An Important Biomarker in Patients with Bell’s Palsy: Serum Calprotectin
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Study Setting and Population
2.4. Blood Sampling
2.5. Methods of Data Collection
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.; Deshmukh, P. Bell’s Palsy: A Review. Cureus 2022, 14, e30186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, L.; Luo, T.; Wu, F.; Zhao, B.; Li, X. The etiology of Bell’s palsy: A review. J. Neurol. 2020, 267, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Wasano, K.; Ishikawa, T.; Kawasaki, T.; Yamamoto, S.; Tomisato, S.; Shinden, S.; Minami, S.; Wakabayashi, T.; Ogawa, K. Novel pre-therapeutic scoring system using patient and haematological data to predict facial palsy prognosis. Clin. Otolaryngol. 2017, 42, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H. Clinical Efficacy of Electroneurography in Acute Facial Paralysis. J. Audiol. Otol. 2016, 20, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Babademez, M.A.; Gul, F.; Kale, H.; Muderris, T.; Bayazit, Y.; Ergin, M.; Erel, O.; Kiris, M. Thiol/disulphide homeostasis in Bell’s palsy as a novel pathogenetic marker. Clin. Otolaryngol. 2017, 42, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Oya, R.; Takenaka, Y.; Imai, T.; Sato, T.; Oshima, K.; Ohta, Y.; Inohara, H. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Prognostic Hematologic Markers of Bell’s Palsy: A Meta-analysis. Otol. Neurotol. 2019, 40, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Kefalidis, G.; Riga, M.; Argyropoulou, P.; Katotomichelakis, M.; Gouveris, C.; Prassopoulos, P.; Danielides, V. Is the width of the labyrinthine portion of the fallopian tube implicated in the pathophysiology of Bell’s palsy?: A prospective clinical study using computed tomography. Laryngoscope 2010, 120, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yeo, S.G.; Byun, J.Y. Role of Biomarkers as Prognostic Factors in Acute Peripheral Facial Palsy. Int. J. Mol. Sci. 2021, 23, 307. [Google Scholar] [CrossRef] [PubMed]
- Kiliçkaya, M.M.; Tuz, M.; Yariktaş, M.; Yasan, H.; Aynalı, G.; Bagci, Ö. The Importance of the Neutrophil-Lymphocyte Ratio in Patients with Idiopathic Peripheral Facial Palsy. Int. J. Otolaryngol. 2015, 2015, 981950. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greco, A.; Gallo, A.; Fusconi, M.; Marinelli, C.; Macri, G.F.; de Vincentiis, M. Bell’s palsy and autoimmunity. Autoimmun. Rev. 2012, 12, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Tarakcioglu, M.; Bayazit, N.; Bayazit, Y.A.; Namiduru, M.; Kanlikama, M. Serum cytokine levels in Bell’s palsy. J. Neurol. Sci. 2002, 197, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Kum, R.O.; Yurtsever Kum, N.; Ozcan, M.; Yilmaz, Y.F.; Gungor, V.; Unal, A.; Ciliz, D.S. Elevated neutrophil-to-lymphocyte ratio in Bell’s palsy and its correlation with facial nerve enhancement on MRI. Otolaryngol. Head Neck Surg. 2015, 152, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Bucak, A.; Ulu, S.; Oruc, S.; Yucedag, F.; Tekin, M.S.; Karakaya, F.; Aycicek, A. Neutrophil-to-lymphocyte ratio as a novel-potential marker for predicting prognosis of Bell palsy. Laryngoscope 2014, 124, 1678–1681. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, H.Y. Hematological Findings in Patients with Acute Peripheral Facial Palsy. J. Int. Adv. Otol. 2020, 16, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Candar, T.; Baklaci, D.; Kuzucu, I.; Kayabasi, S. A proinflammatory marker in chronic rhinosinusitis: Serum calprotectin. Acta Biochim. Pol. 2020, 67, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Kuzucu, İ.; Çandar, T.; Baklacı, D.; Güler, İ.; Kum, R.O.; Arslan, H.; Özcan, M. A Prognostic Marker in Idiopathic Sudden Sensorineural Hearing Loss: Serum Calprotectin. Clin. Exp. Otorhinolaryngol. 2020, 13, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Van Crombruggen, K.; Vogl, T.; Pérez-Novo, C.; Holtappels, G.; Bachert, C. Differential release and deposition of S100A8/A9 proteins in inflamed upper airway tissue. Eur. Respir. J. 2016, 47, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, A.M.; Özdaş, T.; Çakır, A.; Alagoz, S.; Bal, K.K.; Gorgulu, O.; Kuran, G.; Matyar, S.; Gorgulu, F.F. Predictive value of serum calprotectin level in nasal polyposis. B-ENT 2023, 19, 151–156. [Google Scholar] [CrossRef]
- Kum, N.Y.; Kum, R.O.; Candar, T.; Baklaci, D.; Guler, I.; Kuzucu, I.; Ozcan Kursat Murat Ozcan, M.; Dere, H. Elevated serum calprotectin as an inflammatory marker in obstructive sleep apnea. Cranio J. Craniomandib. Sleep Pract. 2020, 41, 160–166. [Google Scholar] [CrossRef]
| BP Group (n = 40) | Control Group (n = 20) | p Value | |
|---|---|---|---|
| Age (year), mean ± SD (Min–Max) | 44.17 ± 18.33 (14–74) | 44.50 ± 10.72 (15–64) | 0.815 a |
| Gender, n (%) | |||
| Male | 20 (50) | 10 (50) | 4.72 b |
| Female | 20 (50) | 10 (50) |
| Pre-Treatment | Post-Treatment | p * | |
|---|---|---|---|
| Mean ± SD Median (Min–Max) | Mean ± SD Median (Min–Max) | ||
| CRP | 3.00 ± 2.77 2.5 (0.2–11) | 1.36 ± 1.36 1.05 (0–6.4) | <0.001 |
| WBC count | 10.94 ± 4.15 10.42 (6.49–20.94) | 7.75 ± 1.62 7.40 (5.24–11.33) | <0.001 |
| Lymphocyte count | 2.17 ± 1.05 2.19 (0.67–4.37) | 1.91 ± 0.58 1.90 (1.03–2.87) | 0.519 |
| Neutrophil count | 8.16 ± 4.31 7.48 (3.30–18.94) | 5.34 ± 1.77 5.08 (2.16–9.04) | 0.001 |
| Neutrophil-to-lymphocyte ratio | 5.29 ± 4.18 4.17 (1.10–13.31) | 3.20 ± 1.89 2.91 (0.86–8.29) | 0.038 |
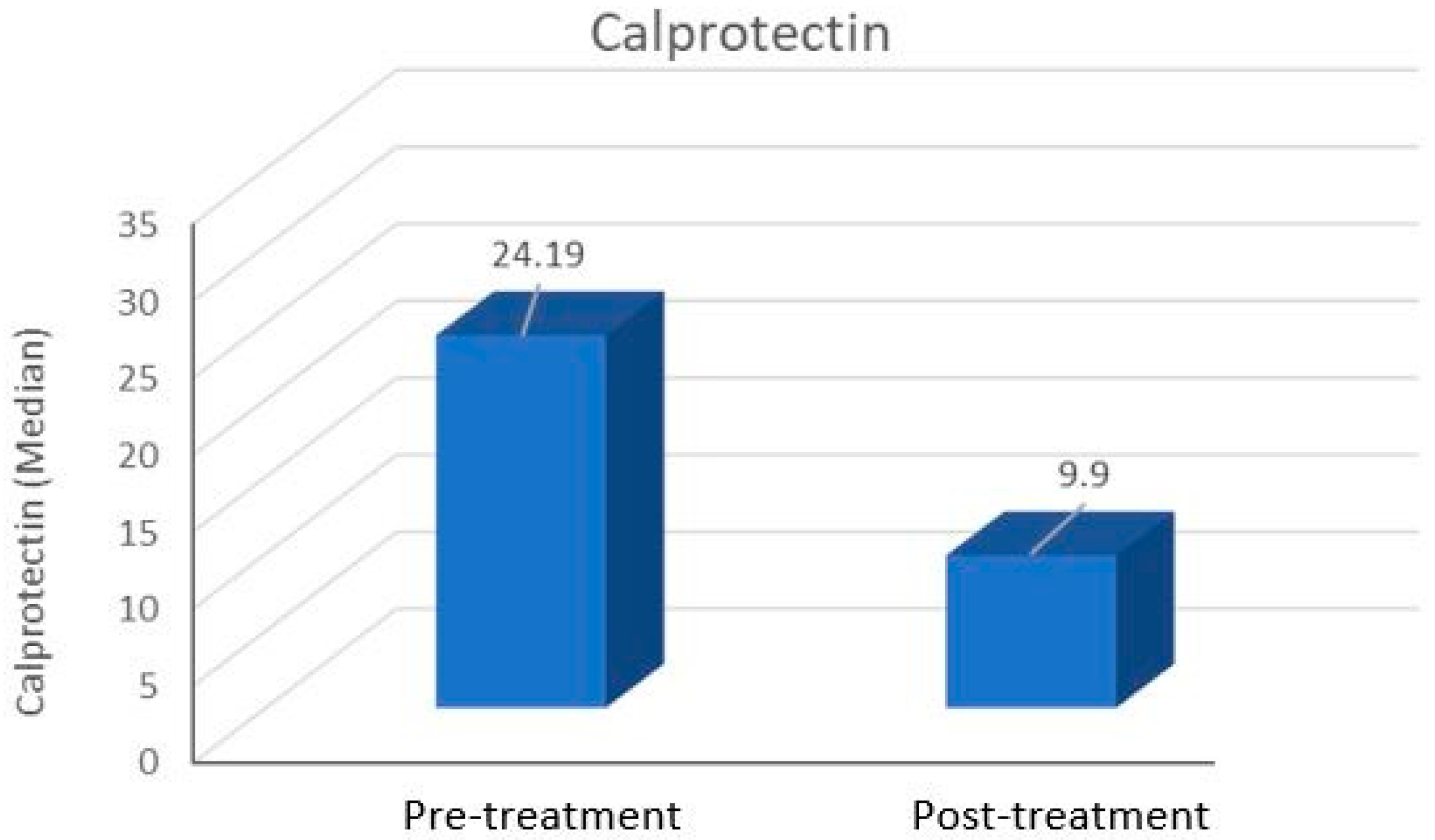
| Calprotectin | 29.94 ± 21.49 24.19 (5.61–73.46) | 14.45 ± 9.91 9.90 (2.80–35.32) | <0.001 |
| Stage | 3 (2–4) | 2 (1–3) | <0.001 |
| BP Group (n = 40) | Control (n = 20) | p * | |
|---|---|---|---|
| Pre-Treatment Parameter | Mean ± SD Median (Min–Max) | Mean ± SD Median (Min–Max) | |
| CRP | 3.00 ± 2.77 2.5 (0.2–11) | 2.05 ± 1.89 1.95 (0.2–9.5) | 0.322 |
| WBC count | 10.94 ± 4.15 10.42 (6.49–20.94) | 7.01 ± 1.43 7 (4.80–9.34) | <0.001 |
| Lymphocyte count | 2.17 ± 1.05 2.19 (0.67–4.37) | 2.17 ± 0.68 2.01 (1.49–3.64) | 0.975 |
| Neutrophil count | 8.16 ± 4.31 7.48 (3.30–18.94) | 3.92 ± 1.42 3.52 (2.37–6.72) | <0.001 |
| Neutrophil-to-lymphocyte ratio | 5.29 ± 4.18 4.17 (1.10–13.31) | 2.03 ± 0.93 1.93 (0.81–3.65) | 0.007 |
| Calprotectin | 29.94 ± 21.49 24.19 (5.61–73.46) | 14.35 ± 7.72 14.69 (2.19–29.40) | 0.012 |
| Complete Response (n = 18) | Partial/No Response (n = 22) | p * | |
|---|---|---|---|
| Mean ± SD Median (Min–Max) | Mean ± SD Median (Min–Max) | ||
| Pre-treatment calprotectin | 33.46 ± 23.95 27.94 (7.48–73.46) | 27.06 ± 19.34 22.06 (5.61–66.02) | 0.381 |
| Post-treatment calprotectin | 12.21 ± 10.91 8.77 (2.8–35.32) | 16.29 ± 8.85 15.23 (4.44–30.02) | 0.089 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türker, C.; Emre, E.; Aydın, S.; Dalgıç, M.; Baklacı, D. An Important Biomarker in Patients with Bell’s Palsy: Serum Calprotectin. Medicina 2025, 61, 747. https://doi.org/10.3390/medicina61040747
Türker C, Emre E, Aydın S, Dalgıç M, Baklacı D. An Important Biomarker in Patients with Bell’s Palsy: Serum Calprotectin. Medicina. 2025; 61(4):747. https://doi.org/10.3390/medicina61040747
Chicago/Turabian StyleTürker, Cihan, Elif Emre, Süleyman Aydın, Mustafa Dalgıç, and Deniz Baklacı. 2025. "An Important Biomarker in Patients with Bell’s Palsy: Serum Calprotectin" Medicina 61, no. 4: 747. https://doi.org/10.3390/medicina61040747
APA StyleTürker, C., Emre, E., Aydın, S., Dalgıç, M., & Baklacı, D. (2025). An Important Biomarker in Patients with Bell’s Palsy: Serum Calprotectin. Medicina, 61(4), 747. https://doi.org/10.3390/medicina61040747





