Hemodynamic Monitoring During Liver Transplantation for Patients on Perioperative Extracorporeal Membrane Oxygenation (ECMO) Support: A Narrative Review
Abstract
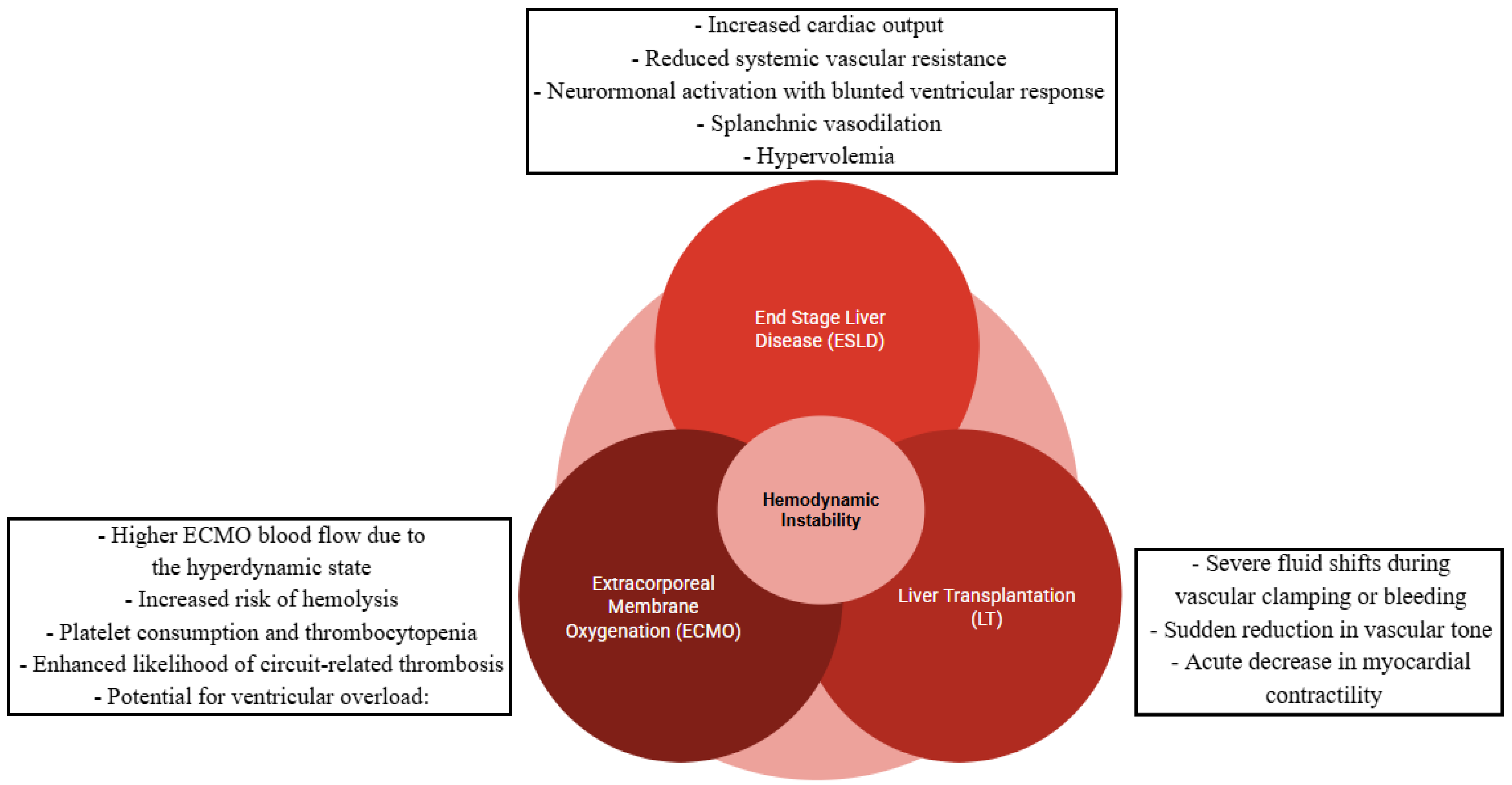
1. Introduction
1.1. Continuous Systemic Arterial Pressure Monitoring
1.2. Pulse Contour Analysis (PCA)
1.3. Neuromonitoring
1.4. ECMO Parameters
1.5. Heart Catheterism
1.6. Echocardiography
1.7. Scope of the Review
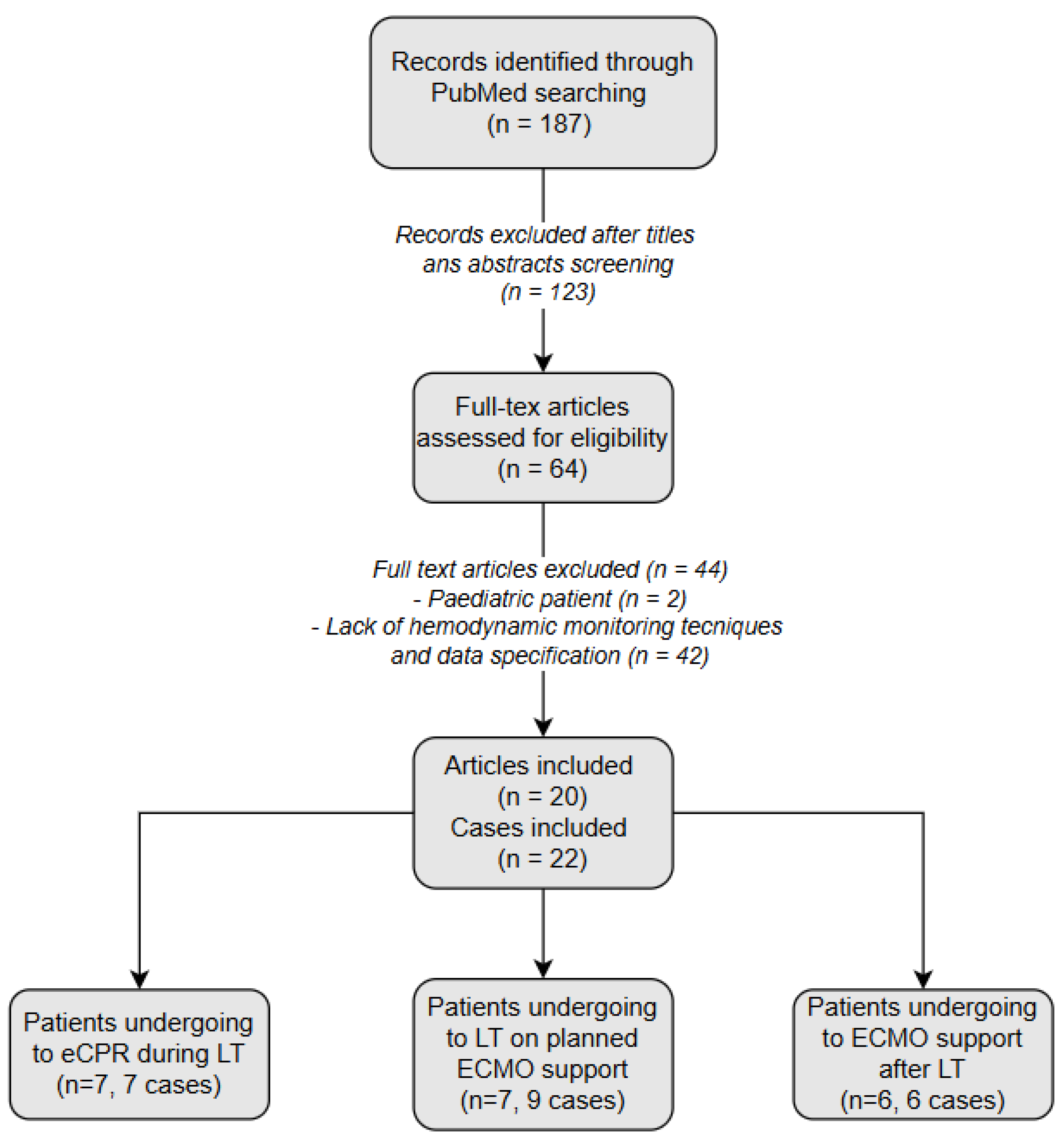
2. Materials and Methods
3. Results and Discussion
- (1)
- Nine cases in which ECMO support was preoperatively planned to optimize the outcome of LT in patients with ESLD and severe cardiorespiratory impairment (Table 5).
- (2)
- Seven cases in which ECMO support was used asrescue support during LT (Table 6).
- (3)
- Six cases in which ECMO support became necessary due to severe cardiorespiratory complications arising post-transplantation (Table 7).
3.1. Patients Undergoing Liver Transplantation on Planned ECMO Support
| Article Journal Year | Study Design | Age Sex (M/F) | LT Indication | ECMO Indication | ECMO Configuration Cannulation | Start/Weaning of ECMO | Hemodynamic Monitoring Before ECMO Support and LT | Hemodynamic Monitoring During ECMO Support and LT | Hemodynamic Monitoring After ECMO Support and LT | Complications | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Barbas et al. Liver Transpl 2021 [50] | Letter | 54 M | Hep C cirrhosis | PoPH | V-V-A Peripheral (femoro-femoral–jugular) | Intraoperative (after GA induction)/POD 1 | Heart Catheterization (HC) Diagnosed moderate PoPH contraindicating LT and initiating anti-PAH therapy. At follow-up, understaging PH permitted re-listing, although two LT attempts aborted for severe PH necessitated preemptive V-A ECMO planning. | No data | Transthoracic Echo (TTE) Detected improved Echoparameters led to weaning of sildenafil and oxygen therapy. | AKI required Continuous Renal Replacement Therapy (CRRT). Hypoxemic respiratory failure requiresdomiciliary oxygen therapy. | Alive at 1 year |
| X. Sun et al. Medicine 2018 [51] | Case report | 44 F | Hep B cirrhosis | PH | V-A Peripheral (femoro-femoral) | Intraoperative (between preanhepatic and anhepatic phases)/30h post-LT | Transthoracic Echo (TTE) Revealed severe MR, TR, LA enlargement, and PH. | No data | Transthoracic Echo (TTE) Revealed improved CVP and progressive decrease in LA size and mitral and tricuspid valve areas. | None | Alive at 48 h post-LT |
| J. Lee et al. J. Clin. Med. 2023 [52] | Case report | 49 F | Cryptogenic ESLD HCC | Severe PoPH with PFO | V-A Peripheral (jugulo-axillary) | 24 h before LT/POD14 | Heart Catheterization (HC) Diagnosed PoPH Transthoracic Echo (TTE) Detected RV overload with borderline systolic function, prompting the decision to plan for V-A ECMO support. | Transesophageal Echo (TEE) Detected acute RV dysfunction at reperfusion, informing for increasing ECMO BFR with subsequent improvement in RV systolic function. | Transthoracic Echo (TTE) Detected RV overload with preserved biventricular systolic function. | Coagulopathy. Refractory hypoxia. Hemodynamic instability. | Died at 6 weeks for asystolia due to refractory hypoxia |
| J. Lee et al. J. Clin. Med. 2023 [52] | Case report | 58 M | Cryptogenic ESLD | PoPH and severe pulmonary valve stenosis | V-A Peripheral (femoro-femoral) | After incision/before abdomen closure | Heart Catheterization (HC) Diagnosed PoPH Transthoracic Echo (TTE) Detected RV overload with high-risk intraoperative dysfunction, prompting the decision to plan for intraoperative V-A ECMO and CRRT | Transesophageal Echo (TEE) Detected acute RV dysfunction at reperfusion, informing for inotropic support initiation with subsequent improvement in RV systolic function. | Transthoracic Echo (TTE) Detected RV overload with preserved biventricular systolic function. | Narrowing at the venous piggyback anastomosis was corrected by balloon angioplasty. | Alive at 1 year |
| C. Laici et al. J. Clin. Med. 2023 [43] | Case report | 59 M | EtOH cirrhosis | Moderate PoPH | V-V Peripheral (femoro-jugular) | After GA induction/14 h post-LT | Transthoracic Echo (TTE) Detected RV dysfunction with high estimated sPAP Heart Catheterization (HC) Diagnosed moderate PoPH prompting anti-PAH therapy escalation; detection of understaged PAH at follow-up unabling LT on planned intraoperative V-V ECMO. | Transesophageal Echo (TEE) Guided intraoperative fluid and vasoactive therapy during V-V ECMO (inhaled NO milrinone NA). | Heart Catheterization (HC) Detected persistent precapillary PH with a reduced CI at rest and right heart failure. | Pleural effusion with complete atelectasis of both lower lobes required thoracic drainage placement. AKI. | Alive at 3 months |
| C. Laici et al. J. Clin. Med. 2023 [43] | Case report | 45 F | EtOH cirrhosis | Group 2 pulmonary hypertension (congestive heart failure) | V-V Peripheral (femoro-jugular) | After GA induction/36 h post-LT | Transthoracic Echo (TTE) Detected RV enlargement with high estimated sPAP. Diagnosis of HPS with bubble test Pulmonary artery catheter (PAC) Diagnosed PH led to perioperative CRRT. | Transesophageal Echo (TEE) Guided for ECMO cannula positioning. | No data | None | Alive at 1 year |
| A. Siniscalchi et al. ASAIO Journal 2023 [53] | Case report | 52 F | HDV/HBV-related chronic cirrhosis | Group 2 pulmonary hypertension (severe mitral regurgitation) | V-V-A Peripheral (femoro-jugular– femoral) | After GA induction/6 h post-LT | Transthoracic Echo (TTE) Detected RV enlargement with high estimated sPAP and severe mitral regurgitation. Pulmonary artery catheter (PAC) Diagnosed PH and fluid tolerance assessment. | No data | Transthoracic Echo (TTE) Detected immediate postoperative improvement in cardiac function, informing for weaning from V-A ECMO 6 h after LT. Transesophageal Echo (TEE) Detected progressive improvement in cardiac function after 10 days, 15 days, and 6 months of LT. | AKI required CRRT. High-flow A-V fistula between the right femoral vessels required surgical treatment. | Alive at 10 months |
| S.R.Choi et al. Medicina 2023 [54] | Case report | 25 F | Exotoxic ALF | ARDS aggravated by HPS | V-V Peripheral (femoro-jugular) | 30h before LT/POD 6 | Transthoracic Echo (TTE) Ruled out cardiogenic pulmonary edema. | Transesophageal Echo (TEE) Carried out as an alternative intraoperative hemodynamic monitoring on PAC for infeasible placement due to ECMO cannula. | No data | Fluid overload required CRRT. | Alive on POD 17 and discharged fromthe general ward |
| J.H. Tyler et al. Cureus 2024 [55] | Case report | 66 M | Cryptogenic ESLD | Severe HPS | V-V Peripheral (femoro-jugular) | Preoperative/POD 11 | Transesophageal Echo (TEE) Detected intrapulmonary shunt with bubble test and small patent foramen ovale (PFO). | No data | Transesophageal Echo (TEE) Bubble test detected enlarged PFO with significant right-to-left shunting during work-up to evaluate pulmonary embolism. | Bleeding required surgical re-exploration. Refractory hypoxemia delirium and right pleural effusion required tracheostomy and chest tube placement. Bile duct stricture required stent placement. Pneumonia. | Alive at 12 months |
3.2. Patients Undergoing eCPR During Liver Transplantation
| Article Journal Year | Study Type | Age Sex (M/F) | LT Indication | ECMO Indication | ECMO Configuration Cannulation | Start/Weaning of ECMO | Preoperative Hemodynamic Data | Intraoperative Hemodynamic Data | Postoperative Hemodynamic Data | Complications | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M. Tejani et al. Liver Transpl 2015 [56] | Letter | 61 M | Hep B cirrhosis | CA | V-A Peripheral (femoro-femoral) trans-diaphragmatic apical venting after CA and distal perfusion cannula (DPC) | Intraoperative (post-reperfusion phase)/POD 2 | Transthoracic Echo (TTE) Revealed normal biventricular function without wall motion abnormalities by a pharmacological stresstest. | Pulmonary Artery Catheter (PAC) Revealed elevated CVP and PAP accompanied by hypotension and bradycardia (post-reperfusion phase) necessitating CPR (external and internal), followed by eCPR. Transesophageal Echo (TEE) Severe LV dilation with systolic dysfunction. | Transesophageal Echo (TEE) Detected progressive improvement in biventricular function with fully restored cardiac function before discharge | Bleeding (left femoral arterial cannulation site and the Jackson–Pratt drain in the pericardium) | Alive at 3 months |
| G. Martucci et al. Minerva Anestesiol 2017 [57] | Letter | 60 M | EtOH cirrhosis | CA | V-A Peripheral (femoro-femoral) | Intraoperative (post-reperfusion phase)/POD 8 (death) | Transthoracic Echo (TTE) Revealed normal biventricular function. | Transesophageal Echo (TEE) Detected PH with normal biventricular function and severe RV dysfunction after reperfusion phase Pulmonary Artery Catheter (PAC) Revealed PH in the preanhepatic phase led to initiation and titration of milrinone and iNO. PAC revealed worsening PH until CA (PEA) occurred, requiring CPR and eCPR. | No data | Septic shock | Died on POD8 for MOF secondary to donor-derived septic shock (Acinetobacter) |
| D. N. Romano et al. Semin Cardiothorac Vasc Anesth 2021 [58] | Case report | 62 M | Hep C and EtOH cirrhosis HCC | CA (cardiac thrombosis) | V-A Peripheral (femoro-femoral) | Intraoperative (post-reperfusion phase)/POD 5 | Transesophageal Echo (TEE) Detected normal heart function at 1 y after CABG. | Pulmonary Artery Catheter (PAC) Detected hyperdynamic circulation and acute PH associated with severe hypotension before CA post-reperfusion requiring CPR and eCPR. Transesophageal Echo (TEE) Preanhepatic phase: revealed mild–moderate reduction in biventricular function and mild MR and TR. Post-reperfusion: detected biventricular thrombosis associated with severe biventricular dysfunction after ROSC led to eCPR initiation | Transesophageal Echo (TEE) Detected improving heart function with apico-inferior akinesis | Coagulopathy Respiratory hypoxemic failure and deterioration of mental status required tracheostomy AKI necessitating CRRT Sepsis | Died on POD 13 secondary to MOF (care withdrawn) |
| J. Szocik et al. Anesthesiology 2002 [59] | Corresp. | 54 F | Primary biliary cirrhosis | CA (cardiac thrombosis) | V-A Peripheral (femoro-femoral) | Intraoperative (late anhepatic phase)/in OR after completion of the biliary anastomoses | No data | Pulmonary Artery Catheter (PAC) Detected acute PH associated with severe hypotension before CA at the end of anhepatic phase requiring CPR and eCPR. Transesophageal Echo (TEE) Detected a massive clot in the RA, RV, and mitral valve. | No data | Extensive caval thrombosis | Died on the ninth week post-LT secondary to MOF |
| J. Y. Lim et al. Korean J Crit Care Med 2016 [60] | Letter | 61 F | Hep B cirrhosis | CA (PE) | V-A Peripheral (femoro-femoral) | Intraoperative (preanhepatic phase)/POD 2 | Transthoracic Echo (TTE) Detected normal biventricular function. | Transesophageal Echo (TEE) Severe RV failure and D-shaped LV and severe biventricular dysfunction after ROSC and during V-A ECMO support. | Transthoracic Echo (TTE) Revealed severe biventricular dysfunction improved by POD 2 facilitating ECMO weaning. TTE on POD 4 demonstrated normal cardiac function. | Operative site bleeding | Alive at POD 64 |
| K. W. Eudailey et al. Perfusion 2015 [61] | Case report | 61 M | Hep B cirrhosis HCC | CA | V-A Peripheral (femoro-femoral) trans-diaphragmatic apical venting after CA and distal perfusion cannula (DPC) | Intraoperative (post-reperfusion phase)/POD 2 | No data | Transesophageal Echo (TEE) Confirmed appropriate venous cannula position. Resuscitative TEE found profound biventricular dysfunction with severe LV overload. TEE guided and confirmed proper placement of the trans diaphragmatic ventricular vent with complete decompression of the LV. | Transesophageal Echo (TEE) Guided weaning and cessation of ECMO support on POD 2 after revealing improved biventricular function. Transthoracic Echo (TTE) Showed normal cardiac function at POD 6 and after 3 months of follow-up | None | Alive at 3 months |
| A. Lauterio et al. Transplantation 2019 [62] | Letter | 53 M | Hep C cirrhosis | CA (MI) | V-A Peripheral (femoro-femoral) | Intraoperative (post-reperfusion phase)/POD 4 | Transthoracic Echo (TTE) Revealed normal biventricular function with normal myocardial perfusion at a rest/stress dipyridamole scintigraphy. | Transthoracic Echo (TTE) Detected severe LV hypokinesia after ROSC (post-reperfusion phase) | Transthoracic Echo (TTE) Detected LVEF of 55% after decannulation | Aminotransferase peak on POD 2 without graft dysfunction | Alive at 10 months |
3.3. Patients Undergoing ECMO Support After Liver Transplantation
| Article Journal Year | Study Type | Age Sex (M/F) | LT Indication | ECMO Indication | ECMO Configuration Cannulation | Start/Weaning of ECMO | Preoperative Hemodynamic Monitoring | Intraoperative Hemodynamic Monitoring | Postoperative Hemodynamic Monitoring | Complications | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N. S. Sharma et al. Int J Artif Organs 2015 [63] | Short comun. | 60 F | NASH cirrhosis | HPS | V-V Peripheral (Avalon catheter or DLC in RIJ vein) | POD 11/POD 24 | Transthoracic Echo (TTE) Bubble test detected right-to-left intrapulmonary shunt Heart Catheterization (HC) Ruled-out PoPH and confirmed diagnosis of HPS | No data | Transthoracic Echo (TTE) Detected worsened right-to-left intrapulmonary shunt informing for postoperative V-V ECMO initiation. Transesophageal Echo (TEE) Guided DLC positioning and detected PFO causing right-to-left shunt, prompting DLC replacement under fluoroscopy guidance. | Left hemothorax requiring multiple blood transfusions and surgical evacuation. Tracheostomy. | Alive on POD 24 |
| C. Stratta et al. Transplantation Proceedings 2013 [64] | Case report | 43 M | EtOH cirrhosis | PoPH and ARDS | V-V Peripheral (femoro- femoral) | POD 1/POD 11 | No data | Pulmonary Artery Catheter (PAC) Diagnosed PH guiding initiation and titration of iNO and epoprostenol infusion. | Transthoracic Echo (TTE) Detected RV overload signs. Pulmonary Artery Catheter (PAC) Detected worsening PoPH guiding the escalation of anti-PAH therapy and initiation of CRRT to avoid fluid overload during V-V ECMO support to manage ARDS. | Right cardiac failure required Levosimendan. CRRT for reducing fluid overload. Pneumonia of the left lobe. | Alive at 1 month |
| G. Caturegli et al. ASAIO Journal 2022 [65] | Case report | 55 M | EtOH cirrhosis | CA | V-A Peripheral (femoro- femoral) | POD 1/POD 7 | Transthoracic Echo (TTE) Revealed normal biventricular function Heart Catheterization (HC) Revealed right-dominant circulation with noncritical mid-right coronary artery (RCA) stenosis | Transesophageal Echo (TEE) Detected biventricular dysfunction with severe hypokinesis of the apex consistent with Takotsubo syndrome | Transesophageal Echo (TEE) Detected normal LV function on ECMO day 7 guiding the decision to decannulate. | Respiratory failure. AKI required CRRT. Aspergillus septic shock. | Died six weeks after LT |
| J.E. Barrueco-Francioni et al. Int J Artif Organs 2024 [66] | Short comun. | 59 yo F | EtOH cirrhosis | Hypoxemia | V-V Peripheral (femoro- jugular) | POD 7/POD 15 | No data | No data | Transesophageal Echo (TEE) Guided ECMO cannulation | Acute graft rejection requiring corticosteroid. Clostridium difficile infection treated with fidaxomicin. PAF managed with beta-blockers. | Alive 6 months after LT |
| R. S. Biondi et al. Rev Bras Ter Intensiva. 2018 [67] | Case report | 58 F | Cryptogenic cirrhosis | CS | V-A Peripheral (femoro- femoral) | POD 2/POD 6 | Transthoracic Echo (TTE) Revealed normal biventricular function with normal LVEF on pharmacologic echo stress | No data | Transthoracic Echo (TTE) Showed severe dysfunction of the left ventricle (EF: 12%), with severe distension of the left ventricle, diffuse hypokinesia and dyskinesia of the apex and signs of high cardiac filling pressures | Hemoperitoneum and hepatic ischemia required re-laparotomy. AKI required CRRT. Coagulopathy. | Alive 2 months after LT |
| A. Lauterio et al., Minerva Anestesiologica 2022 [68] | Case report | 56 F | EtOH cirrhosis | CS | V-A Peripheral (femoro- femoral) | POD 1/POD 6 | Transthoracic Echo (TTE) Revealed normal biventricular function and sizes | No data | Pulmonary Artery Catheter (PAC) Revealed high PCWP (29 mmHg) Transthoracic Echo (TTE) Showed severe LV systolic dysfunction (LV EF 15%) and basal/middle segment akinesia with hyperkinetic apex consistent with inverted Takotsubo cardiomyopathy. TTE-guided femoral cannulation. | Hypoxemia | Alive on POD 10 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LT | Liver Transplantation |
| ECMO | Extracorporeal Membrane Oxygenation |
| V-V | Veno-Venous |
| V-A | Veno-Arterial |
| V-V-A | Veno-Venous-Arterial |
| BFR | Blood Flow Rate |
| DO2 | Oxygen Delivery |
| FiO2 | Fraction Inspired Oxygen |
| PoPH | Portopulmonary Hypertension |
| PH | Pulmonary Hypertension |
| RHC | Right Heart Catheterization |
| PAC | Pulmonary Artery Catheter |
| PVR | Pulmonary Vascular Resistance |
| PCWP | Pulmonary Capillary Wedge Pressure |
| GA | General Anesthesia |
| POD | Postoperative Day |
| HC | Heart Catheterization |
| TTE | Transthoracic Echocardiography |
| TEE | Transesophageal Echocardiography |
| MR | Mitral Regurgitation |
| TR | Tricuspid Regurgitation |
| LA | Left Atrium |
| RV | Right Ventricle |
| BFR | Blood Flow Rate |
| sPAP | Systolic Pulmonary Arterial Pressure |
| mPAP | Mean Pulmonary Arterial Pressure |
| TCD | Transcranial Doppler |
| ABI | Acute Brain Injury |
| MES | Microembolic Signals |
| CI | Cardiac Index |
| CO | Cardiac Output |
| SvO2 | Venous Oxygen Saturation |
| CRRT | Continuous Renal Replacement Therapy |
| AKI | Acute Kidney Injury |
| EtOH | Cirrhosis Alcohol-Related Liver Cirrhosis |
| ESLD | End-Stage Liver Disease |
| HCC | Hepatocellular Carcinoma |
| PFO | Patent Foramen Ovale |
| PAC | Pulmonary Artery Catheter |
| PoPH | PortoPulmonary Hypertension |
| HPS | Hepatopulmonary Syndrome |
| RWMA | Regional Wall Motion Abnormalities |
| LVEF | Left Ventricular Ejection Fraction |
| VTI | Velocity–Time Integral |
| eCPR | extracorporeal Cardiopulmonary Resuscitation |
| ECLS | Extracorporeal Life Support |
| A-VFistula | Arteriovenous Fistula |
| ARDS | Acute Respiratory Distress Syndrome |
| MOF | Multiorgan Failure |
| DLC | Double-Lumen-Cannula |
References
- Yang, J.D.; Larson, J.J.; Watt, K.D.; Allen, A.M.; Wiesner, R.H.; Gores, G.J.; Roberts, L.R.; Heimbach, J.A.; Leise, M.D. Hepatocellular carcinoma is the most common indication for liver transplantation and placement on the waitlist in the United States. Clin. Gastroenterol. Hepatol. 2017, 15, 767–775.e3. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Bruix, J. Liver transplantation for hepatocellular carcinoma: Outcomes and novel surgical approaches. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 203–217. [Google Scholar] [CrossRef]
- Goldberg, D.; Ditah, I.C.; Saeian, K.; Lalehzari, M.; Aronsohn, A.; Gorospe, E.C.; Charlton, M. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017, 152, 1090–1099.e1. [Google Scholar] [CrossRef] [PubMed]
- Verna, E.C.; Patel, Y.A.; Aggarwal, A.; Desai, A.P.; Frenette, C.; Pillai, A.A.; Salgia, R.; Seetharam, A.; Sharma, P.; Sherman, C.; et al. Liver transplantation for hepatocellular carcinoma: Management after the transplant. Am. J. Transplant. 2020, 20, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Tabrizian, P.; Holzner, M.L.; Zaret, D.; Meyerovich, G.; Fagenson, A.; Schiano, T. Liver transplantation and hepatocellular carcinoma 2023, a narrative review of management and outcomes. Ann. Palliat. Med. 2024, 13, 126–140. [Google Scholar] [CrossRef]
- Ince, V.; Sahin, T.T.; Akbulut, S.; Yilmaz, S. Liver transplantation for hepatocellular carcinoma: Historical evolution of transplantation criteria. World J. Clin. Cases 2022, 10, 10413–10427. [Google Scholar] [CrossRef]
- Dimou, F.M.; Mehta, H.B.; Adhikari, D.; Harland, R.C.; Riall, T.S.; Kuo, Y.-F. The role of extended criteria donors in liver transplantation for nonalcoholic steatohepatitis. Surgery 2016, 160, 1533–1543. [Google Scholar] [CrossRef]
- VanWagner, L.B.; Serper, M.; Kang, R.; Levitsky, J.; Hohmann, S.; Abecassis, M.; Skaro, A.; Lloyd-Jones, D.M. Factors associated with major adverse cardiovascular events after liver transplantation among a national sample. Am. J. Transplant. 2016, 16, 2684–2694. [Google Scholar] [CrossRef]
- Eckman, P.M.; Katz, J.N.; El Banayosy, A.; Bohula, E.A.; Sun, B.; van Diepen, S. Veno-arterial extracorporeal membrane oxygenation for cardiogenic shock: An introduction for the busy clinician: An introduction for the busy clinician. Circulation 2019, 140, 2019–2037. [Google Scholar] [CrossRef]
- Contento, C.; Battisti, A.; Agrò, B.; De Marco, M.; Iaiza, A.; Pietraforte, L.; Pisani, P.; Proietti, A.; Vitalini, E.; Montalto, A.; et al. A novel veno-arteriovenous extracorporeal membrane oxygenation with double pump for the treatment of Harlequin syndrome. Perfusion 2020, 35, 65–72. [Google Scholar] [CrossRef]
- Alwardt, C.M.; Patel, B.M.; Lowell, A.; Dobberpuhl, J.; Riley, J.B.; DeValeria, P.A. Regional perfusion during venoarterial extracorporeal membrane oxygenation: A case report and educational modules on the concept of dual circulations. J. Extra Corpor. Technol. 2013, 45, 187–194. [Google Scholar] [CrossRef]
- Biancofiore, G.; Critchley, L.A.H.; Lee, A.; Bindi, L.; Bisà, M.; Esposito, M.; Meacci, L.; Mozzo, R.; DeSimone, P.; Urbani, L.; et al. Evaluation of an uncalibrated arterial pulse contour cardiac output monitoring system in cirrhotic patients undergoing liver surgery. Br. J. Anaesth. 2009, 102, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Su, B.C.; Tsai, Y.F.; Cheng, C.W.; Yu, H.P.; Yang, M.W.; Lee, W.C.; Lin, C.C. Stroke volume variation derived by arterial pulse contour analysis is a good indicator for preload estimation during liver transplantation. Transplant. Proc. 2012, 44, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Nigatu, A.; Yap, J.E.; Lee Chuy, K.; Go, B.; Doukky, R. Bleeding risk of transesophageal echocardiography in patients with esophageal varices. J. Am. Soc. Echocardiogr. 2019, 32, 674–676.e2. [Google Scholar] [CrossRef] [PubMed]
- Souto Moura, T.; Aguiar Rosa, S.; Germano, N.; Cavaco, R.; Sequeira, T.; Alves, M.; Papoila, A.L.; Bento, L. The accuracy of PiCCO® in measuring cardiac output in patients under therapeutic hypothermia: Comparison with transthoracic echocardiography. Med Intensiv. (Engl. Ed.) 2018, 42, 92–98. [Google Scholar] [CrossRef]
- Zhao, D.; Shou, B.L.; Caturegli, G.; Whitman, G.J.R.; Kim, B.S.; Cho, S.-M.; on behalf of Herald Investigators. Trends on near-infrared spectroscopy associated with acute brain injury in venoarterial extracorporeal membrane oxygenation. ASAIO J. 2023, 69, 1083–1089. [Google Scholar] [CrossRef]
- Marinoni, M.; Migliaccio, M.L.; Trapani, S.; Bonizzoli, M.; Gucci, L.; Cianchi, G.; Gallerini, A.; Buoninsegni, L.T.; Cramaro, A.; Valente, S.; et al. Cerebral microemboli detected by transcranial doppler in patients treated with extracorporeal membrane oxygenation. Acta Anaesthesiol. Scand. 2016, 60, 934–944. [Google Scholar] [CrossRef]
- Kalra, A.; Kang, J.K.; Wilcox, C.; Shou, B.L.; Brown, P.; Rycus, P.; Anders, M.M.; Zaaqoq, A.M.; Brodie, D.; Whitman, G.J.R.; et al. Pulse pressure and acute brain injury in venoarterial extracorporeal membrane oxygenation: An Extracorporeal Life Support Organization Registry analysis. ASAIO J. 2025, 71, 99–108. [Google Scholar] [CrossRef]
- Caturegli, G.; Zhang, L.Q.; Mayasi, Y.; Gusdon, A.M.; Ergin, B.; Ponomarev, V.; Kim, B.S.; Keller, S.; Geocadin, R.G.; Whitman, G.J.R.; et al. Characterization of cerebral hemodynamics with TCD in patients undergoing VA-ECMO and VV-ECMO: A prospective observational study. Neurocrit Care 2023, 38, 407–413. [Google Scholar] [CrossRef]
- Kato, T. The relationship between pulsatility index and left ventricular function in patients on venoarterial extracorporeal membrane oxygenation. J. Card. Fail. 2020, 26. [Google Scholar]
- Tigano, S.; Sanfilippo, F.; Capuano, P.; Arcadipane, A.; Martucci, G. Current practice optimization suggestions and future perspectives on transfusion in patients supported by extracorporeal membrane oxygenation: A narrative review. Ann. Blood 2024, 9, 9. [Google Scholar] [CrossRef]
- Patel, B.; Arcaro, M.; Chatterjee, S. Bedside troubleshooting during venovenous extracorporeal membrane oxygenation (ECMO). J. Thorac. Dis. 2019, 11, S1698–S1707. [Google Scholar] [CrossRef] [PubMed]
- Tralhão, A.; Fortuna, P. Hypoxemia during veno-venous extracorporeal membrane oxygenation. When two is not better than one. Rev. Bras. Ter. Intensiv. 2022, 34, 400–401. [Google Scholar] [CrossRef]
- Krivitski, N.; Galyanov, G.; Cooper, D.; Said, M.M.; Rivera, O.; Mikesell, G.T.; Rais-Bahrami, K. In vitro and in vivo assessment of oxygenator blood volume for the prediction of clot formation in an ECMO circuit (theory and validation). Perfusion 2018, 33, 51–56. [Google Scholar] [CrossRef]
- Wickramarachchi, A.; Burrell, A.J.C.; Joyce, P.R.; Bellomo, R.; Raman, J.; Gregory, S.D.; Rais-Bahrami, K. Flow capabilities of arterial and drainage cannulae during venoarterial extracorporeal membrane oxygenation: A simulation model. Perfusion 2024, 40, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Bartolome, S.; Denton, C.P.; Gatzoulis, M.A.; Gu, S.; Khanna, D.; Badesch, D.; Montani, D. Definition, classification and diagnosis of pulmonary hypertension. Eur. Respir. J. 2024, 64, 2401324. [Google Scholar] [CrossRef]
- Lechartier, B.; Kularatne, M.; Jaïs, X.; Humbert, M.; Montani, D. Updated hemodynamic definition and classification of pulmonary hypertension. Semin. Respir. Crit. Care Med. 2023, 44, 721–727. [Google Scholar] [CrossRef]
- Kularatne, M.; Gerges, C.; Jevnikar, M.; Humbert, M.; Montani, D. Updated clinical classification and hemodynamic definitions of pulmonary hypertension and its clinical implications. J. Cardiovasc. Dev. Dis. 2024, 11, 78. [Google Scholar] [CrossRef]
- Salzano, A.; Sirico, D.; Golia, L.; Faga, V.; Flora, M.; Bossone, E.; Cittadini, A. The portopulmonary hypertension: An overview from diagnosis to treatment. Monaldi Arch. Chest Dis. 2013, 80, 66–68. [Google Scholar] [CrossRef][Green Version]
- Kandil, S.; Sedra, A. Hemodynamic monitoring in liver transplantation “the hemodynamic system”. Curr. Opin. Organ. Transplant. 2024, 29, 72–81. [Google Scholar] [CrossRef]
- Guyen-Buckley, C.; Bezinover, D.; Bhangui, P. International Liver Transplantation Society/Society for Advancement of Transplant Anesthesia Consensus Statement on Essential Attributes of a Liver Transplant Anesthesiologist. Transplantation 2023, 107, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, T.M.A.; Schofield, N.; Krenn, C.G.; Rizkalla, N.; Spiro, M.; Raptis, D.A.; De Wolf, A.M.; Merritt, W.T.; the ERAS4OLT.org Working Group. What is the optimal anesthetic monitoring regarding immediate and short-term outcomes after liver transplantation?-A systematic review of the literature and expert panel recommendations. Clin. Transplant. 2022, 36, e14643. [Google Scholar] [CrossRef]
- Uriel, N.; Sayer, G.; Addetia, K.; Fedson, S.; Kim, G.H.; Rodgers, D.; Kruse, E.; Collins, K.; Adatya, S.; Sarswat, N.; et al. Hemodynamic ramp tests in patients with left ventricular assist devices. JACC Heart Fail. 2016, 4, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.; Zöllner, C.; Manert, W.; Briegel, J.; Kilger, E.; Polasek, J.; Hummel, T.; Forst, H.; Peter, K. Thermodilution cardiac output may be incorrect in patients on venovenous extracorporeal lung assist. Am. J. Respir. Crit. Care Med. 1995, 152, 1812–1817. [Google Scholar] [CrossRef]
- Silva, F.L.; Gomes, J.L.U.M.; Boas, W.W.V.; de Freitas, G.V. Pulmonary artery catheter knot in a liver transplantation, a rare complication. Saudi. J. Anaesth. 2022, 16, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Valencia, L.; López, S.; Olivas, A.; Becerra, Á.; Alemán-Segura, M.D.; Évora-García, M.; Ojeda, N.; Cabrera, L.; Rodríguez-Pérez, A.; Pérez-Peñate, G. Venoarterial extracorporeal membrane oxygenation for vasoplegic shock after treprostinil refill of an implanted intravenous pump: A case report. Front. Cardiovasc. Med. 2024, 11, 1348311. [Google Scholar] [CrossRef]
- Burkhoff, D.; Sayer, G.; Doshi, D.; Uriel, N. Hemodynamics of mechanical circulatory support. J. Am. Coll. Cardiol. 2015, 66, 2663–2674. [Google Scholar] [CrossRef]
- Lee, M.; Oh, J.H. Echocardiographic diagnosis of right-to-left shunt using transoesophageal and transthoracic echocardiography. Open Heart 2020, 7, e001150. [Google Scholar] [CrossRef]
- Platts, D.G.; Sedgwick, J.F.; Burstow, D.J.; Mullany, D.V.; Fraser, J.F. The role of echocardiography in the management of patients supported by extracorporeal membrane oxygenation. J. Am. Soc. Echocardiogr. 2012, 25, 131–141. [Google Scholar] [CrossRef]
- Millan, P.D.; Thiele, R.H. Agreement between transesophageal echocardiography and thermodilution-based cardiac output. Anesth. Analg. 2018, 127, 329–330. [Google Scholar] [CrossRef]
- Swenson, J.D.; Bull, D.; Stringham, J. Subjective assessment of left ventricular preload using transesophageal echocardiography: Corresponding pulmonary artery occlusion pressures. J. Cardiothorac. Vasc. Anesth. 2001, 15, 580–583. [Google Scholar] [CrossRef]
- Hemamalini, P.; Dutta, P.; Attawar, S. Transesophageal echocardiography compared to fluoroscopy for Avalon bicaval dual-lumen cannula positioning for venovenous ECMO. Ann. Card. Anaesth. 2020, 23, 283. [Google Scholar] [CrossRef] [PubMed]
- Laici, C.; Bianchini, A.; Miglionico, N.; Bambagiotti, N.; Vitale, G.; Fallani, G.; Ravaioli, M.; Siniscalchi, A. Planned extracorporeal life support employment during liver transplantation: The potential of ECMO and CRRT as preventive therapies-case reports and literature review. J. Clin. Med. 2023, 12, 1239. [Google Scholar] [CrossRef]
- Douflé, G.; Roscoe, A.; Billia, F.; Fan, E. Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit Care 2015, 19, 326, Erratum in Crit. Care 2016, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Cavarocchi, N.C.; Pitcher, H.T.; Yang, Q.; Karbowski, P.; Miessau, J.; Hastings, H.M.; Hirose, H. Weaning of extracorporeal membrane oxygenation using continuous hemodynamic transesophageal echocardiography. J. Thorac. Cardiovasc. Surg. 2013, 146, 1474–1479. [Google Scholar] [CrossRef]
- Mathur, S.K.; Singh, P. Transoesophageal echocardiography related complications. Ind. J. Anaesth. 2009, 53, 567–574. [Google Scholar]
- Arcas-Bellas, J.J.; Siljeström, R.; Sánchez, C.; González, A.; García-Fernández, J. Use of transesophageal echocardiography during orthotopic liver transplantation: Simplifying the procedure. Transplant. Direct 2024, 10, e1564. [Google Scholar] [CrossRef]
- Da Costa Rodrigues, J.; Gazarian, C.; Maillard, J.; Albu, G.; Assouline, B.; Lador, F.; Schiffer, E. Emergency ECMO deployment during liver transplantation in Portopulmonary hypertension patients. Am. J. Case Rep. 2025, 26, e946268. [Google Scholar] [CrossRef]
- Kumar, L.; Balakrishnan, D.; Varghese, R.; Surendran, S. Extracorporeal membrane oxygenation for post-transplant hypoxaemia following very severe hepatopulmonary syndrome. BMJ Case Rep. 2017, 2017, bcr–2017-221381. [Google Scholar] [CrossRef]
- Barbas, A.S.; Schroder, J.N.; Borle, D.P.; Suarez, A.; Abraham, N.; Manning, M.W.; Miller, T.E.; Berg, C.L.; Fortin, T.A.; Sudan, D.L.; et al. Planned initiation of venoarterial extracorporeal membrane oxygenation prior to liver transplantation in a patient with severe portopulmonary hypertension. Liver Transpl. 2021, 27, 760–762. [Google Scholar] [CrossRef]
- Sun, X.; Qiu, W.; Chen, Y.; Lv, G.; Fan, Z. Utilization of extracorporeal membrane oxygenation for a severe cardiocirculatory dysfunction recipient in liver transplantation: A case report: A case report. Medicine 2018, 97, e12407. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Allen, W.L.; Scott, C.L.; Aniskevich, S.; Pai, S.-L. Preemptive venoarterial extracorporeal membrane oxygenation for liver transplantation-judicious candidate selection. J. Clin. Med. 2023, 12, 4965. [Google Scholar] [CrossRef]
- Siniscalchi, A.; Laici, C.; Facciotto, L.; Vitale, G.; Fallani, G.; Ravaioli, M.; Bianchini, A. VA-ECMO cardiac support during liver transplant: A case report. ASAIO J. 2023, 69, e411–e414. [Google Scholar] [CrossRef]
- Choi, S.R.; Lee, S.C.; Lee, T.Y.; Jung, J.W.; Kim, M.A.; Park, S.Y. Perioperative extracorporeal membrane oxygenation support for acute respiratory distress syndrome aggravated by hepatopulmonary syndrome in deceased donor liver transplantation: A case report. Medicina 2023, 59, 1422. [Google Scholar] [CrossRef]
- Tyler, J.H.; Fleetwood, V.; Kamel, G.; Verma, D.R.; Rangrass, G. Planned venovenous-extracorporeal membrane oxygenation as a bridge to orthotopic liver transplant performed for very severe hepatopulmonary syndrome: A case report. Cureus 2024, 16, e63962. [Google Scholar] [CrossRef] [PubMed]
- Tejani, M.; Yi, S.Y.; Eudailey, K.W.; George, I.; Guarrera, J.V.; Wagener, G. Extracorporeal membrane oxygenation as a rescue device for postreperfusion cardiac arrest during liver transplantation. Liver Transpl. 2015, 21, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Martucci, G.; Burgio, G.; Lullo, F.; Panarello, G.; Arcadipane, A. Veno-arterial extracorporeal membrane oxygenation as an intraoperative rescue option in case of portopulmonary hypertension recognized during liver transplantation. Minerva Anestesiol. 2017, 83, 1336–1337. [Google Scholar] [CrossRef]
- Romano, D.N.; Smith, N.K.; Itagaki, S.; Bekki, Y.; Gunasekaran, G.; Zerillo, J. A case report of venoarterial ECMO as salvage therapy for prolonged cardiac arrest following post-reperfusion intracardiac thrombosis during orthotopic liver transplantation. Semin. Cardiothorac. Vasc. Anesth. 2021, 25, 62–66. [Google Scholar] [CrossRef]
- Szocik, J.; Rudich, S.; Csete, M. ECMO resuscitation after massive pulmonary embolism during liver transplantation. Anesthesiology 2002, 97, 763–764. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kang, P.J.; Kim, D.H. Successful extracorporeal membrane oxygenation support for acute pulmonary thromboembolism during adult liver transplantation. Korean J. Crit. Care Med. 2016, 31, 371–374. [Google Scholar] [CrossRef]
- Eudailey, K.W.; Yi, S.Y.; Mongero, L.B.; Wagener, G.; Guarrera, J.V.; George, I. Trans-diaphragmatic left ventricular venting during peripheral venous-arterial extracorporeal membrane oxygenation. Perfusion 2015, 30, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Lauterio, A.; De Carlis, R.; Cannata, A.; Di Sandro, S.; De Gasperi, A.; Russo, C.; De Carlis, L. Emergency intraoperative implantation of ECMO for refractory cardiogenic shock arising during liver transplantation as a bridge to myocardial surgical revascularization. Transplantation 2019, 103, e317–e318. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.S.; Wille, K.M.; Diaz Guzman, E. Extracorporeal membrane oxygenation after liver transplantation in a patient with hepatopulmonary syndrome and an atrial septal defect. Int. J. Artif. Organs 2015, 38, 170–172. [Google Scholar] [CrossRef]
- Stratta, C.; Lavezzo, B.; Ballaris, M.A.; Panio, A.; Crucitti, M.; Andruetto, P.; Fanelli, V.; Marra, W.G.; Ranieri, M.; Salizzoni, M. Extracorporeal membrane oxygenation rescue therapy in a case of portopulmonary hypertension during liver transplantation: A case report. Transplant. Proc. 2013, 45, 2774–2775. [Google Scholar] [CrossRef]
- Caturegli, G.; Crane, M.A.; Etchill, E.; Giuliano, K.; Nguyen, M.; Philosophe, B.; Cho, S.-M.; Wittstein, I.S.; Whitman, G.J. Stress-induced (Takotsubo) cardiomyopathy after liver transplant rescued with venoarterial extracorporeal membrane oxygenation. ASAIO J. 2022, 68, e66–e68. [Google Scholar] [CrossRef]
- Barrueco-Francioni, J.E.; Martínez-González, M.C.; Martínez-Carmona, J.F.; Benítez-Moreno, M.P.; Aragón-González, C.; Herrera-Gutiérrez, M.E. ECMO in severe hypoxemia post liver transplant for hepatopulmonary syndrome. Int. J. Artif. Organs 2024, 47, 858–861. [Google Scholar] [CrossRef]
- Biondi, R.S.; Barzilai, V.S.; Watanabe, A.L.C.; Ferreira Gde, S.A.; Atik, F.A. Use of extracorporeal membrane oxygenation for treating acute cardiomyopathy after liver transplantation: A case report. Rev. Bras. Ter. Intensiv. 2018, 30, 233–236. [Google Scholar] [CrossRef]
- Lauterio, A.; Bottiroli, M.; Cannata, A.; DECarlis, R.; Valsecchi, M.; Perricone, G.; Colombo, S.; Buscemi, V.; Zaniboni, M.; Pedrazzini, G.; et al. Successful recovery from severe inverted Takotsubo cardiomyopathy after liver transplantation: The efficacy of extracorporeal membrane oxygenation (ECMO). Minerva Anestesiol. 2022, 88, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Mehdiani, A.; Immohr, M.B.; Boettger, C.; Dalyanoglu, H.; Scheiber, D.; Westenfeld, R.; Aubin, H.; Akhyari, P.; Saeed, D.; Lichtenberg, A.; et al. Extracorporeal membrane oxygenation after heart transplantation: Impact of type of cannulation. Thorac. Cardiovasc. Surg. 2021, 69, 263–270. [Google Scholar] [CrossRef]
- Giovannico, L.; Fischetti, G.; Parigino, D.; Savino, L.; Di Bari, N.; Milano, A.D.; Padalino, M.; Bottio, T. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) support in New Era of heart transplant. Transpl. Int. 2024, 37, 12981. [Google Scholar] [CrossRef]
- Shudo, Y.; Elde, S.; Lingala, B.; He, H.; Casselman, K.G.; Zhu, Y.; Kasinpila, P.; Woo, Y.J. Extracorporeal membrane oxygenation bridge to heart-lung transplantation. ASAIO J. 2022, 68, e44–e47. [Google Scholar] [CrossRef] [PubMed]
- Hayanga, J.W.A.; Chan, E.G.; Musgrove, K.; Leung, A.; Shigemura, N.; Hayanga, H.K. Extracorporeal membrane oxygenation in the perioperative care of the lung transplant patient. Semin. Cardiothorac. Vasc. Anesth. 2020, 24, 45–53. [Google Scholar] [CrossRef]
- Konoeda, C.; Sato, M.; Nakajima, J. Extracorporeal membrane oxygenation in lung transplantation. Kyobu Geka 2022, 75, 254–258. [Google Scholar] [PubMed]
- Martin, A.K.; Jayaraman, A.L.; Nabzdyk, C.G.; Wilkey, B.J.; Fritz, A.V.; Kolarczyk, L.; Ramakrishna, H. Extracorporeal membrane oxygenation in lung transplantation: Analysis of techniques and outcomes. J. Cardiothorac. Vasc. Anesth. 2021, 35, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Siebenmann, C.; Lundby, C. Regulation of cardiac output in hypoxia. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S4), 53–59. [Google Scholar] [CrossRef]
- Phillips, B.A.; McConnell, J.W.; Smith, M.D. The effects of hypoxemia on cardiac output. A Dose-Response Curve. Chest 1988, 93, 471–475. [Google Scholar] [CrossRef]
- Møller, S.; Bendtsen, F. The pathophysiology of arterial vasodilatation and hyperdynamic circulation in cirrhosis. Liver Int. 2018, 38, 570–580. [Google Scholar] [CrossRef]
- Tomarchio, E.; Momigliano, F.; Giosa, L.; Collins, P.D.; Barrett, N.A.; Camporota, L. The intricate physiology of veno-venous extracorporeal membrane oxygenation: An overview for clinicians. Perfusion 2024, 39, 49S–65S. [Google Scholar] [CrossRef]
- Bartlett, R.H. Physiology of gas exchange during ECMO for respiratory failure. J. Intensive Care Med. 2017, 32, 243–248. [Google Scholar] [CrossRef]
- Lorusso, R.; Meani, P.; Raffa, G.M.; Kowalewski, M. Extracorporeal membrane oxygenation and left ventricular unloading: What is the evidence? JTCVS Tech. 2022, 13, 101–114. [Google Scholar] [CrossRef]
- Sabaté, A.; Figueras, J.; Segura, R.; Fuentelsanz, T.; Camprubí, I.; Jaurrieta, E. Utilization of veno-venous bypass in orthotopic liver transplantation. Rev. Esp. Anestesiol. Reanim. 1993, 40, 12–16. [Google Scholar]
- Voulgarelis, S.; Hong, J.C.; Zimmerman, M.A.; Kim, J.; Scott, J.P. A novel escalation from veno-venous bypass to veno-venous ECMO during orthotopic liver transplantation. A Case report. Perfusion 2021, 36, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Feltracco, P.; Barbieri, S.; Carollo, C.; Bortolato, A.; Michieletto, E.; Bertacco, A.; Gringeri, E.; Cillo, U. Early circulatory complications in liver transplant patients. Transplant. Rev. 2019, 33, 219–230. [Google Scholar] [CrossRef]
- Hirani, N.; Chatterjee, P. A systematic review on Artificial Intelligence applied to predictive cardiovascular risk analysis in liver transplantation. F1000Research 2024, 13, 701. [Google Scholar] [CrossRef]
- Stephens, A.F.; Šeman, M.; Diehl, A.; Pilcher, D.; Barbaro, R.P.; Brodie, D.; Pellegrino, V.; Kaye, D.M.; Gregory, S.D.; Hodgson, C.; et al. ECMO PAL: Using deep neural networks for survival prediction in venoarterial extracorporeal membrane oxygenation. Intensive Care Med. 2023, 49, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Holste, G.; Oikonomou, E.K.; Wang, Z.; Khera, R. PanEcho: Complete AI-enabled echocardiography interpretation with multi-task deep learning. medRxiv 2024. [Google Scholar] [CrossRef]
- Berg, E.A.R.; Taskén, A.A.; Nordal, T.; Grenne, B.; Espeland, T.; Kirkeby-Garstad, I.; Dalen, H.; Holte, E.; Stølen, S.; Aakhus, S.; et al. Fully automatic estimation of global left ventricular systolic function using deep learning in transoesophageal echocardiography. Eur. Heart J. Imaging Methods Pract. 2023, 1, qyad007. [Google Scholar] [CrossRef]
- Shin, W.-J.; Song, J.-G.; Jun, I.-G.; Moon, Y.-J.; Kwon, H.-M.; Jung, K.; Kim, S.-O.; Hwang, G.-S. Effect of ventriculo-arterial coupling on transplant outcomes in cirrhotics: Analysis of pressure-volume curve relations. J. Hepatol. 2017, 66, 328–337. [Google Scholar] [CrossRef]
- Brener, M.I.; Masoumi, A.; Ng, V.G.; Tello, K.; Bastos, M.B.; Cornwell, W.K., 3rd; Hsu, S.; Tedford, R.J.; Lurz, P.; Rommel, K.-P.; et al. Invasive right ventricular pressure-volume analysis: Basic principles, clinical applications, and practical recommendations. Circ. Heart Fail. 2022, 15, e009101. [Google Scholar] [CrossRef]
| Classification | Hemodynamic Criteria |
|---|---|
| Precapillary PH | mPAP > 20 mmHg PCWP ≤ 15 mmHg PVR > 2 WU |
| Isolated postcapillary PH | mPAP > 20 mmHg PCWP ≤ 15 mmHg PVR > 2 WU |
| Combined pre- and postcapillary PH | mPAP > 20 mmHg PCWP > 15 mmHg PVR > 2 WU |
| Group | Description | Examples |
|---|---|---|
| Group 1 | PH | Idiopathic PH, heritable PH, drug- or toxin-induced PH-associated conditions (e.g., connective tissue disease, HIV infection, portal hypertension, congenital heart disease) |
| Group 2 | PH due to left heart disease | Left ventricular systolic or diastolic dysfunction, valvular heart disease |
| Group 3 | PH due to lung diseases and/or hypoxia | COPD, ILD, sleep-disordered breathing, alveolar hypoventilation syndromes |
| Group 4 | PH due to pulmonary artery obstructions | CTEPH and other rare causes of pulmonary artery obstruction |
| Group 5 | PH with unclear and/or multifactorial mechanisms | Hematological disorders (e.g., sickle cell disease), systemic disorders (e.g., sarcoidosis), metabolic disorders (e.g., glycogen storage diseases), CKD |
| Absolute | Relative |
|---|---|
| Esophageal tumour or mass | Cervical spine instability |
| Esophageal stricture perforation or trauma | Dysphagia odynophagia |
| Esophageal diverticulum | Gastroesophageal reflux esophagitis |
| Esophagectomy | History of chest radiation |
| Esophageal spasm or contraction | Pharyngeal tumour or facial trauma |
| Scleroderma | Symptomatic hiatal hernia peptic ulcer |
| Mallory–Weiss syndrome | Barrett’s esophagus |
| Recent upper gastrointestinal surgery | Thoracoabdominal aneurysm |
| Active gastrointestinal bleeding | Coagulopathy thrombocytopenia, recent upper gastrointestinal bleeding, history of gastrointestinal surgery |
| Category | Complications | IBP and Pulse Contour | PAC Data | Neuro- Monitoring (NIRS and TCD) | ECMO Parameters (BFR and ΔP) | Transesophageal Echo (TEE) |
|---|---|---|---|---|---|---|
| Drainage Cannula/PAC-Related Issues | Iatrogenic cardiovascular injuries (i.e., great vessels rupture/dissection) | MAP ↓\n SVV ↑ SVRI ↑/↓ dP/dt ↑/↓ CI ↓ Eadyn ↑ | EDVI ↓ RVEF ↓ SvO2 ↓ | NIRS: rSO2 ↓ TCD: PI ↑ RI ↑ | BFR ↓ ΔP ↑/↓ (V-V = V-A) | Pericardial effusion EDV ↓ kissing wall |
| Cannula/PAC malposition or kinking | MAP ↓ SVV ↑ SVRI ↑/↓ dP/dt ↑/↓ CI ↓ Eadyn ↑ | EDVI ↑/↓ RVEF ↓/= SvO2 ↓ | NIRS: rSO2 ↓ TCD: PI ↑ RI ↑ | BFR ↓ ΔP ↑/↓ (V-V = V-A) | Malpositioned cannula ↑ turbulent flow | |
| Oxygenation-Related Complications | Intracardiac shunt (i.e., PFO) | MAP =/↑ SVV↑ SVRI ↑ dP/dt =/↑ CI ↑ Eadyn ↓ | EDVI ↓/↑ RVEF ↓ SvO2 ↓ | NIRS: rSO2 ↓ TCD: PI ↑ RI ↑ | BFR ↑/= ΔP ↑/= (V-V = V-A) | Bubble test: bubble appears in LH within 3–5 heart cycles |
| Extracardiac shunt (i.e., HPS) | MAP ↓ SVV ↑ SVRI ↓ dP/dt = CI ↑ Eadyn ↓ | EDVI ↓ RVEF ↓ SvO2 ↓ | NIRS: rSO2 ↓ TCD: PI ↑ RI ↑ | BFR ↓/= ΔP ↑/= (V-V = V-A) | Bubble test: bubble appears in LH after 3–5 heart cycles | |
| Recirculation (during V-V ECMO) | MAP ↓ SVV ↑ SVRI ↓ dP/dt ↓ CI ↓ Eadyn ↓ | EDVI ↓ RVEF ↓ SvO2 ↓ | NIRS: rSO2 ↓ TCD: PI ↑ RI ↑ | BFR =/↑ ΔP ↑ | ↑ Turbulent flow in RH between cannulae | |
| Differential hypoxia (i.e., Harlequin syndrome in V-A ECMO) | No changes | No changes | NIRS: rSO2 ↓ (and right arm SpO2) TCD: PI ↑ RI ↑ | No changes | No changes | |
| Pulmonary Circulation Complications | Acute right ventricle overload (i.e., graft reperfusion) | MAP ↓ SVV ↑ SVRI ↑/= dP/dt ↓ CI ↓ Eadyn =/↑ | EDVI ↑ RVEF ↓ SvO2 ↓ | NIRS: rSO2 ↓ TCD: PI ↑ RI ↑ | BFR ↓ ΔP =/↑ (V-V > V-A) | RV and RA enlargement TAPSE ↓ FAC ↓ TR septal shift towards LV (D shape of LV) |
| Post-Obstructive Complications | Pneumothorax (i.e., transdiaphragmatic pleural perforation) | MAP ↓ SVV ↑ SVRI ↑ dP/dt ↓ CI ↓ Eadyn ↑ | EDVI ↓ RVEF ↓ SvO2 ↓ | NIRS: rSO2 ↓ TCD: PI ↑ RI ↑ | BFR ↓ ΔP ↑ (V-V > V-A) | Lung point associated withRH overload or dysfunction |
| Pericardial tamponade (i.e., ECMO cannulation) | MAP ↓ SVV ↑ SVRI ↑ dP/dt ↓ CI ↓ Eadyn ↑ | EDVI ↓ RVEF ↓ SvO2 ↓ | NIRS: rSO2 ↓ TCD: PI ↑ RI ↑ | BFR ↓ ΔP ↑ (V-V > V-A) | Pericardial effusion diastolic collapse of RV | |
| Pulmonary embolism (i.e., intracardiac thrombosis) | MAP ↓ SVV ↑ SVRI ↑ dP/dt ↓ CI ↓ Eadyn ↓/↑ | EDVI ↓ RVEF ↓ SvO2 ↓ | NIRS: rSO2 ↓ TCD: PI ↑ RI ↑ | BFR ↓ ΔP ↑ (V-V > V-A) | Thrombotic mass in right chambers RH dysfunction | |
| Systolic anterior motion (SAM) of the mitral valve | MAP ↓ SVV ↑ SVRI =/↑ dP/dt =/↑ CI ↓ Eadyn ↑ | EDVI ↓ RVEF ↓ SvO2 ↓ | NIRS: rSO2 ↓ TCD: PI ↑ RI ↑ | BFR ↓ ΔP ↑ (V-V > V-A) | ↓ C-sept ↑ VTI and pressure gradient across LVOT | |
| Systemic Circulation Complications | Acute left ventricle overload (V-A ECMO) | MAP ↓ SVV ↓ SVRI ↑ dP/dt ↓ CI ↓ Eadyn ↑/= | EDVI ↑ RVEF ↓ SvO2 ↓ | NIRS: rSO2 ↓ TCD: PI ↑ RI ↑ | BFR ↓ ΔP ↓ (V-A > V-V) | LA and LV enlargement restrictive filling pattern of the mitral valve with MR ↓ LVEF and MAPSE |
| Ischemic heart events | MAP ↓ SVV ↓ SVRI ↑ dP/dt ↓ CI ↓ Eadyn ↑/= | EDVI ↓ RVEF ↓ SvO2 ↓ | NIRS: rSO2 ↓ TCD: PI ↑ RI ↑ | BFR ↓ ΔP ↑ (V-A > V-V) | RWMA and hypokinesia | |
| Vasoplegia syndrome (i.e., reperfusion phase) | MAP ↓ SVV ↑ SVRI ↓ dP/dt ↓ CI ↓ Eadyn ↓ | EDVI ↓ RVEF ↓ SvO2 ↓ | NIRS: rSO2 ↓ TCD: PI ↑ RI ↑ | BFR ↑/= ΔP ↓/= (V-V >V-A) | Hyperdynamic heart and ↑ LVEF | |
| Valvular disease | New onset or worsening of pre-existing valvular stenosis/regurgitation | Depending on the valve and grading of valvulopathy | ||||
| Monitoring Technique | Veno-Venous (V-V) ECMO | Veno-Arterial (V-A) ECMO | Rationale |
|---|---|---|---|
| Bilateral and central invasive blood pressure (IBP) monitoring | ++ | ++++ | More critical in V-A-ECMO to detect differential hypoxia (e.g., Harlequin syndrome). |
| Pulse contour analysis (PCA) | +++ | ++ | More critical in V-V ECMO due to preserved native (pulsatile) flow. |
| Near-Infrared- Spectroscopy (NIRS) | ++ | +++ | More critical in V-A ECMO to detect cerebral hypoxia (e.g., Harlequin syndrome). |
| Transcranial echo-color-doppler (ECD) | ++ | +++ | More critical in V-A ECMO to detect acute brain embolic or hemorrhagic events |
| ECMO parameters (BFR and trans-oxygenator pressures) | +++ | +++ | Essential for both V-V and V-A configurations to optimize flow, detect thrombosis, and assess oxygenator function |
| Pulmonary artery catheter (PAC) | +++ | +++ | Critical in both V-V and V-A configurations to inform for recovery of cardiorespiratory native function (e.g., increase in SvO2) |
| Echocardiography (transthoracic and transesophageal) | +++ | ++++ | Crucial in both configurations: monitoring RV function (V-V ECMO) and assessing recovery of native CI (V-A ECMO) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tigano, S.; Casolaro, G.; Bianchini, A.; Bernardi, E.; Laici, C.; Ramahi, L.; Vitale, G.; Siniscalchi, A. Hemodynamic Monitoring During Liver Transplantation for Patients on Perioperative Extracorporeal Membrane Oxygenation (ECMO) Support: A Narrative Review. Medicina 2025, 61, 768. https://doi.org/10.3390/medicina61040768
Tigano S, Casolaro G, Bianchini A, Bernardi E, Laici C, Ramahi L, Vitale G, Siniscalchi A. Hemodynamic Monitoring During Liver Transplantation for Patients on Perioperative Extracorporeal Membrane Oxygenation (ECMO) Support: A Narrative Review. Medicina. 2025; 61(4):768. https://doi.org/10.3390/medicina61040768
Chicago/Turabian StyleTigano, Stefano, Giulio Casolaro, Amedeo Bianchini, Enrico Bernardi, Cristiana Laici, Linda Ramahi, Giovanni Vitale, and Antonio Siniscalchi. 2025. "Hemodynamic Monitoring During Liver Transplantation for Patients on Perioperative Extracorporeal Membrane Oxygenation (ECMO) Support: A Narrative Review" Medicina 61, no. 4: 768. https://doi.org/10.3390/medicina61040768
APA StyleTigano, S., Casolaro, G., Bianchini, A., Bernardi, E., Laici, C., Ramahi, L., Vitale, G., & Siniscalchi, A. (2025). Hemodynamic Monitoring During Liver Transplantation for Patients on Perioperative Extracorporeal Membrane Oxygenation (ECMO) Support: A Narrative Review. Medicina, 61(4), 768. https://doi.org/10.3390/medicina61040768






