Role of Nutritional Status in Acute Coronary Syndrome Patients with Diabetes
Abstract
1. Introduction
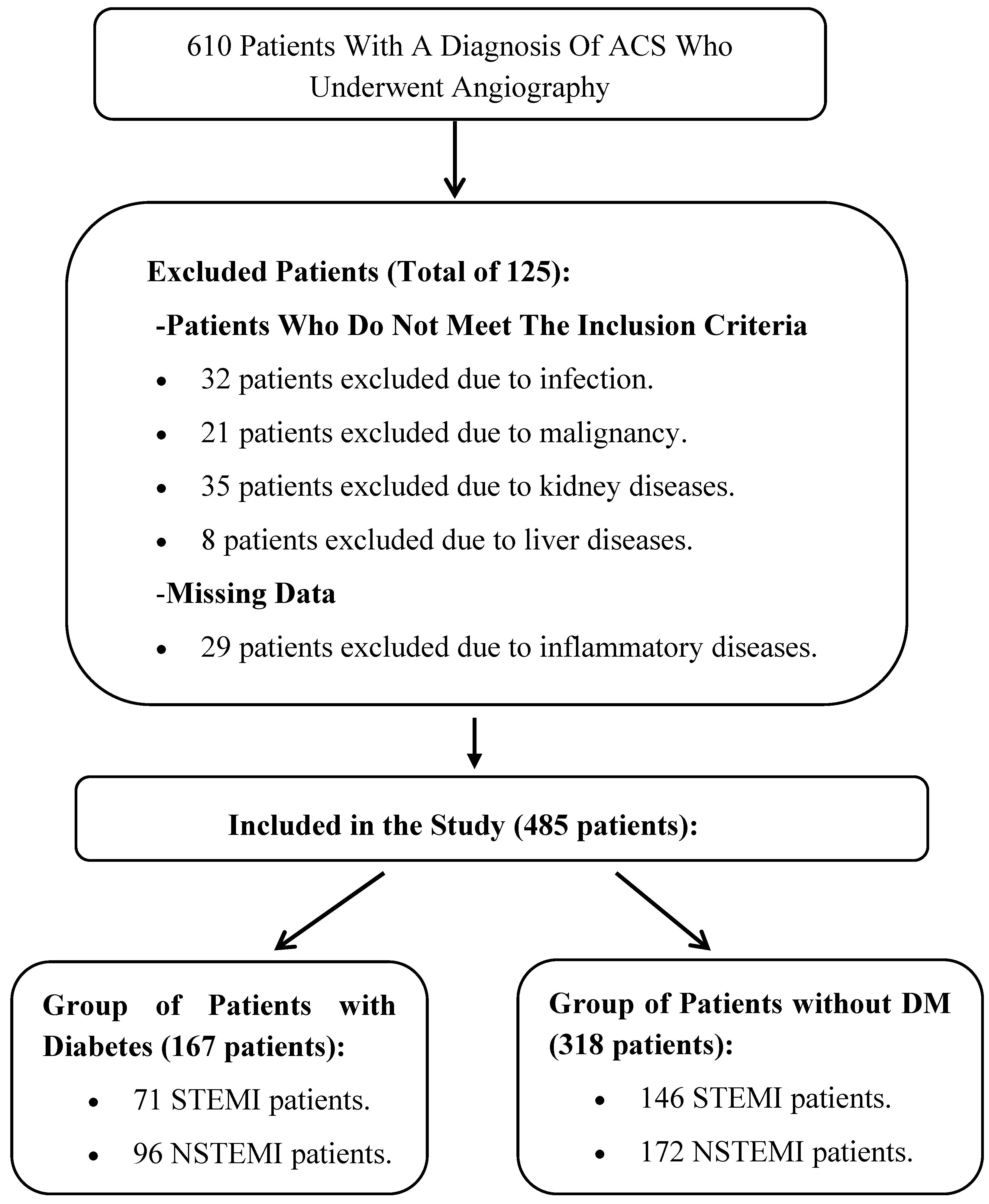
2. Materials and Methods
2.1. Participants and Study Design
2.2. Methods of Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndrome |
| DM | Diabetes mellitus |
| STEMI | ST-elevation myocardial infarction |
| NSTEMI | Non-ST-elevation myocardial infarction |
| CVD | Cardiovascular disease |
| CAD | Coronary artery disease |
| AMI | Acute myocardial infarction |
| pPCI | Primary percutaneous coronary intervention |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein cholesterol |
| SPSS | Statistical Package for the Social Sciences |
| TG | Triglycerides |
| TC | Total cholesterol |
| PNI | Prognostic Nutrition Index |
| CONUT | Controlling Nutritional Status |
| EF | Ejection fraction |
| eGFR | Estimated glomerular filtration rate |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.; Irfan, U.; Abdelkhalek, A.; Javed, I.; Khokhar, M.I.; Shakil, F.; Raza, S.; Salim, S.S.; Altaf, M.M.; Habib, R.; et al. Comprehensive Quality Analysis of Conventional and Novel Biomarkers in Diagnosing and Predicting Prognosis of Coronary Artery Disease, Acute Coronary Syndrome, and Heart Failure, a Comprehensive Literature Review. J. Cardiovasc. Transl. Res. 2024, 17, 1258–1285. [Google Scholar] [CrossRef]
- Bishop, A.J.; Nehme, Z.; Nanayakkara, S.; Anderson, D.; Stub, D.; Meadley, B.N. Artificial neural networks for ECG interpretation in acute coronary syndrome: A scoping review. Am. J. Emerg. Med. 2024, 83, 1–8. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Kumar, V.; Rosenzweig, R.; Asalla, S.; Nehra, S.; Prabhu, S.D.; Bansal, S.S. TNFR1 Contributes to Activation-Induced Cell Death of Pathological CD4+T Lymphocytes During Ischemic Heart Failure. JACC Basic. Transl. Sci. 2022, 7, 1038–1049. [Google Scholar] [CrossRef]
- Bodí, V.; Sanchis, J.; Núñez, J.; Rumiza, E.; Mainar, L.; López-Lereu, M.P.; Monmeneu, J.V.; Oltra, R.; Forteza, M.J.; Chorro, F.J.; et al. Post-reperfusion lymphopenia and microvascular obstruction in ST-segment elevation acute myocardial infarction. Rev. Esp. Cardiol. 2009, 62, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wan, Z.; Zhang, Y.; Fan, Y.; Gu, W.; Li, F.; Meng, L.; Zeng, X.; Han, D.; Li, X. Neutrophil count improves the GRACE risk score prediction of clinical outcomes in patients with ST-elevation myocardial infarction. Atherosclerosis 2015, 241, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Heshmat-Ghahdarijani, K.; Talaei, M.; Safaei, A.; Sarrafzadegan, N.; Roohafza, H. The predictive value of atherogenic index of plasma in the prediction of cardiovascular events; a fifteen-year cohort study. Adv. Med. Sci. 2021, 66, 418–423. [Google Scholar] [CrossRef]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid. Med. Cell Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef]
- Hetherington, I.; Totary-Jain, H. Anti-atherosclerotic therapies: Milestones, challenges, and emerging innovations. Mol. Ther. 2022, 30, 3106–3117. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, X.; Feng, J.; Chen, X. Association of metabolic syndrome with atherogenic index of plasma in an urban Chinese population: A 15-year prospective study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Yandrapalli, S.; Nabors, C.; Goyal, A.; Aronow, W.S.; Frishman, W.H. Modifiable Risk Factors in Young Adults with First Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 73, 573–584. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, C.; Yang, J.; Seery, S.; Qi, Y.; Wang, W.; Zhang, K.; Shao, C.; Tang, Y.D. Atherogenic index of plasma for non-diabetic, coronary artery disease patients after percutaneous coronary intervention: A prospective study of the long-term outcomes in China. Cardiovasc. Diabetol. 2022, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Czapla, M.; Karniej, P.; Juarez-Vela, R.; Lokiec, K. The association between nutritional status and in-hospital mortality among patients with acute coronary syndrome: A result of the retrospective nutritional status heart study (NSHS). Nutrients 2020, 12, 3091. [Google Scholar] [CrossRef]
- Raposeiras, R.S.; Abu Assi, E.; Cespon, F.M.; Barreiro, P.C.; Lizancos, C.A.; Parada, J.A.; Perez, D.D.; Blanco, P.S.; Rossello, X.; Ibanez, B.; et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J. Am. Coll. Cardiol. 2020, 76, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Tonet, E.; Campo, G.; Maietti, E.; Formiga, F.; Martinez-Selles, M.; Pavasini, R.; Biscaglia, S.; Serenelli, M.; Sanchis, J.; Diez-Villanueva, P.; et al. Nutritional status and all-cause mortality in older adults with acute coronary syndrome. Clin. Nutr. 2020, 39, 1572–1579. [Google Scholar] [CrossRef]
- Yoo, S.H.; Kook, H.Y.; Hong, Y.J.; Kim, J.H.; Ahn, Y.; Jeong, M.H. Influence of undernutrition at admission on clinical outcomes in patients with acute myocardial infarction. J. Cardiol. 2017, 69, 555–560. [Google Scholar] [CrossRef]
- Sze, S.; Pellicori, P.; Kazmi, S.; Rigby, A.; Cleland, J.G.F.; Wong, K.; Clark, A.L. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: A comparison with body mass index. JACC Heart Fail. 2018, 6, 476–486. [Google Scholar] [CrossRef]
- Raposeiras-Roubin, S.; Abu-Assi, E.; Paz, R.C.; Rossello, X.; Barreiro, P.C.; Pinon, E.M.; Pascual, C.R.; Garcia, C.J.; Gonzalez-Carrero, L.A.; Caneiro-Queija, B.; et al. Impact of malnutrition in the embolic–haemorrhagic trade-off of elderly patients with atrial fibrillation. EP Eur. 2020, 22, 878–887. [Google Scholar] [CrossRef]
- Czapla, M.; Juarez-Vela, R.; Lokiec, K.; Karniej, P. The association between nutritional status and in-hospital mortality among patients with heart failure: A result of the retrospective nutritional status heart study 2 (NSHS2). Nutrients 2021, 13, 1669. [Google Scholar] [CrossRef]
- Freeman, A.M.; Morris, P.B.; Barnard, N.; Esselstyn, C.B.; Ros, E.; Agatston, A.; Devries, S.; O’Keefe, J.; Miller, M.; Ornish, D.; et al. Trending cardiovascular nutrition controversies. J. Am. Coll. Cardiol. 2017, 69, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Tomas, N.; Blanco, M.S.; Viguiliouk, E.; Khan, T.; Kendall, C.W.C.; Kahleova, H.; Rahelic, D.; Sievenpiper, J.L.; Salas-Salvado, J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1207–1227. [Google Scholar] [CrossRef]
- Ahmed, N.; Choe, Y.; Mustad, V.A.; Chakraborty, S.; Goates, S.; Luo, M.; Mechanick, J.I. Impact of malnutrition on survival and healthcare utilization in Medicare beneficiaries with diabetes: A retrospective cohort analysis. BMJ Open Diabetes Res. Care 2018, 6, e000471. [Google Scholar] [CrossRef]
- Vischer, U.M.; Perrenoud, L.; Genet, C.; Ardigo, S.; Registe-Rameau, Y.; Herrmann, F.R. The high prevalence of malnutrition in elderly diabetic patients: Implications for anti-diabetic drug treatments. Diabetic Med. J. Br. Diabetic Assoc. 2010, 27, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Sueta, D.; Hokimoto, S.; Sakamoto, K.; Akasaka, T.; Tabata, N.; Kaikita, K.; Honda, O.; Naruse, M.; Ogawa, H. Multi-center study of hemodialysis patients undergoing invasive cardiovascu-lar procedures study I: Validation of the high mortality rate of malnutri-tion-inflammation-atherosclerosis syndrome: Community-based observational study. Int. J. Cardiol. 2017, 230, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Reber, E.; Schönenberger, K.A.; Vasiloglou, M.F.; Stanga, Z. Nutritional risk screening in cancer patients: The first step toward better clinical outcome. Front Nutr. 2021, 8, 152. [Google Scholar] [CrossRef]
- Buzby, G.P.; Mullen, J.L.; Matthews, D.C.; Hobbs, C.L.; Rosato, E.F. Prognostic nutritional index in gastrointestinal surgery. Am. J. Surg. 1980, 139, 160–167. [Google Scholar] [CrossRef]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005, 20, 38–45. [Google Scholar]
- Wada, H.; Dohi, T.; Miyauchi, K.; Doi, S.; Konishi, H.; Naito, R.; Tsuboi, S.; Ogita, M.; Kasai, T.; Okazaki, S.; et al. Prognostic impact of nutritional status assessed by the Controlling Nutritional Status score in patients with stable coronary artery disease undergoing percutaneous coronary intervention. Clin. Res. Cardiol. 2017, 106, 875–883. [Google Scholar] [CrossRef]
- Takahashi, T.; Watanabe, T.; Otaki, Y.; Kato, S.; Tamura, H.; Nishiyama, S.; Arimoto, T.; Takahashi, H.; Shishido, T.; Watanabe, M. Prognostic significance of the controlling nutritional (CONUT) score in patients with acute coronary syndrome. Heart Vessel. 2021, 36, 1109–1116. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, L.T.; Lai, W.; Yang, F.; Zhou, D.; Xu, R.; Tong, G. Prognostic value of inflammatory and nutritional indexes among patients with unresectable advanced gastric cancer receiving immune checkpoint inhibitors combined with chemotherapy-a retrospective study. PeerJ 2024, 1, e18659. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Ren, J.; Zhao, Y. Prognostic value of prognostic nutritional index in nasopharyngeal carcinoma: A meta-analysis containing 4511 patients. Oral Oncol. 2020, 110, 104991. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, G.; Chen, Z.; Zhuang, Y.; Li, G. Prognostic role of the prognostic nutritional index in pancreatic cancer: A meta-analysis. Nutr. Cancer 2019, 71, 207–213. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Zhang, X.; Zhang, T. Pretreatment prognostic nutritional index as a prognostic factor in lung cancer: Review and meta-analysis. Clin. Chim. Acta 2018, 486, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, H.; Chen, S.; Cai, S.; Zhou, S.; Wang, C.; Ni, X. Prognostic nutritional index and prognosis of patients with coronary artery disease: A systematic review and meta-analysis. Front Nutr. 2023, 10, 1114053. [Google Scholar] [CrossRef]
- Keskin, M.; Hayiroglu, M.I.; Keskin, T.; Kaya, A.; Tatlisu, M.A.; Altay, S.; Uzun, A.O.; Börklü, E.B.; Güvenç, T.S.; Avcı, I.I.; et al. A novel and useful predictive indicator of prognosis in ST-segment elevation myocardial infarction, the prognostic nutritional index. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 438–446. [Google Scholar] [CrossRef]
- Wada, H.; Dohi, T.; Miyauchi, K.; Jun, S.; Endo, H.; Doi, S.; Konishi, H.; Naito, R.; Tsuboi, S.; Ogita, M.; et al. Relationship between the prognostic nutritional index and long-term clinical outcomes in patients with stable coronary artery disease. J. Cardiol. 2018, 72, 155–161. [Google Scholar] [CrossRef]
- Li, T.; Yuan, D.; Wang, P.; Zeng, G.; Jia, S.; Zhang, C.; Zhu, P.; Song, Y.; Tang, X.; Gao, R.; et al. Association of prognostic nutritional index level and diabetes status with the prognosis of coronary artery disease: A cohort study. Diabetol. Metab. Syndr. 2023, 15, 58. [Google Scholar] [CrossRef]
- Kalyoncuoğlu, M.; Katkat, F.; Biter, H.I.; Cakal, S.; Tosu, A.R.; Can, M.M. Predicting one-year deaths and major adverse vascular events with the controlling nutritional status score in elderly patients with Non-ST-elevated myocardial ınfarction undergoing percutaneous coronary ıntervention. J. Clin. Med. 2021, 10, 2247. [Google Scholar] [CrossRef]
- Boyraz, B.; Ibisoglu, E.; Aslan, B. The prognostic value of the nutritional prognostic index (NPI) and controlling nutritional status (CONUT) scoring systems in non-ST elevated myocardial infarction patients over 65 years of age. Aging Clin. Exp. Res. 2022, 34, 555–562. [Google Scholar] [CrossRef]
- Lai, A.R.; Warrier, M.; Ng, E.Z.; Lin, C.; Chin, Y.H.; Kong, G.; Anand, V.V.; Lee, E.C.; Lai, H.; Ng, H.W.; et al. Cardiovascular outcomes in acute coronary syndrome and malnutrition: A meta-analysis of nutritional assessment tools. JACC Adv. 2023, 2, 100635. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yuan, L.; Chen, X.; Li, J.; Tao, J.; Li, W.; Zheng, R. Correlations and Prognostic Roles of the Nutritional Status and Neutrophil-to-lymphocyte Ratio in Elderly Patients with Acute Myocardial Infarction Undergoing Primary Coronary Intervention. Int. Heart J. 2020, 61, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, G.; Pizzino, F.; Lilli, A.; De Caterina, A.R.; Esposito, A.; Dalmiani, S.; Mazzone, A.; Di Bella, G.; Berti, S.; Paradossi, U. Advanced Lung Cancer Inflammation Index as Pre-dictor of All-Cause Mortality in ST-Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention. J. Clin. Med. 2024, 13, 6059. [Google Scholar] [CrossRef] [PubMed]
| GROUPS | Patients with DM | Patients Without DM | p | |
|---|---|---|---|---|
| N (F/M) | 167 (62/105) | 318 (105/253) | <0.001 | |
| Age | 65.72 ± 11.10 | 64.12 ± 13.18 | 0.234 | |
| Glucose (mg/dL) | 186.11 ± 95.71 | 129.54 ± 49.23 | <0.001 | |
| LDH (U/L) | 409.90 ± 288.51 | 415.81 ± 463.00 | 0.648 | |
| Albumin (g/L) | 36.63 ± 4.77 | 37.84 ± 5.11 | 0.010 | |
| Triglyceride (mg/dL) | 167.74 ± 160.57 | 134.88 ± 75.93 | 0.014 | |
| LDL (mg/dL) | 99.65 ± 37.09 | 111.31 ± 40.83 | 0.001 | |
| Cholesterol (mg/dL) | 164.95 ± 45.53 | 177.22 ± 49.52 | 0.008 | |
| HDL (mg/dL) | 39.35 ± 10.21 | 39.81 ± 9.81 | 0.611 | |
| Uric Acid (mg/dL) | 5.78 ± 2.02 | 5.87 ± 2.23 | 0.697 | |
| WBC (109/L) | 11.32 ± 3.63 | 11.46 ± 4.45 | 0.957 | |
| Hemoglobin (g/dL) | 13.36 ± 2.00 | 14.15 ± 1.98 | <0.001 | |
| Platelet (109/L) | 250.74 ± 69.35 | 241.45 ± 67.86 | 0.067 | |
| Neutrophil (109/L) | 10.30 ± 25.95 | 7.16 ± 4.06 | 0.192 | |
| Lymphocyte (109/L) | 2.89 ± 3.10 | 3.22 ± 3.60 | 0.348 | |
| Creatinine (mg/dL) | 1.16 ± 0.99 | 1.00 ± 0.54 | 0.118 | |
| EF (%) | 47.4 ± 11.19 | 49.73 ± 10.27 | 0.037 | |
| eGFR | 73.37 ± 25.59 | 81.18 ± 23.54 | <0.001 | |
| Hyperlipidemia | Yes, n (%) | 97 (58.1) | 152 (47.8) | 0.031 |
| No, n (%) | 70 (41.9) | 156 (52.2) | ||
| PNİ Score | 51.09 ± 18.93 | 53.92 ± 18.88 | 0.014 | |
| Malnutrition state | Normal, n (%) | 137 (82) | 292 (91.8) | 0.004 |
| Moderate, n (%) | 13 (7.8) | 8 (2.5) | ||
| Severe, n (%) | 17 (10.2) | 18 (5.7) | ||
| CONUT Score | 2.44 ± 2.66 | 2.02 ± 2.12 | 0.267 | |
| Malnutrition state | Normal, n (%) | 86 (51.5) | 165 (51.9) | 0.038 |
| Mild, n (%) | 45 (26.9) | 113 (35.5) | ||
| Moderate, n (%) | 30 (18) | 35 (11) | ||
| Severe, n (%) | 6 (3.6) | 5 (1.6) |
| Patients with DM | Patients Without DM | P* | |||||
|---|---|---|---|---|---|---|---|
| MI GROUPS | STEMI | NSTEMI | p | STEMI | NSTEMI | p | |
| N (F/M) | 71 (29/42) | 96 (33/63) | 0.392 | 146 (26/120) | 172 (40/132) | 0.233 | |
| Age | 65.45 ± 11.50 | 65.92 ± 10.85 | 0.719 | 63.12 ± 13.39 | 64.97 ± 12.98 | 0.162 | 0.315 |
| Glucose (mg/dL) | 189.87 ± 96.33 a | 183.33 ± 95.66 a | 0.503 | 130.21 ± 55.72 b | 128.97 ± 43.14 b | 0.836 | <0.001 |
| LDH (U/L) | 422.71 ± 274.44 | 400.33 ± 299.81 | 0.437 | 418.64 ± 354.04 | 413.66 ± 532.40 | 0.369 | 0.986 |
| Albumin (g/L) | 36.54 ± 5.49 | 36.70 ± 4.19 | 0.675 | 38.03 ± 4.93 | 37.67 ± 5.27 | 0.627 | 0.078 |
| Triglyceride (mg/dL) | 138.30 ± 101.35 a | 189.66 ± 190.87 b | 0.007 | 132.08 ± 71.41 a | 137.23 ± 79.67 a | 0.648 | <0.001 |
| LDL (mg/dL) | 102.57 ± 38.46 a,b | 97.42 ± 36.07 a | 0.588 | 113.45 ± 39.01 b,c | 109.50 ± 42.32 a,b | 0.342 | 0.015 |
| Cholesterol (mg/dL) | 163.41 ± 48.40 | 166.09 ± 43.52 | 0.649 | 178.53 ± 45.24 | 176.11 ± 52.99 | 0.414 | 0.062 |
| HDL (mg/dL) | 40.65 ± 9.06 | 38.38 ± 10.94 | 0.120 | 40.20 ± 9.71 | 39.49 ± 9.91 | 0.500 | 0.431 |
| Uric Acid (mg/dL) | 5.78 ± 2.13 | 5.78 ± 1.95 | 0.891 | 6.04 ± 2.55 | 5.74 ± 1.94 | 0.527 | 0.683 |
| WBC (109/L) | 11.00 ± 3.97 | 11.56 ± 3.35 | 0.140 | 11.62 ± 3.65 | 11.33 ± 5.04 | 0.150 | 0.744 |
| Hemoglobin (g/dL) | 13.50 ± 1.90 a,b | 13.26 ± 2.07 b | 0.590 | 14.42 ± 1.96 b,c | 13.91 ± 1.98 a,c | 0.022 | <0.001 |
| Platelet (109/L) | 247.66 ± 67.05 | 253.02 ± 71.26 | 0.445 | 250.09 ± 68.61 | 234.07 ± 66.51 | 0.019 | 0.086 |
| Neutrophil (109/L) | 6.87 ± 3.75 a,b,c | 12.84 ± 33.93 a | 0.016 | 7.03 ± 4.26 c | 7.26 ± 3.89 b,c | 0.534 | 0.015 |
| Lymphocyte (109/L) | 3.39 ± 4.20 | 2.52 ± 1.85 | 0.597 | 3.48 ± 3.14 | 3.00 ± 3.94 | 0.298 | 0.161 |
| Creatinine (mg/dL) | 1.12 ± 1.01 | 1.18 ± 0.99 | 0.116 | 1.00 ± 0.55 | 1.01 ± 0.53 | 0.826 | 0.156 |
| EF (%) | 46.06 ± 10.48 a | 48.39 ± 11.64 a,b | 0.184 | 48.9 ± 10.52 a,b | 50.44 ± 10.01 b | 0.185 | 0.030 |
| eGFR | 75.55 ± 27.33 a,b,c | 71.76 ± 24.24 c | 0.346 | 82.68 ± 23.16 a,d | 79.9 ± 23.85 b,d | 0.296 | 0.004 |
| PNİ Score | 53.50 ± 24.75 | 49.31 ± 12.94 | 0.600 | 55.42 ± 17.65 | 52.65 ± 19.83 | 0.292 | 0.105 |
| CONUT Score | 2.59 ± 2.99 | 2.33 ± 2.40 | 0.886 | 1.97 ± 2.05 | 2.07 ± 2.18 | 0.818 | 0.235 |
| B | SE | % 95 CI | Exp(B) | p | ||
|---|---|---|---|---|---|---|
| Exitus | Constant | −9.388 | 3.320 | 0.000 | 0.005 | |
| Age | 0.009 | 0.003 | 1.003–1.154 | 1.076 | 0.041 | |
| Glucose | 0.009 | 0.003 | 1.004–1.015 | 1.009 | 0.001 | |
| Platelet | 0.007 | 0.004 | 1.000–1.014 | 1.007 | 0.064 | |
| eGFR | −0.041 | 0.012 | 0.937–0.984 | 0.960 | 0.001 | |
| R2 (Cox–Snell) = 0.237 R2 (Nagelkerke) = 0.347 Model: X2(2) = 50.95. p < 0.001 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seçen, Ö.; Uslu, M.F. Role of Nutritional Status in Acute Coronary Syndrome Patients with Diabetes. Medicina 2025, 61, 740. https://doi.org/10.3390/medicina61040740
Seçen Ö, Uslu MF. Role of Nutritional Status in Acute Coronary Syndrome Patients with Diabetes. Medicina. 2025; 61(4):740. https://doi.org/10.3390/medicina61040740
Chicago/Turabian StyleSeçen, Özlem, and Muhammed Fuad Uslu. 2025. "Role of Nutritional Status in Acute Coronary Syndrome Patients with Diabetes" Medicina 61, no. 4: 740. https://doi.org/10.3390/medicina61040740
APA StyleSeçen, Ö., & Uslu, M. F. (2025). Role of Nutritional Status in Acute Coronary Syndrome Patients with Diabetes. Medicina, 61(4), 740. https://doi.org/10.3390/medicina61040740






