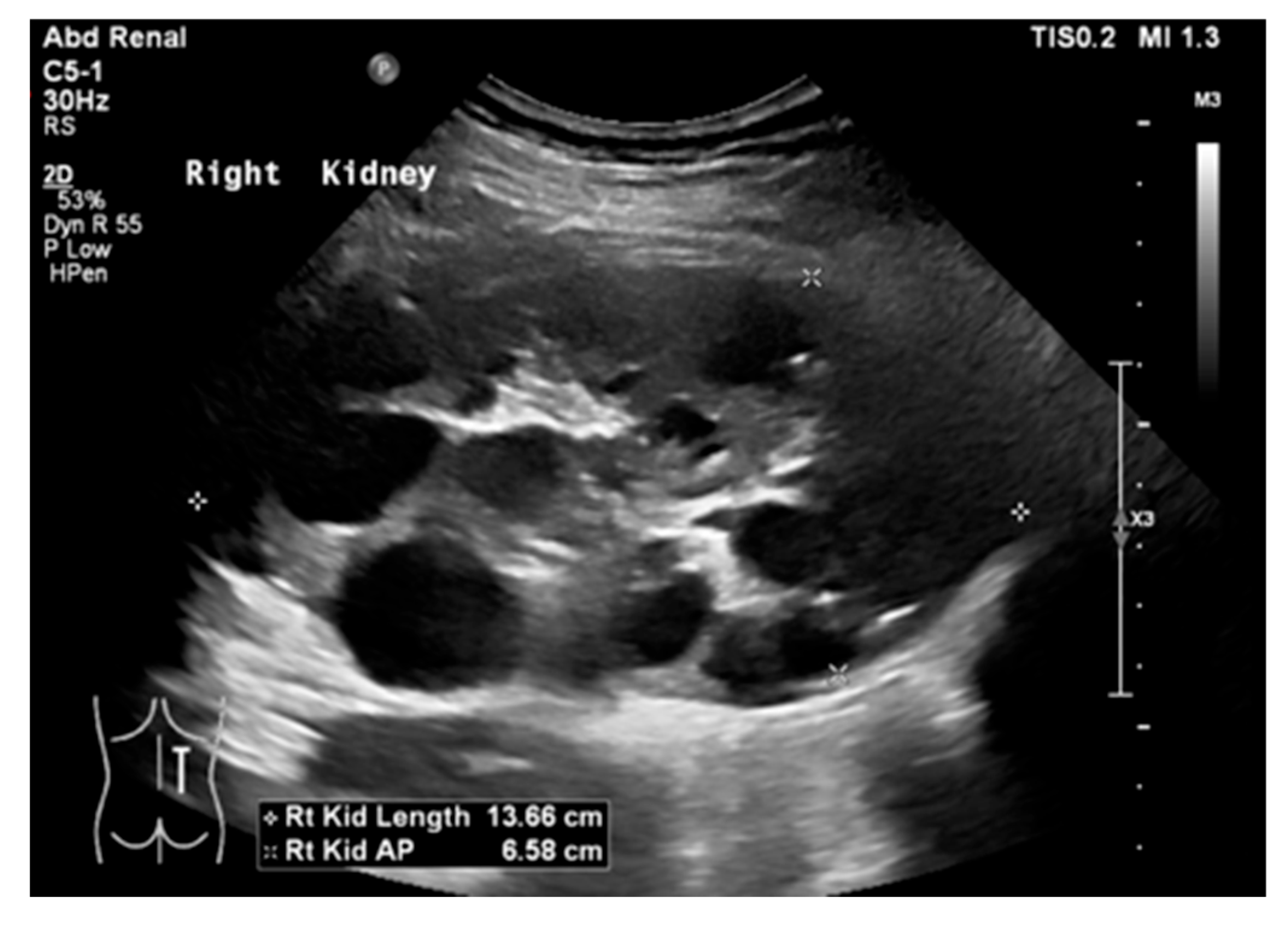
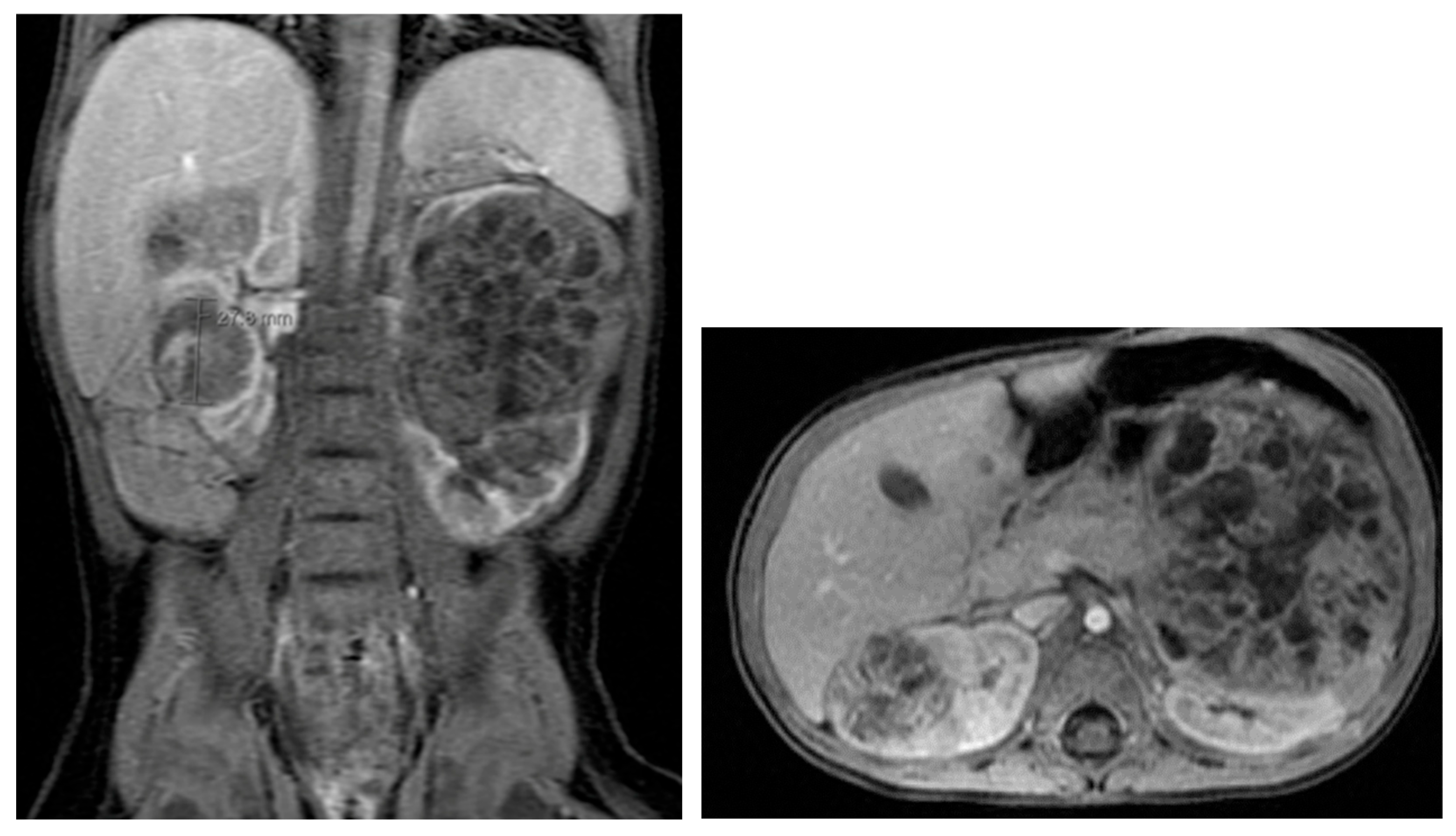
17. Cystic Kidney Disease
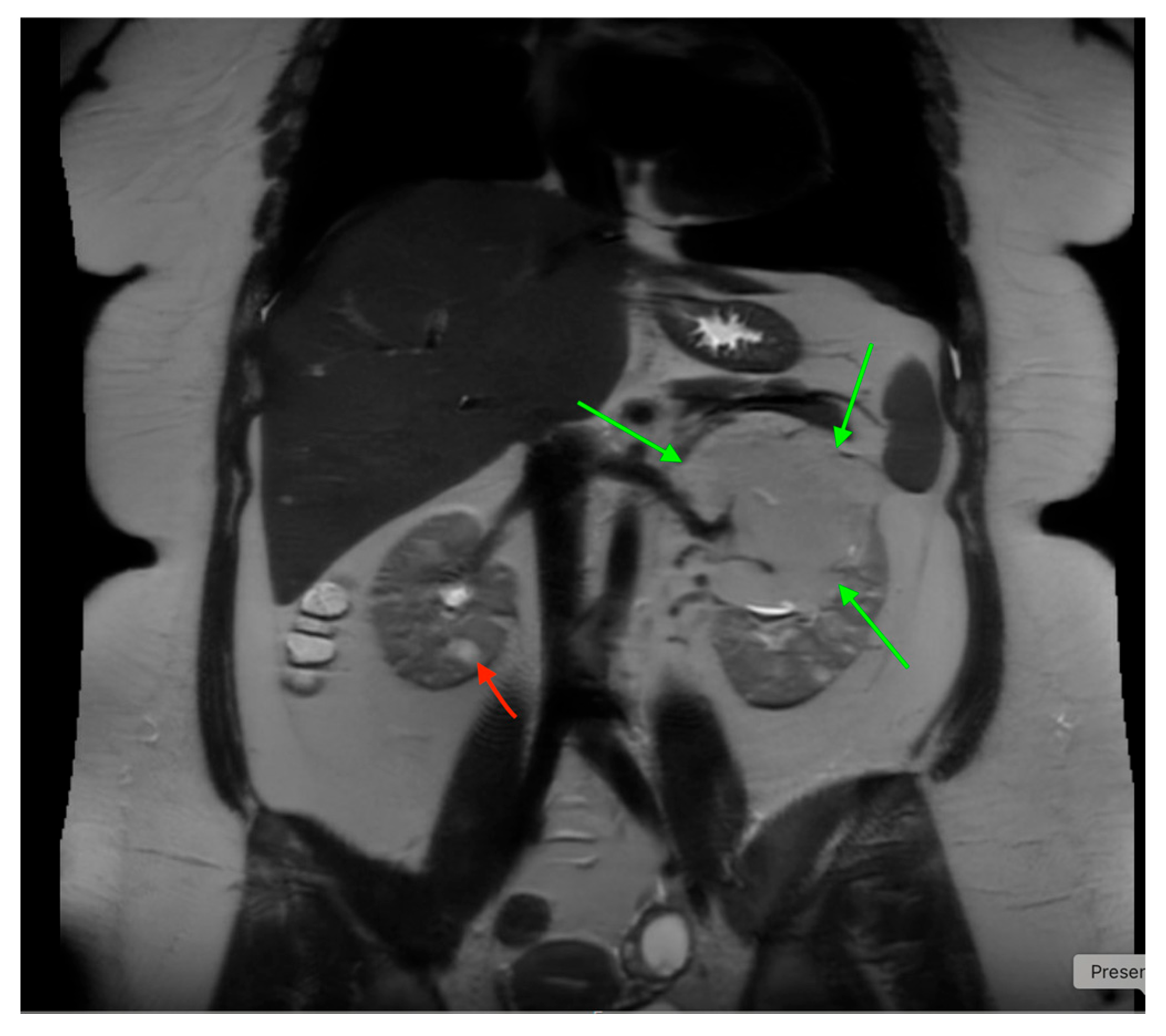
18. Tuberous Sclerosis
19. Kidney Failure
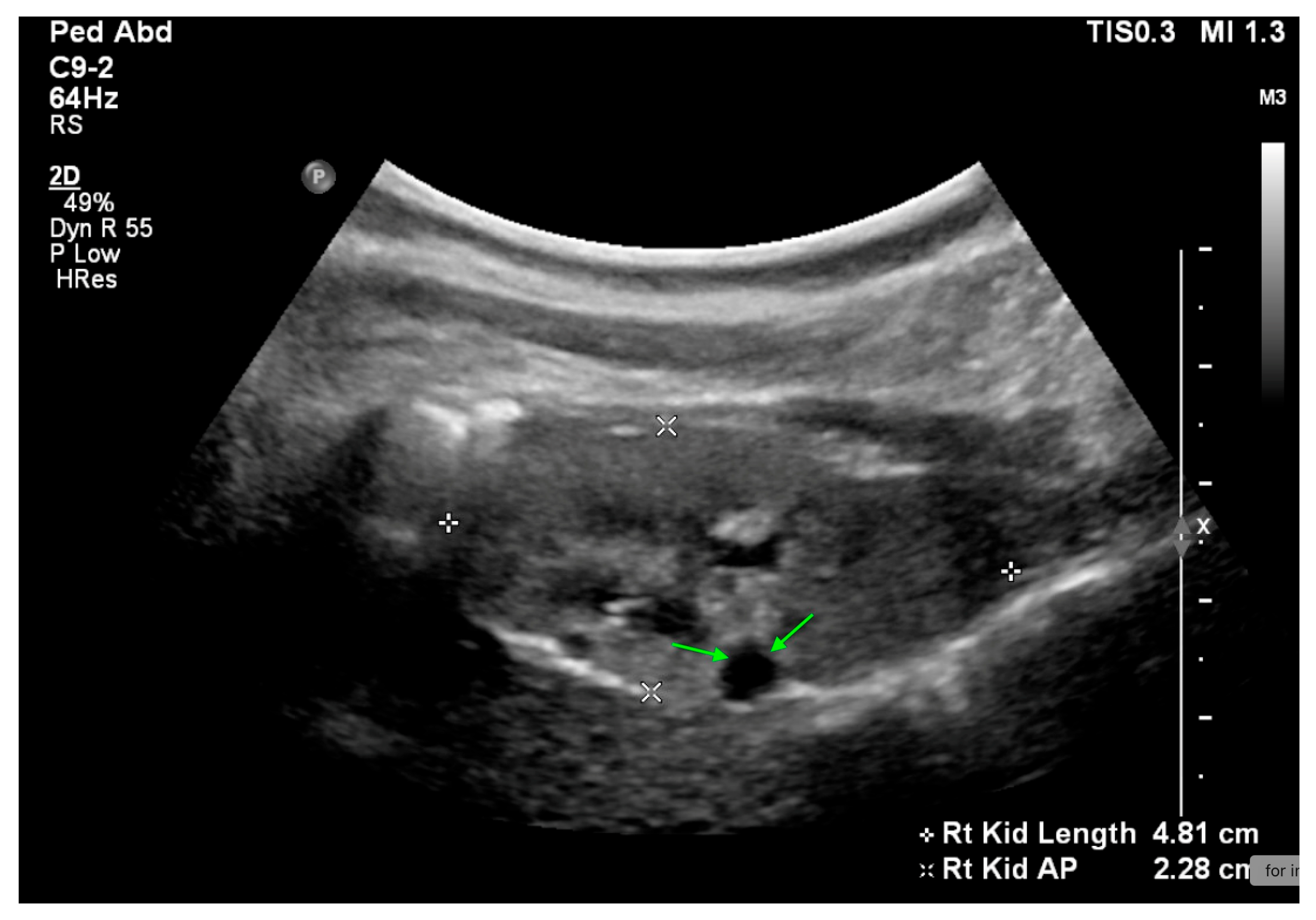
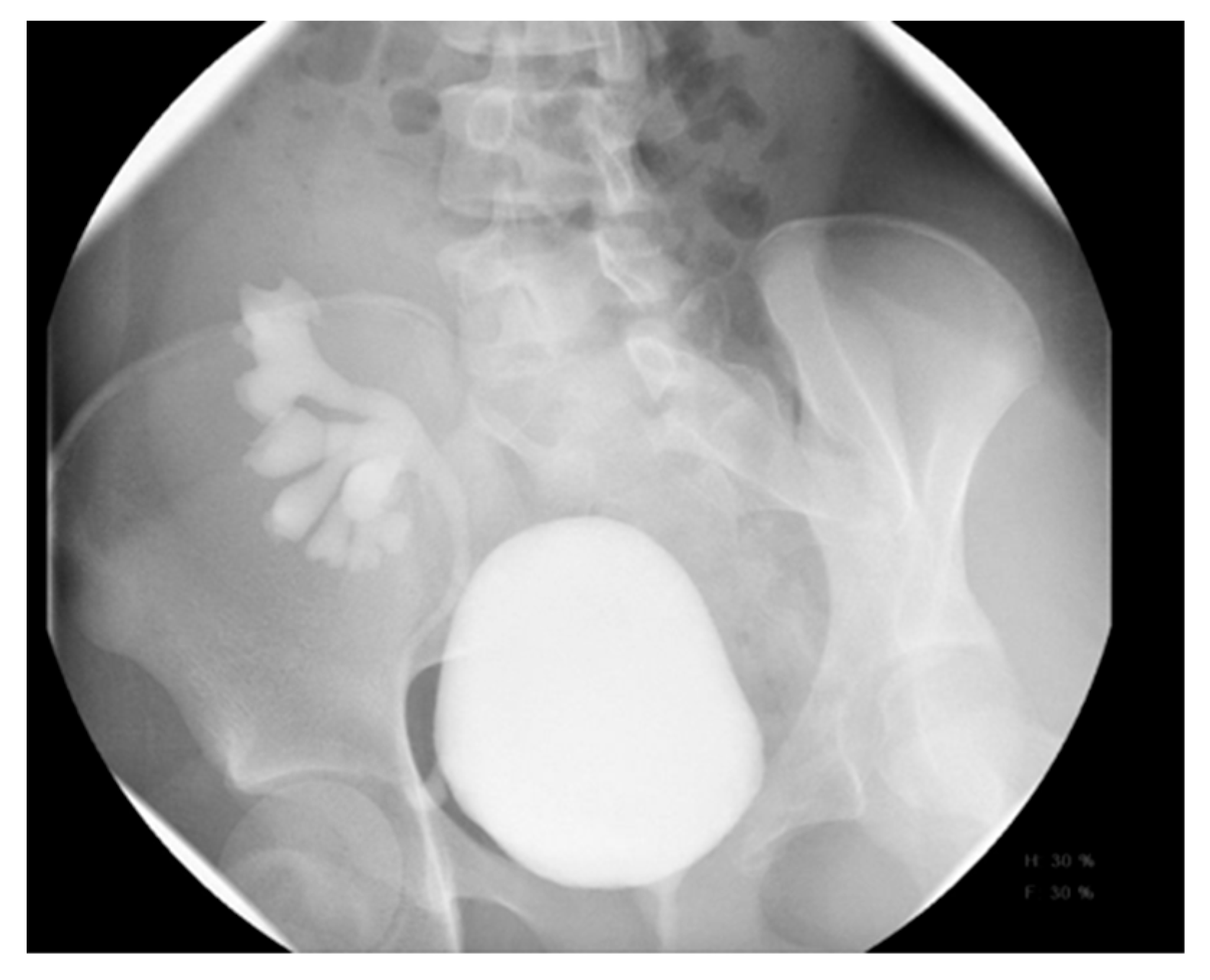
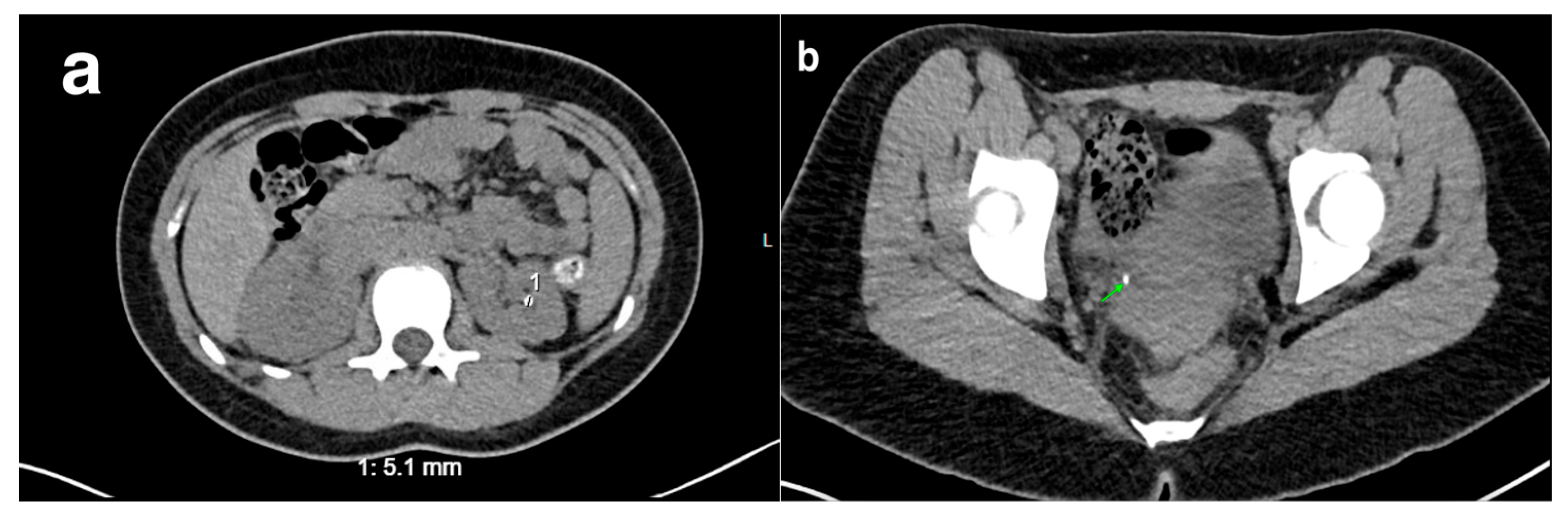
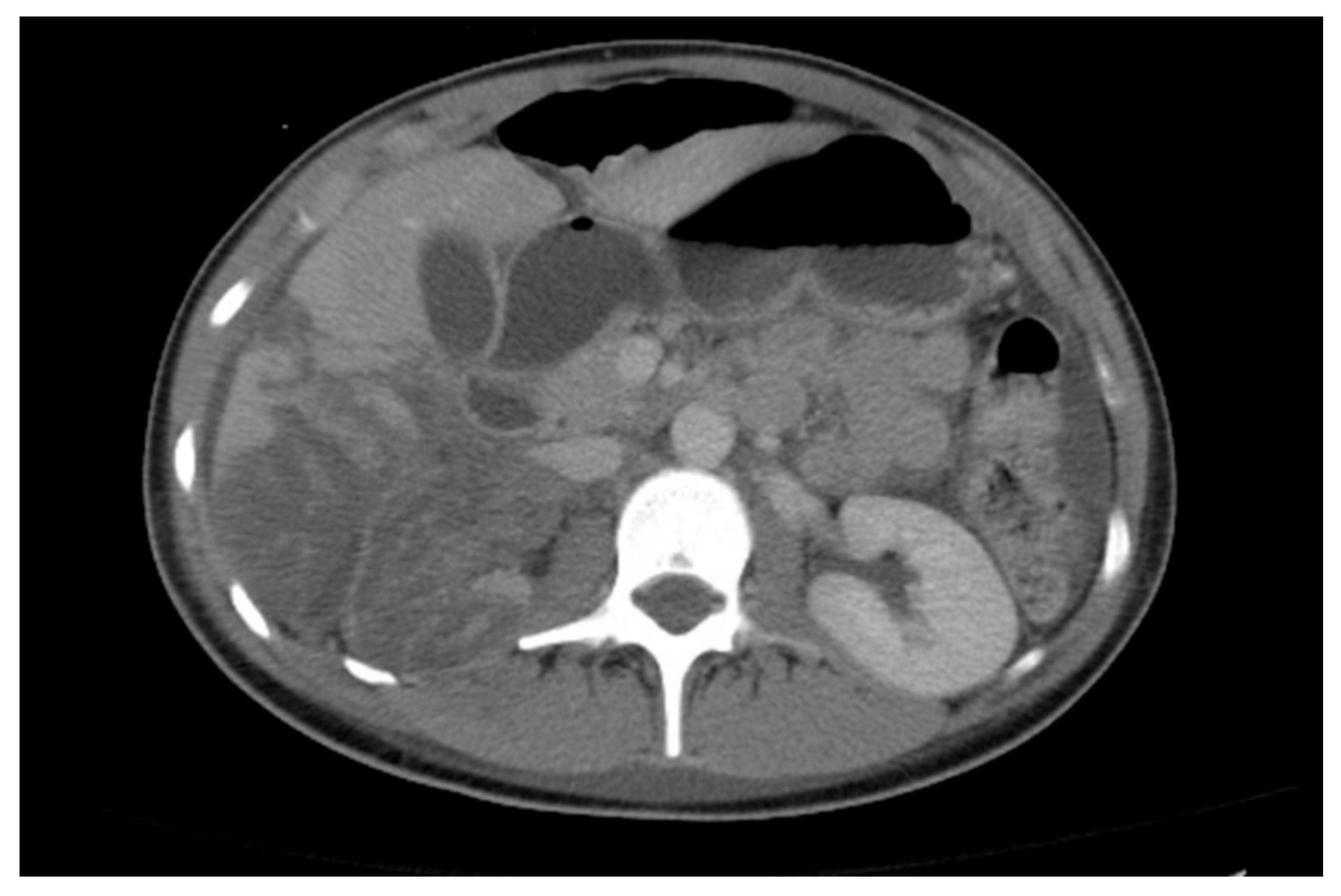
20. Nephrolithiasis
21. Trauma to the Urinary Tract
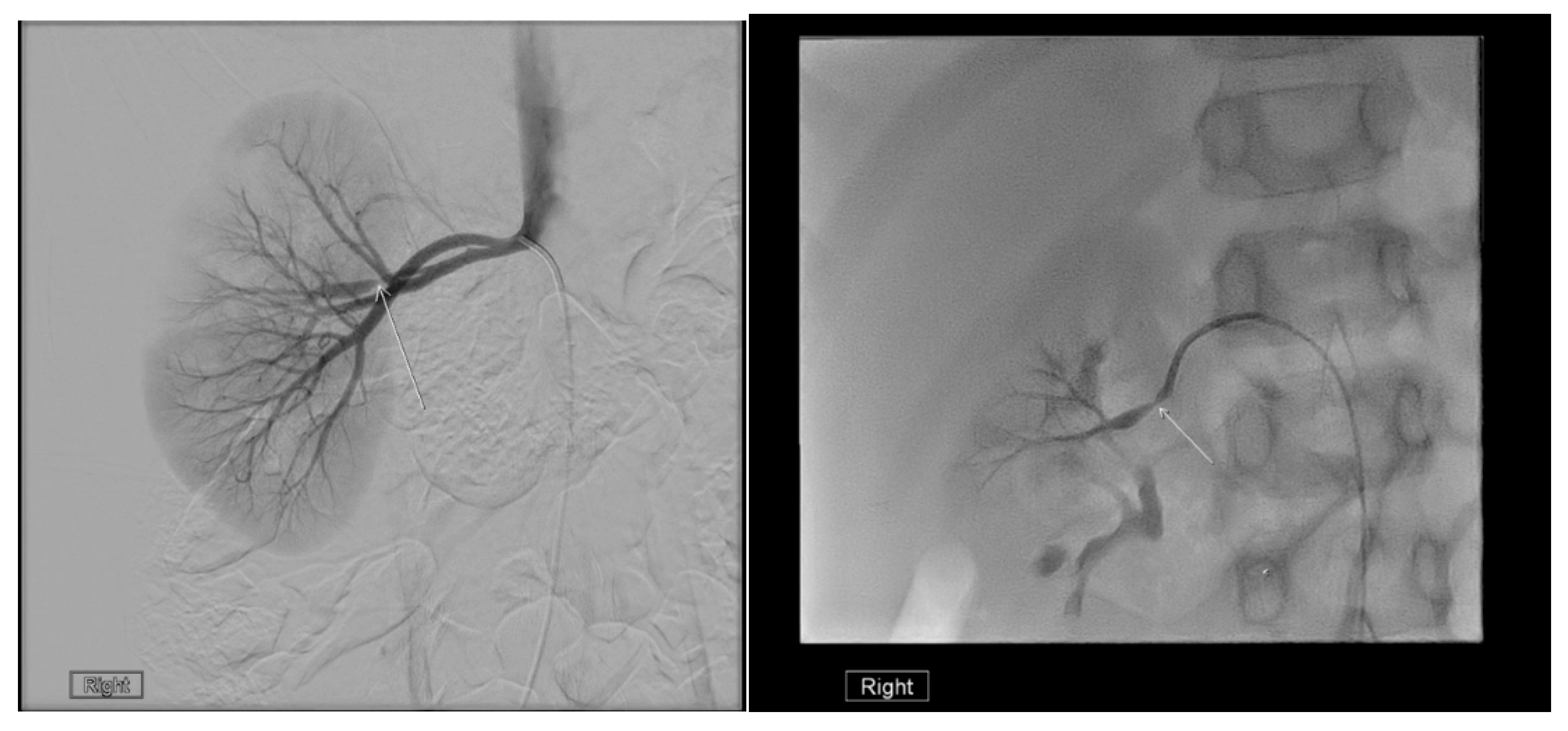
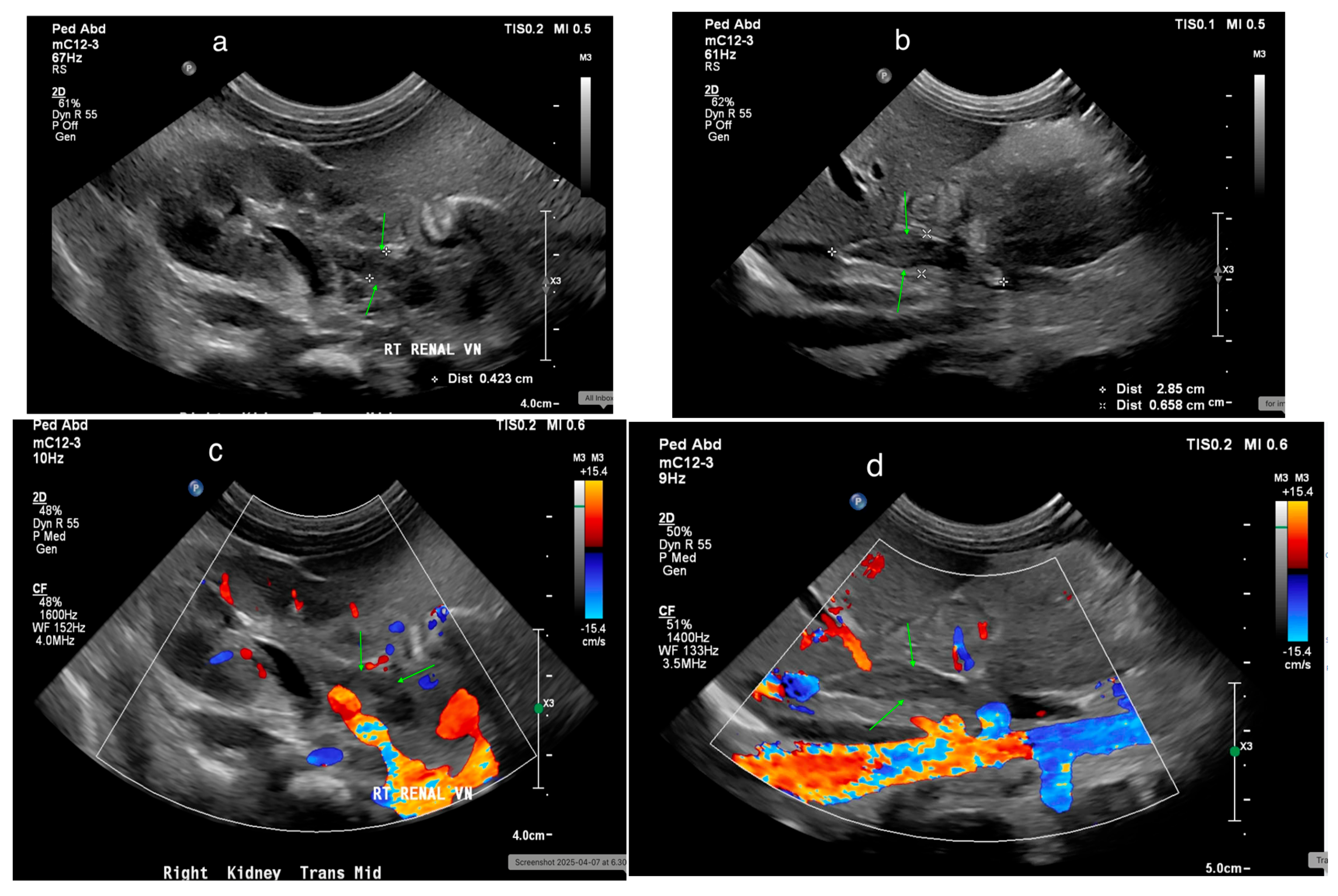
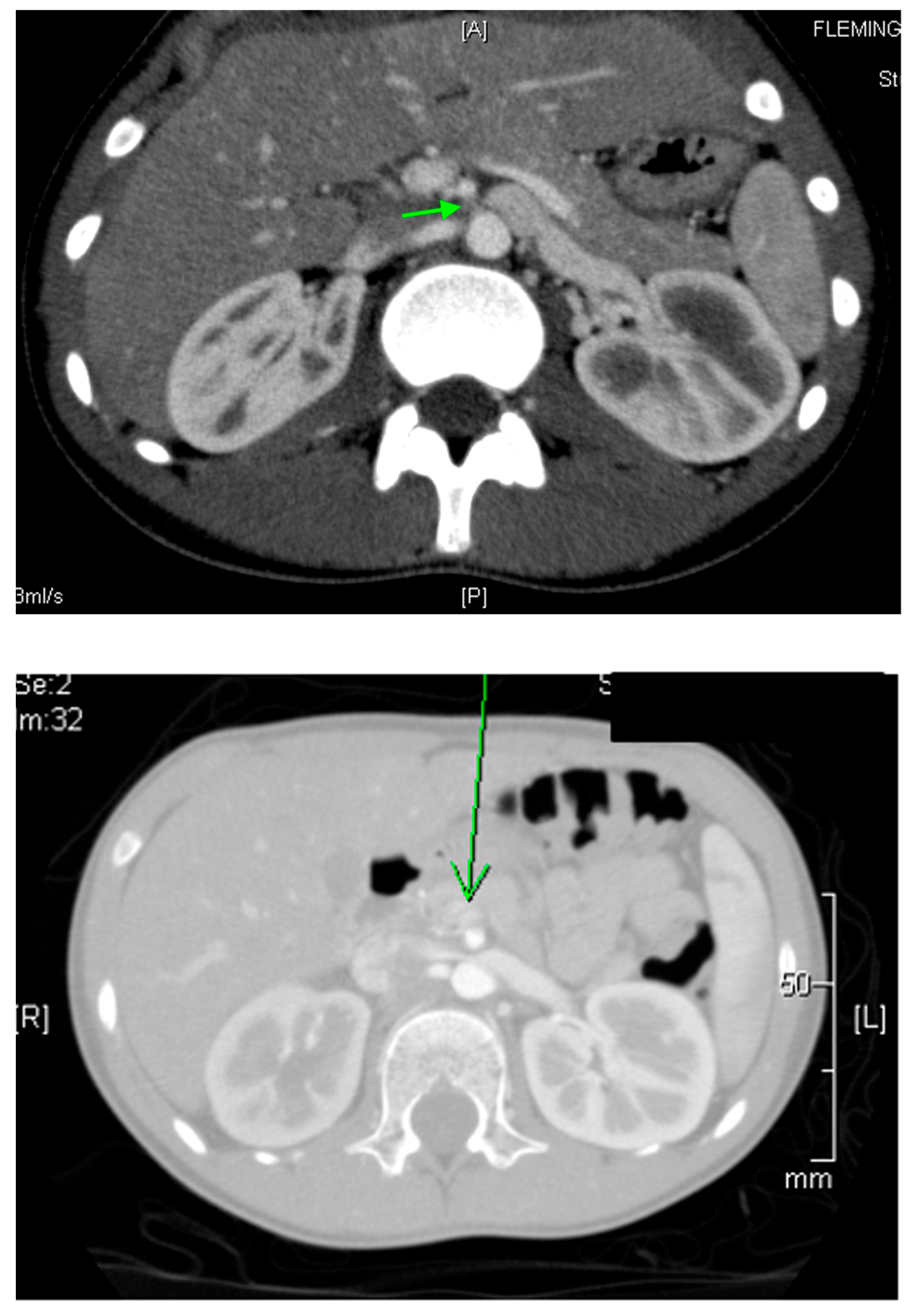
22. Renal Vascular Disease
22.1. Renal Artery Stenosis
22.2. Renal Vein Thrombosis
22.3. Nutcracker Syndrome
23. Urinary Tract Tumors
24. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fried, J.G.; Morgan, M.A. Renal Imaging: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 73, 552–565. [Google Scholar] [CrossRef]
- Viteri, B.; Calle-Toro, J.S.; Furth, S.; Darge, K.; Hartung, E.A.; Otero, H. State-of-the-Art Renal Imaging in Children. Pediatrics 2020, 145, e20190829. [Google Scholar] [CrossRef]
- Maliborski, A.; Zegadło, A.; Placzyńska, M.; Sopińska, M.; Lichosik, M.; Jobs, K. The role of modern diagnostic imaging in diagnosing and differentiating kidney diseases in children. Dev. Period Med. 2018, 22, 81–87. [Google Scholar] [CrossRef]
- Emma, F.; Goldstein, S.L.; Bagga, A.; Bates, C.M.; Shroff, R. (Eds.) Diagnostic imaging in pediatric nephrology. In Pediatric Nephrology, 8th ed.; Springer: Cham, Switzerland, 2022; pp. 173–211. [Google Scholar]
- Kher, K.; Schnaper, H.W.; Greenbaum, L.A. Diagnostic imaging of the urinary tract. In Clinical Pediatric Nephrology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 87–103. [Google Scholar]
- O’neill, W.C. Ultrasound in Nephrology: Applications in Kidney Disease and Procedures. Clin. J. Am. Soc. Nephrol. 2014, 9, 373–381. [Google Scholar] [CrossRef]
- Riccabona, M. Basics, principles, techniques and modern methods in paediatric ultrasonography. Eur. J. Radiol. 2014, 83, 1487–1494. [Google Scholar] [CrossRef]
- Kher, K.; Schnaper, H.W.; Greenbaum, L.A. Radionuclide renal imaging. In Clinical Pediatric Nephrology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 105–114. [Google Scholar]
- Chiara, A.; Chirico, G.; Barbarini, M.; De Vecchi, E.; Rondini, G. Ultrasonic evaluation of kidney length in term and preterm infants. Eur. J. Pediatr. 1989, 149, 94–95. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Korngold, E.; Teele, R.L. Sonographic assessment of renal length in normal children. Am. J. Roentgenol. 1984, 142, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.S.; Donaher, E.R.; O’Riordan, M.A.; Dell, K.M. Diagnosis of pediatric urolithiasis: Role of ultrasound and computerized tomography. J. Urol. 2005, 174 Pt 1, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Benson, C.B.; Bromley, B.; Campbell, J.B.; Chow, J.; Coleman, B.; Cooper, C.; Crino, J.; Darge, K.; Herndon, C.D.; et al. Multidisciplinary consensus on the classification of prenatal and postnatal urinary tract dilation (UTD classification system). J. Pediatr. Urol. 2014, 10, 982–998. [Google Scholar] [CrossRef] [PubMed]
- Darge, K.; Grattan-Smith, J.D.; Riccabona, M. Pediatric uroradiology: State of the art. Pediatr. Radiol. 2011, 41, 82–91. [Google Scholar] [CrossRef]
- Jequier, S.; Jequier, J.C. Reliability of Voiding Cystourethrography to Detect Reflux. Am. J. Roentgenol. 1989, 153, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Piepsz, A.; Ham, H.R. Pediatric applications of renal nuclear medicine. Semin. Nucl. Med. 2006, 36, 16–35. [Google Scholar] [CrossRef]
- Blane, C.E.; Bookstein, F.L.; DiPietro, M.A.; Kelsch, R.C. Sonographic standards for normal infant kidney length. Am. J. Roentgenol. 1985, 145, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimons, R.B. Measurement of Kidney Length in Newborns Using Ultrasound. Acta Paediatr. Scand. 1983, 72, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Han, B.K.; Babcock, D.S. Sonographic Evaluation of Normal Renal Size in Children. Am. J. Roentgenol. 1985, 145, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.L.; Cooper, J.; Eisenberg, P.; Mandel, F.S.; Gross, B.R.; Goldman, M.A.; Barzel, E.; Rawlinson, K.F. Sonographic Evaluation of Fetal Kidney Length in 397 Obstetric Cases. Am. J. Roentgenol. 1991, 157, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.Y.; Chu, W.C.; Yeung, C.K.; Sreedhar, B.; Liu, J.X.; Wong, E.M.; Metreweli, C. Nomograms of total renal volume, urinary bladder volume and bladder wall thickness index in 3376 children with a normal urinary tract. Pediatr. Radiol. 2007, 37, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, A.E.; Hernandez, R.J.; Zerin, J.M.; Marks, T.I.; Kelsch, R.C. Interobserver and intraobserver variations in sonographic renal length measurements in children. Am. J. Roentgenol. 1991, 156, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Dinkel, E.; Ertel, M.; Dittrich, M.; Peters, H.; Berres, M.; Schulte-Wissermann, H. Kidney size in childhood. Sonographical growth charts for kidney length and volume. Pediatr. Radiol. 1985, 15, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hricak, H.; Slovis, T.L.; Callen, C.W.; Callen, P.W.; Romanski, R.N. Neonatal kidneys: Sonographic anatomic correlation. Radiology 1983, 147, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, F.; Toorenvliet, B.R.; Ruige, M.; Holscher, H.C. Increased echogenicity of renal cortex: A transient feature in acutely ill children. Am. J. Roentgenol. 2008, 190, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Fernbach, S.K.; Maizels, M.; Conway, J.J. Ultrasound grading of hydronephrosis: Introduction to the system used by the Society for Fetal Urology. Pediatr. Radiol. 1993, 23, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, M.; Hori, C.; Tsuchida, S.; Sudo, M. Ultrasonographic evaluation of bladder volume in young children. Pediatr. Nephrol. 1995, 9, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Kuzmić, A.C.; Brkljačić, B.; Ivanković, D.; Galešić, K. Doppler sonographic renal resistance index in healthy children. Eur. Radiol. 2000, 10, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Bude, R.O.; Rubin, J.M.; Adler, R.S. Power versus conventional color Doppler sonography: Comparison in the depiction of normal intrarenal vasculature. Radiology 1994, 192, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Riccabona, M.; Nelson, T.R.; Pretorius, D.H.; E Davidson, T. Distance and volume measurement using three-dimensional ultrasonography. J. Ultrasound Med. 1995, 14, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.-S.; Liu, M.; Luo, J.; Tian, W.-S.; Liang, J.-Y.; Xu, M.; Zheng, Y.-L.; Xie, X.-Y. Transplant renal artery stenosis: Evaluation with contrast-enhanced ultrasound. Eur. J. Radiol. 2017, 90, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Duran, C.; Beltrán, V.P.; González, A.; Gómez, C.; Riego, J.D. Contrast-enhanced Voiding Urosonography for Vesicoureteral Reflux Diagnosis in Children. Radiographics 2017, 37, 1854–1869. [Google Scholar] [CrossRef] [PubMed]
- Goodman, T.R.; Mustafa, A.; Rowe, E. Pediatric CT radiation exposure: Where we were, and where we are now. Pediatr. Radiol. 2019, 49, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Odle, T.G. Radiation Dose Reduction in Pediatric Computed Tomography. Radiol. Technol. 2019, 91, 161CT–180CT. [Google Scholar] [PubMed]
- Asif, A.; Epstein, M. Prevention of radiocontrast-induced nephropathy. Am. J. Kidney Dis. 2004, 44, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Aspelin, P.; Aubry, P.; Fransson, S.-G.; Strasser, R.; Willenbrock, R.; Berg, K.J. Nephrotoxicity in High-Risk Patients Study of Iso-Osmolar and Low-Osmolar Non-Ionic Contrast Media Study Investigators. Nephrotoxic effects in high-risk patients undergoing angiography. N. Engl. J. Med. 2003, 348, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Falesch, L.A.; Foley, W.D. Computed Tomography Angiography of the Renal Circulation. Radiol. Clin. N. Am. 2016, 54, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Herd, D.W. Anxiety in children undergoing VCUG: Sedation or no sedation? Adv. Urol. 2008, 2008, 498614. [Google Scholar] [CrossRef]
- Lebowitz, R.L.; Olbing, H.; Parkkulainen, K.V.; Smellie, J.M.; Tamminen-Möbius, T.E. International system of radiographic grading of vesicoureteric reflux. International Reflux Study in Children. Pediatr. Radiol. 1985, 15, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Vates, T.S.; Shull, M.J.; Underberg-Davis, S.J.; Fleisher, M.H. Complications of voiding cystourethrography in the evaluation of infants with prenatally detected hydronephrosis. J. Urol. 1999, 162 Pt 2, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Taleda, M.; Katayama, K.; Tsutsui, T.; Takahashi, H.; Komeyama, T.; Mizusawa, T.; Sato, S. Value of dimercaptosuccinic acid single photon emission computed tomography and magnetic resonance imaging in detecting renal injury in pediatric patients with vesicoureteral reflux. Comparison with dimercaptosuccinic acid planar scintigraphy and intravenous pyelography. Eur. Urol. 1994, 25, 320–325. [Google Scholar] [CrossRef]
- Itoh, K.; Yamashita, T.; Tsukamoto, E.; Nonomura, K.; Furudate, M.; Koyanagi, T. Qualitative and quantitative evaluation of renal parenchymal damage by 99mTc-DMSA planar and SPECT scintigraphy. Ann. Nucl. Med. 1995, 9, 23–28. [Google Scholar] [CrossRef]
- Majd, M.; Gil Rushton, H.; Jantausch, B.; Wiedermann, B.L. Relationship among vesicoureteral reflux, P-fimbriated Escherichia coli, and acute pyelonephritis in children with febrile urinary tract infection. J. Pediatr. 1991, 119, 578–585. [Google Scholar] [CrossRef]
- Parkhouse, H.F.; Godley, M.L.; Cooper, J.; Risdon, R.A.; Ransley, P.G. Renal imaging with 99Tcm-labelled DMSA in the detection of acute pyelonephritis: An experimental study in the pig. Nucl. Med. Commun. 1989, 10, 63–70. [Google Scholar] [CrossRef]
- Arnold, A.; Brownless, S.; Carty, H.; Rickwood, A. Detection of renal scarring by DMSA scanning—An experimental study. J. Pediatr. Surg. 1990, 25, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, D.L.; Flink, C.C.; Johnson, E.K.; Maizels, M.; Yerkes, E.B.; Lindgren, B.W.; Liu, D.B.; Rosoklija, I.; Cheng, E.Y.; Gong, E.M. The Correlation between Serial Ultrasound and Diuretic Renography in Children with Severe Unilateral Hydronephrosis. J. Urol. 2018, 200, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kandur, Y.; Salan, A.; Guler, A.G.; Tuten, F. Diuretic renography in hydronephrosis: A retrospective single-center study. Int. Urol. Nephrol. 2018, 50, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Deo, A.; Fogel, M.; Cowper, S.E. Nephrogenic Systemic Fibrosis: A Population Study Examining the Relationship of Disease Development to Gadolinium Exposure. Clin. J. Am. Soc. Nephrol. 2007, 2, 264–267. [Google Scholar] [CrossRef]
- Nardone, B.; Saddleton, E.; Laumann, A.E.; Edwards, B.J.; Raisch, D.W.; McKoy, J.M.; Belknap, S.M.; Bull, C.; Haryani, A.; Cowper, S.; et al. Pediatric nephrogenic systemic fibrosis is rarely reported: A RADAR report. Pediatr. Radiol. 2014, 44, 173–180. [Google Scholar] [CrossRef]
- Grattan-Smith, J.D.; Jones, R.A. MR Urography in Children. Pediatr. Radiol. 2006, 36, 1119–1132, quiz 1228–1229. [Google Scholar] [CrossRef]
- Moore, K.I.; Persaud, T.V.E. The Urogenital System. In The Developing Human: Clinically Oriented Embryology, 6th ed.; Moore, K.I., Ed.; WB Saunders: Philadelphia, PA, USA, 1998; p. 303. [Google Scholar]
- Nguyen, H.T.; Herndon, C.D.; Cooper, C.; Gatti, J.; Kirsch, A.; Kokorowski, P.; Lee, R.; Perez-Brayfield, M.; Metcalfe, P.; Yerkes, E.; et al. The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J. Pediatr. Urol. 2010, 6, 212–231. [Google Scholar] [CrossRef]
- Bassanese, G.; Travan, L.; D’ottavio, G.; Monasta, L.; Ventura, A.; Pennesi, M. Prenatal anteroposterior pelvic diameter cutoffs for postnatal referral for isolated pyelectasis and hydronephrosis: More is not always better. J. Urol. 2013, 190, 1858–1863. [Google Scholar] [CrossRef]
- Lee, R.S.; Cendron, M.; Kinnamon, D.D.; Nguyen, H.T. Antenatal hydronephrosis as a predictor of postnatal outcome: A meta-analysis. Pediatrics 2006, 118, 586–593. [Google Scholar] [CrossRef]
- Dejter, S.W., Jr.; Gibbons, M.D. The Fate of Infant Kidneys with Fetal Hydronephrosis but Initially Normal Postnatal Sonography. J. Urol. 1989, 142 Pt 2, 661–662. [Google Scholar] [CrossRef]
- van Eerde, A.M.; Meutgeert, M.H.; de Jong, T.P.V.M.; Giltay, J.C. Vesico-ureteral reflux in children with prenatally detected hydronephrosis: A systematic review. Ultrasound Obstet. Gynecol. 2007, 29, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, N.A.; Epelman, M.; Victoria, T.; Johnson, A.M. Complex genitourinary abnormalities on fetal MRI: Imaging findings and approach to diagnosis. Am. J. Roentgenol. 2012, 199, W222–W231. [Google Scholar] [CrossRef] [PubMed]
- Dillon, E.; Ryall, A. A 10-Year Audit of Antenatal Ultrasound Detection of Renal Disease. Br. J. Radiol. 1998, 71, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Sharma, D.; Singh, N.; Jain, B.K.; Minocha, V. Hydronephrotic obstructed kidney mimicking a congenital multicystic kidney: Case report with review of literature. Int. Urol. Nephrol. 2002, 34, 179–182. [Google Scholar] [CrossRef]
- Eickmeyer, A.B.; Casanova, N.F.; He, C.; Smith, E.A.; Wan, J.; Bloom, D.A.; Dillman, J.R. The natural history of the multicystic dysplastic kidney—Is limited follow-up warranted? J. Pediatr. Urol. 2014, 10, 655–661. [Google Scholar] [CrossRef]
- Strife, J.L.; Souza, A.S.; Kirks, D.R.; Strife, C.F.; Gelfand, M.J.; Wacksman, J. Multicystic dysplastic kidney in children: US follow-up. Radiology 1993, 186, 785–788. [Google Scholar] [CrossRef]
- Adeb, M.; Darge, K.; Dillman, J.R.; Carr, M.; Epelman, M. Magnetic resonance urography in evaluation of duplicated renal collecting systems. Magn. Reson. Imaging Clin. N. Am. 2013, 21, 717–730. [Google Scholar] [CrossRef]
- Zerin, J.M.; Baker, D.R.; Casale, J.A. Single-System Ureteroceles in Infants and Children: Imaging Features. Pediatr. Radiol. 2000, 30, 139–146. [Google Scholar] [CrossRef]
- Berrocal, T.; Pinilla, I.; Gutiérrez, J.; Prieto, C.; de Pablo, L.; del Hoyo, M.-L. Mild hydronephrosis in newborns and infants: Can ultrasound predict the presence of vesicoureteral reflux. Pediatr. Nephrol. 2007, 22, 91–96. [Google Scholar] [CrossRef]
- Foresman, W.H.; Hulbert, W.C., Jr.; Rabinowitz, R. Does urinary tract ultrasonography at hospitalization for acute pyelonephritis predict vesicoureteral reflux? J. Urol. 2001, 165 Pt 2, 2232–2234. [Google Scholar] [CrossRef]
- Mercado-Deane, M.-G.; Beeson, J.E.; John, S.D. US of Renal Insufficiency in Neonates. Radiographics 2002, 22, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Gagnadoux, M.F.; Habib, R.; Levy, M.; Brunelle, F.; Broyer, M. Cystic renal diseases in children. Adv. Nephrol. Necker Hosp. 1989, 18, 33–57. [Google Scholar] [PubMed]
- Avni, F.E.; Guissard, G.; Hall, M.; Janssen, F.; DeMaertelaer, V.; Rypens, F. Hereditary polycystic kidney diseases in children: Changing sonographic patterns through childhood. Pediatr. Radiol. 2002, 32, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Rahbari-Oskoui, F.; Mittal, A.; Mittal, P.; Chapman, A. Renal relevant radiology: Radiologic imaging in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2014, 9, 406–415. [Google Scholar] [CrossRef]
- Fick, G.M.; Duley, I.T.; Johnson, A.M.; Strain, J.D.; Manco-Johnson, M.L.; A Gabow, P. The spectrum of autosomal dominant polycystic kidney disease in children. J. Am. Soc. Nephrol. 1994, 4, 1654–1660. [Google Scholar] [CrossRef]
- Carmichael, J.; Easty, M. Imaging Chronic Renal Disease and Renal Transplant in Children. Pediatr. Radiol. 2010, 40, 963–974. [Google Scholar] [CrossRef]
- Epelman, M.; Victoria, T.; Meyers, K.E.; Chauvin, N.; Servaes, S.; Darge, K. Postnatal imaging of neonates with prenatally diagnosed genitourinary abnormalities: A practical approach. Pediatr. Radiol. 2012, 42 (Suppl. S1), S124–S141. [Google Scholar] [CrossRef]
- Sampson, J.R.; Maheshwar, M.M.; Aspinwall, R.; Thompson, P.; Cheadle, J.P.; Ravine, D.; Roy, S.; Haan, E.; Bernstein, J.; Harris, P.C. Renal cystic disease in tuberous sclerosis: Role of the polycystic kidney disease 1 gene. Am. J. Hum. Genet. 1997, 61, 843–851. [Google Scholar] [CrossRef]
- Amin, S.; Kingswood, J.C.; Bolton, P.F.; Elmslie, F.; Gale, D.P.; Harland, C.; Johnson, S.R.; Parker, A.; Sampson, J.R.; Smeaton, M.; et al. The UK guidelines for management and surveillance of Tuberous Sclerosis Complex. QJM Int. J. Med. 2019, 112, 171–182. [Google Scholar] [CrossRef]
- Lam, H.C.; Siroky, B.J.; Henske, E.P. Renal disease in tuberous sclerosis complex: Pathogenesis and therapy. Nat. Rev. Nephrol. 2018, 14, 704–716. [Google Scholar] [CrossRef]
- Bissler, J.J.; Kingswood, J.C. Renal Manifestation of Tuberous Sclerosis Complex. Am. J. Med. Genet. Part C Semin. Med Genet. 2018, 178, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Kenney, P.J.; E Brinsko, R.; Patel, D.V.; E Spitzer, R.; Farrar, F.M. Sonography of the kidneys in hemolytic uremic syndrome. Investig. Radiol. 1986, 21, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.H.; Cornella, R.; Loevinger, E.; Schabel, S.I.; Curry, N.S. Sonography of systemic lupus nephritis. Am. J. Roentgenol. 1984, 142, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, M.V.; Watson, T.A.; Hickson, M.; Smeulders, N.; Humphries, P.D. Acoustic shadowing in pediatric kidney stone ultrasound: A retrospective study with non-enhanced computed tomography as reference standard. Pediatr. Radiol. 2019, 49, 777–783. [Google Scholar] [CrossRef]
- Fernández-Ibieta, M. Renal Trauma in Pediatrics: A Current Review. Urology 2018, 113, 171–178. [Google Scholar] [CrossRef]
- Trautmann, A.; Roebuck, D.J.; McLaren, C.A.; Brennan, E.; Marks, S.D.; Tullus, K. Non-invasive imaging cannot replace formal angiography in the diagnosis of renovascular hypertension. Pediatr. Nephrol. 2017, 32, 495–502. [Google Scholar] [CrossRef]
- Kurian, J.; Epelman, M.; Darge, K.; Meyers, K.; Nijs, E.; Hellinger, J.C. The role of CT angiography in the evaluation of pediatric renovascular hypertension. Pediatr. Radiol. 2013, 43, 490–501. [Google Scholar] [CrossRef]
- Mikołajczak, A.; Tytkowska, A.; Jaworska, A.; Wesołowska, A.; Borszewska-Kornacka, M.K.; Bokiniec, R. Sequential sonographic features in neonatal renal vein thrombosis. Ginekol. Polska 2018, 89, 271–275. [Google Scholar] [CrossRef]
- Kraft, J.K.; Brandão, L.R.; Navarro, O.M. Sonography of renal venous thrombosis in neonates and infants: Can we predict outcome? Pediatr. Radiol. 2011, 41, 299–307. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Jin, W.; San, P.; Xu, P.; Pan, S. The Left Renal Entrapment Syndrome: Diagnosis and Treatment. Ann. Vasc. Surg. 2007, 21, 198–203. [Google Scholar] [CrossRef]
- Dennery, M.P.; Rushton, H.G.; Belman, A.B. Sonography for the detection and follow-up of primary nonsarcomatous bladder tumors in children. Urology 2002, 59, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Servaes, S.E.; Hoffer, F.A.; Smith, E.A.; Khanna, G. Imaging of Wilms tumor: An update. Pediatr. Radiol. 2019, 49, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.; Oostveen, M.; Rogers, H.; Pritchard-Jones, K.; Olsen, Ø. The role of imaging in the initial investigation of paediatric renal tumours. Lancet Child Adolesc. Health 2020, 4, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.S.; Saade-Lemus, S.; Servaes, S.E.; Acord, M.R.; Reid, J.R.; Anupindi, S.A.; States, L.J. Imaging surveillance for children with predisposition to renal tumors. Pediatr. Radiol. 2019, 49, 1453–1462. [Google Scholar] [CrossRef]









| Study | Key Objective | Methodology/Type of Imaging | Findings/Results | Clinical Relevance |
|---|---|---|---|---|
| Fried JG, Morgan MA (2019) [1]. | Overview of renal imaging techniques | Imaging techniques for kidneys (US, CT, MRI) | Summarizes core imaging methods | Comprehensive review of renal imaging applications |
| Viteri B, et al. (2020) [2]. | Renal imaging in pediatric patients | Pediatric-specific renal imaging (US, MRI) | Discusses renal imaging modalities in children | Highlights importance of specialized pediatric imaging |
| Maliborski A, et al. (2018) [3]. | Diagnostic imaging in pediatric kidney diseases | Multiple methods (US, MRI, CT, Nuclear Medicine) | Differentiates kidney diseases in children | Useful for diagnosing congenital/acquired pediatric kidney conditions |
| Kher KK, et al. (2017) [5]. | Diagnostic imaging of the urinary tract | Pediatric nephrology imaging (US, VCUG, MRI) | Reviews urinary tract disease imaging in children | Key resource for pediatric nephrology professionals |
| O’Neill WC (2014) [6]. | Applications of ultrasound in nephrology | Ultrasound for kidney disease | US essential for evaluating kidney function | Practical guide for nephrologists/radiologists |
| Riccabona M (2014) [7]. | Pediatric ultrasonography techniques | Pediatric ultrasound | Discusses techniques, principles, and applications | Essential for clinicians using US in pediatric care |
| Chiara A, et al. (1989) [9]. | Measurement of kidney length in infants | US for kidney length measurement | Provides kidney length norms for full/preterm infants | Crucial for diagnosing developmental kidney anomalies |
| Rosenbaum DM, et al. (1984) [10]. | Normal renal size assessment in children | US | Examines normal kidney length in healthy children | Important for establishing normal kidney size |
| Palmer JS, et al. (2005) [11]. | Diagnosis of pediatric urolithiasis | US and CT | Reviews role of imaging in kidney stone diagnosis | Guides imaging decisions for suspected pediatric urolithiasis |
| Nguyen HT, et al. (2014) [12]. | Classification of urinary tract dilation | US for prenatal/postnatal diagnosis | Establishes UTD classification consensus | Key for assessing urinary tract dilation in children |
| Darge K, et al. (2011) [13]. | MRI in pediatric nephro-urology | MRI for pediatric renal/urinary imaging | MRI offers superior contrast without radiation | Supports increased MRI use in pediatric imaging |
| Jequier S, Jequier JC (1989) [14]. | Assess accuracy of VCUG in detecting vesicoureteral reflux | VCUG for vesicoureteral reflux detection | Evaluates reliability of VCUG for reflux diagnosis | Helps refine diagnostic protocols for pediatric urinary reflux |
| Piepsz A, et al. (2006) [15] | Radionuclide imaging in pediatric nephrology | DMSA and MAG3 renal scans | Highlights nuclear medicine’s diagnostic value | Critical for functional renal damage assessment |
| Age | Mean Length (cm) | Range (±2 SD in cm) |
|---|---|---|
| Term newborn | 4.48 | 3.86–5.10 |
| 2 months | 5.28 | 3.96–6.60 |
| 6 months | 6.15 | 4.81–7.49 |
| 1.5 years | 6.65 | 5.57–7.73 |
| 2.5 years | 7.36 | 6.28–8.44 |
| 3.5 years | 7.36 | 6.18–8.54 |
| 4.5 years | 7.78 | 6.87–8.87 |
| 5.5 years | 8.09 | 7.01–9.17 |
| 6.5 years | 7.83 | 6.39–9.27 |
| 7.5 years | 8.33 | 7.31–9.35 |
| 8.5 years | 8.90 | 7.14–10.66 |
| 9.5 years | 9.20 | 7.40–11.00 |
| 10.5 years | 9.17 | 7.53–10.81 |
| 11.5 years | 9.60 | 8.32–10.88 |
| 12.5 years | 10.42 | 8.68–12.16 |
| 13.5 years | 9.79 | 8.29–11.29 |
| 14.5 years | 10.05 | 8.81–11.29 |
| 15.5 years | 10.93 | 9.41–12.45 |
| 16.5 years | 10.04 | 8.32–11.76 |
| 17.5 years | 10.53 | 9.95–11.11 |
| 18.5 years | 10.81 | 8.55–13.07 |
| Grade | Ultrasound Description |
|---|---|
| 0 | No hydronephrosis |
| 1 | Only the renal pelvis is visible |
| 2 | Renal pelvis and a few calyces visible |
| 3 | Virtually all calyces visible |
| 4 | Similar to grade 3, but with parenchymal thinning |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldughiem, A. Imaging Diagnosis of Major Kidney and Urinary Tract Disorders in Children. Medicina 2025, 61, 696. https://doi.org/10.3390/medicina61040696
Aldughiem A. Imaging Diagnosis of Major Kidney and Urinary Tract Disorders in Children. Medicina. 2025; 61(4):696. https://doi.org/10.3390/medicina61040696
Chicago/Turabian StyleAldughiem, Ahmad. 2025. "Imaging Diagnosis of Major Kidney and Urinary Tract Disorders in Children" Medicina 61, no. 4: 696. https://doi.org/10.3390/medicina61040696
APA StyleAldughiem, A. (2025). Imaging Diagnosis of Major Kidney and Urinary Tract Disorders in Children. Medicina, 61(4), 696. https://doi.org/10.3390/medicina61040696





