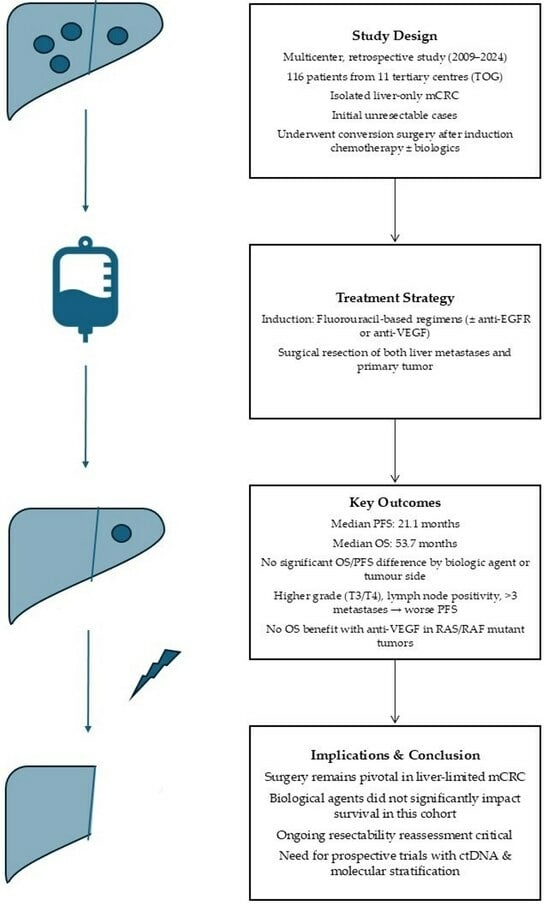
Long-Term Outcomes of Patients Undergoing Conversion Surgery After Induction Chemotherapy: Turkish Oncology Group Study
Abstract
1. Introduction
2. Material and Methods
2.1. Treatment Regimens
2.2. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Progression-Free Survival
3.3. Overall Survival
3.4. Radiographic Response and Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Aykan, N.F.; Yalçın, S.; Turhal, N.S.; Özdoğan, M.; Demir, G.; Özkan, M.; Yaren, A.; Camcı, C.; Akbulut, H.; Artaç, M.; et al. Epidemiology of colorectal cancer in Turkey: A cross-sectional disease registry study (A Turkish Oncology Group trial). Turk. J. Gastroenterol. 2015, 26, 145–153. [Google Scholar] [CrossRef]
- Martin, J.; Petrillo, A.; Smyth, E.C.; Shaida, N.; Khwaja, S.; Cheow, H.K.; Duckworth, A.; Heister, P.; Praseedom, R.; Jah, A.; et al. Colorectal liver metastases: Current management and future perspectives. World J. Clin. Oncol. 2020, 11, 761–808. [Google Scholar] [CrossRef]
- Bond, M.J.G.; Kuiper, B.I.; Bolhuis, K.; Komurcu, A.; van Amerongen, M.J.; Chapelle, T.; Dejong, C.H.C.; Engelbrecht, M.R.W.; Gerhards, M.F.; Grünhagen, D.J.; et al. Intersurgeon Variability in Local Treatment Planning for Patients with Initially Unresectable Colorectal Cancer Liver Metastases: Analysis of the Liver Expert Panel of the Dutch Colorectal Cancer Group. Ann. Surg. Oncol. 2023, 30, 5376–5385. [Google Scholar] [CrossRef]
- De Kleijn, E.M.; Punt, C.J. Biological therapy of colorectal cancer. Eur. J. Cancer 2002, 38, 1016–1022. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.-Q.; Luo, Q.; Wang, L.; Song, G.-B.; Sheng, J.-P.; Xu, B. Signaling pathways involved in colorectal cancer: Pathogenesis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Bachler, J.L.; Khan, G.N.; Wollner, I.S.; Philip, P.A. Treatment of unresectable and resectable stage IV colorectal cancer. Clin. Adv. Hematol. Oncol. 2024, 22, 455–463. [Google Scholar]
- Mace, A.G.; Pai, R.K.; Stocchi, L.; Kalady, M.F. American Joint Committee on Cancer and College of American Pathologists regression grade: A new prognostic factor in rectal cancer. Dis. Colon Rectum 2015, 58, 32–44. [Google Scholar] [CrossRef]
- Washington, M.K.; Berlin, J.; Branton, P.; Burgart, L.J.; Carter, D.K.; Fitzgibbons, P.L.; Halling, K.; Frankel, W.; Jessup, J.; Kakar, S.; et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch. Pathol. Lab. Med. 2009, 133, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Seymour, L.; Litière, S.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur. J. Cancer 2016, 62, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Chrabaszcz, S.; Rajeev, R.; Witmer, H.D.D.; Dhiman, A.; Klooster, B.; Gamblin, T.C.; Banerjee, A.; Johnston, F.M.; Turaga, K.K. A Systematic Review of Conversion to Resectability in Unresectable Metastatic Colorectal Cancer Chemotherapy Trials. Am. J. Clin. Oncol. 2022, 45, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.P.A.; Nogueiro, J.; Oliveira, A.; Sousa, J.P.; Guimarães, A.V.; Teixeira, I.; Bouça-Machado, T.; Aral, M.; Graça, L.; Barbosa, E. Predicting factors for disease-free survival and overall survival in patients submitted to liver resection for colorectal liver metastases: A retrospective study in a tertiary hospital. Eur. J. Surg. Oncol. 2024, 50, 107743. [Google Scholar] [CrossRef]
- Rivera, F.; Karthaus, M.; Hecht, J.R.; Sevilla, I.; Forget, F.; Fasola, G.; Canon, J.-L.; Guan, X.; Demonty, G.; Schwartzberg, L.S. Final analysis of the randomised PEAK trial: Overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int. J. Colorectal Dis. 2017, 32, 1179–1190. [Google Scholar] [CrossRef]
- Arnold, D.; Lueza, B.; Douillard, J.-Y.; Peeters, M.; Lenz, H.-J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.-P.; Tabernero, J. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann. Oncol. 2017, 28, 1713–1729. [Google Scholar] [CrossRef]
- Fernandez, F.G.; Drebin, J.A.; Linehan, D.C.; Dehdashti, F.; Siegel, B.A.; Strasberg, S.M. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann. Surg. 2004, 240, 438–447, discussion 447–450. [Google Scholar] [CrossRef]
- Zeineddine, F.A.; Zeineddine, M.A.; Yousef, A.; Gu, Y.; Chowdhury, S.; Dasari, A.; Huey, R.W.; Johnson, B.; Kee, B.; Lee, M.S.; et al. Survival improvement for patients with metastatic colorectal cancer over twenty years. NPJ Precis. Oncol. 2023, 7, 16. [Google Scholar] [CrossRef]
- Creasy, J.M.; Sadot, E.; Koerkamp, B.G.; Chou, J.F.; Gonen, M.; Kemeny, N.E.; Balachandran, V.P.; Kingham, T.P.; DeMatteo, R.P.; Allen, P.J.; et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: What factors preclude cure? Surgery 2018, 163, 1238–1244. [Google Scholar] [CrossRef]
- Bridgewater, J.A.; Pugh, S.A.; Maishman, T.; Eminton, Z.; Mellor, J.; Whitehead, A.; Stanton, L.; Radford, M.; Corkhill, A.; Griffiths, G.O.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): Long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 398–411. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O'Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.S.; Rivera, F.; Karthaus, M.; Fasola, G.; Canon, J.L.; Hecht, J.R.; Yu, H.; Oliner, K.S.; Go, W.Y. PEAK: A randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J. Clin. Oncol. 2014, 32, 2240–2247. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Lv, Y.; Li, J.; Cao, C.; Lu, J.; Wang, S.; Du, B.; Yang, X. Chemotherapy plus panitumumab/cetuximab versus chemotherapy plus bevacizumab in wild-type KRAS/RAS metastatic colorectal cancer: A meta-analysis. Expert Rev. Anticancer Ther. 2022, 22, 1333–1347. [Google Scholar] [CrossRef]
- Sorich, M.J.; Wiese, M.D.; Rowland, A.; Kichenadasse, G.; McKinnon, R.A.; Karapetis, C.S. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: A meta-analysis of randomized, controlled trials. Ann. Oncol. 2015, 26, 13–21. [Google Scholar] [CrossRef]
- Uetake, H.; Yamashita, R.; Shitara, K.; Yoshino, T.; Watanabe, J.; Yasui, H.; Ohori, H.; Shiozawa, M.; Muro, K.; Yamazaki, K.; et al. Acquired gene alteration patterns and post-progression survival: PARADIGM study analysis. J. Clin. Oncol. 2024, 42, 3507. [Google Scholar] [CrossRef]
- Ychou, M.; Rivoire, M.; Thezenas, S.; Guimbaud, R.; Ghiringhelli, F.; Mercier-Blas, A.; Mineur, L.; Francois, E.; Khemissa, F.; Chauvenet, M.; et al. Chemotherapy (doublet or triplet) plus targeted therapy by RAS status as conversion therapy in colorectal cancer patients with initially unresectable liver-only metastases. The UNICANCER PRODIGE-14 randomised clinical trial. Br. J. Cancer 2022, 126, 1264–1270. [Google Scholar] [CrossRef]
- Bond, M.J.G.; Bolhuis, K.; Loosveld, O.J.L.; de Groot, J.W.B.; Droogendijk, H.; Helgason, H.H.; Hendriks, M.P.; Klaase, J.M.; Kazemier, G.; Liem, M.S.L.; et al. First-line systemic treatment strategies in patients with initially unresectable colorectal cancer liver metastases (CAIRO5): An open-label, multicentre, randomised, controlled, phase 3 study from the Dutch Colorectal Cancer Group. Lancet Oncol. 2023, 24, 757–771. [Google Scholar] [CrossRef]
- Nakamura, Y.; Watanabe, J.; Akazawa, N.; Hirata, K.; Kataoka, K.; Yokota, M.; Kato, K.; Kotaka, M.; Kagawa, Y.; Yeh, K.-H.; et al. ctDNA-based molecular residual disease and survival in resectable colorectal cancer. Nat. Med. 2024, 30, 3272–3283. [Google Scholar] [CrossRef]
- Tenekeci, A.K.; Unal, A.A.; Ceylan, F.; Nahit Sendur, M.A. An updated overview of K-RAS G12C inhibitors in advanced stage non-small cell lung cancer. Future Oncol. 2024, 20, 3019–3038. [Google Scholar] [CrossRef]
- Chen, K.; Collins, G.; Wang, H.; Toh, J.W.T. Pathological Features and Prognostication in Colorectal Cancer. Curr. Oncol. 2021, 28, 5356–5383. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.L.; Wang, Y.Y.; Liu, W.; Xing, B.C. Prognostic factors in colorectal liver metastases patients with various tumor numbers treated by liver resection: A single-center, retrospective study. World J. Surg. Oncol. 2022, 20, 237. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, Y.; Wen, N.; Wang, S.; Li, B.; Liu, G. Prognostic factors associated with early recurrence following liver resection for colorectal liver metastases: A systematic review and meta-analysis. BMC Cancer 2024, 24, 426. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-J.; Chern, Y.-J.; Wu, Z.-E.; Yu, Y.-L.; Liao, C.-K.; Tsai, W.-S.; You, J.-F.; Lee, C.-W. The oncologic outcome and prognostic factors for solitary colorectal liver metastasis after liver resection. J. Gastrointest. Surg. 2024, 28, 267–275. [Google Scholar] [CrossRef]
- Iwatsuki, S.; Dvorchik, I.; Madariaga, J.R.; Marsh, J.W.; Dodson, F.; Bonham, A.C.; Geller, D.A.; Gayowski, T.J.; Fung, J.J.; Starzl, T.E. Hepatic resection for metastatic colorectal adenocarcinoma: A proposal of a prognostic scoring system. J. Am. Coll. Surg. 1999, 189, 291–299. [Google Scholar] [CrossRef]
- Okano, K.; Yamamoto, J.; Kosuge, T.; Yamamoto, S.; Sakamoto, M.; Nakanishi, Y.; Hirohashi, S. Fibrous pseudocapsule of metastatic liver tumors from colorectal carcinoma. Clinicopathologic study of 152 first resection cases. Cancer 2000, 89, 267–275. [Google Scholar] [CrossRef]



| Variables | Whole Group (n = 116) | Right Colon (n = 28) | Left Colon (n = 40) | Rectum (n = 48) | p | |
|---|---|---|---|---|---|---|
| Gender | Female | 44 (38%) | 11 | 19 | 14 | 0.208 |
| Male | 72 (62%) | 17 | 21 | 34 | ||
| Age | 57 | 59 | 57 | 55 | 0.229 | |
| ECOG PS | 0 | 61 (52%) | 15 | 18 | 28 | 0.532 |
| 1 | 52 (45%) | 13 | 20 | 19 | ||
| 2 | 3 (3%) | 0 | 2 | 1 | ||
| Metastases in the same segment | Yes | 56 (48%) | 15 | 13 | 28 | 0.044 |
| No | 60 (52%) | 13 | 27 | 20 | ||
| Number of Liver metastases | ≤3 | 84 (72%) | 21 | 28 | 35 | 0.897 |
| >3 | 32 (28%) | 7 | 12 | 13 | ||
| Largest Metastasis Diameter (mm) | 38 | 46 | 38 | 36 | 0.484 | |
| Chemotherapy | Doublet | 14 (13%) | 3 | 7 | 4 | 0.529 |
| Anti-VEGF Group | 56 (52%) | 15 | 16 | 25 | ||
| Anti-EGFR Group | 37 (35%) | 7 | 15 | 15 | ||
| Chemotherapy Regimes | Capox | 1 (1%) | 0 | 0 | 1 | 0.689 |
| Folfox | 13 (11%) | 3 | 7 | 3 | ||
| Folfox + Anti-EGFR | 25 (22%) | 6 | 9 | 10 | ||
| Folfox + Anti-VEGF | 33 (28%) | 8 | 12 | 13 | ||
| Folfiri + Anti-EGFR | 12 (10%) | 1 | 6 | 5 | ||
| Folfiri + Anti-VEGF | 11 (10%) | 5 | 1 | 5 | ||
| Capox+ Anti-VEGF | 12 (10%) | 2 | 3 | 7 | ||
| Folfoxiri | 1 (1%) | 0 | 1 | 0 | ||
| Folfoxiri + Anti-EGFR | 1 (1%) | 0 | 0 | 1 | ||
| Folfoxiri+ Anti-VEGF | 7 (6%) | 3 | 1 | 3 | ||
| Number Of Chemotherapy Cycles Administered | 8 | 9 | 9 | 8 | 0.462 | |
| Grade 3/4 AE | Absence | 97 (84%) | 25 | 32 | 40 | 0.594 |
| Presence | 19 (16%) | 3 | 8 | 8 | ||
| Radiographic response | CR | 24 (21%) | 4 | 6 | 14 | 0.268 |
| PR | 83 (71%) | 21 | 29 | 33 | ||
| SD | 8 (7%) | 3 | 4 | 1 | ||
| PD | 1 (1%) | 0 | 1 | 0 | ||
| ypT | T1 | 7 (6%) | 1 | 1 | 5 | 0.543 |
| T2 | 15 (13%) | 5 | 2 | 7 | ||
| T3 | 70 (60%) | 17 | 26 | 27 | ||
| T4 | 24 (21%) | 5 | 10 | 9 | ||
| ypN | N0 | 40 (35%) | 7 | 14 | 0.569 | |
| N1 | 54 (47%) | 15 | 19 | |||
| N2 | 21 (18%) | 5 | 7 | |||
| N3 | 1 (1%) | 1 | 0 | |||
| Time to Surgery (Months) Median, IQR | 6.0 | 7.2 | 9.8 | 9.2 | 0.446 | |
| Number of lymph nodes removed | <12 | 44 (38%) | 8 | 14 | 22 | 0.292 |
| ≥12 | 72 (62%) | 20 | 26 | 26 | ||
| Grade | N/A | 37 (32%) | 8 | 16 | 13 | 0.743 |
| Grade1 | 29 (25%) | 7 | 9 | 13 | ||
| Grade2 | 32 (28%) | 10 | 7 | 15 | ||
| Grade3 | 16 (14%) | 3 | 7 | 6 | ||
| Grade4 | 2 (1%) | 0 | 1 | 1 | ||
| Differentiation | N/A | 19 (16%) | 6 | 7 | 6 | 0.831 |
| Good | 35 (30%) | 7 | 11 | 17 | ||
| Intermediate | 54 (47%) | 14 | 18 | 22 | ||
| Poor | 8 (7%) | 1 | 4 | 3 | ||
| Tumor Regression Score | Grade 0 | 1 (1%) | 0 | 1 | 0 | 0.488 |
| Grade 1 | 7 (6%) | 4 | 2 | 1 | ||
| Grade 2 | 42 (36%) | 9 | 16 | 17 | ||
| Grade 3 | 38 (33%) | 8 | 12 | 18 | ||
| N/A | 28 (24%) | 7 | 9 | 12 | ||
| Resection | N/A | 14 (12%) | 3 | 2 | 9 | 0.227 |
| R0 | 88 (76%) | 22 | 32 | 34 | ||
| R1 | 11 (9%) | 2 | 6 | 3 | ||
| R2 | 3 (3%) | 1 | 0 | 2 | ||
| KRAS | Wild | 65 (56%) | 13 | 25 | 27 | 0.421 |
| Mutant | 51 (44%) | 15 | 15 | 21 | ||
| NRAS | Wild | 111 (96%) | 28 | 39 | 44 | 0.290 |
| Mutant | 5 (4%) | 0 | 1 | 4 | ||
| BRAF | Wild | 103 (89%) | 27 | 36 | 40 | 0.465 |
| Mutant | 4 (3%) | 0 | 1 | 3 | ||
| N/A | 9 (8%) | 1 | 3 | 5 | ||
| MSI | MSS | 79 (99%) | 16 | 29 | 34 | 0.153 |
| MSI-H | 1 (1%) | 1 | 0 | 0 | ||
| Radiotherapy (for rectum adenocarcinoma) | Received | 16 (57%) | 0 | 0 | 16 | |
| Not Received | 12 (43%) | 28 | 40 | 12 | ||
| Adjuvant Chemotherapy | Received | 38 (72%) | ||||
| Not Received | 15 (28%) | |||||
| Recurrence | Presence | 86 (74%) | ||||
| Chemotherapy after recurrence | Administered | 84 (98%) | ||||
| Univariate Analyses | Multivariate Analyses | |||
|---|---|---|---|---|
| HR (95% Cl) | p | HR (95% CI) | p | |
| Age | 0.339 | |||
| <65 | 1 | |||
| ≥65 | 0.78 (0.47–1.31) | |||
| Gender | 0.590 | |||
| Female | 1 | |||
| Male | 1.13 (0.72–1.77) | |||
| ECOG | 0.181 | |||
| 0 | 1 | |||
| 1 | 1.35 (0.87–2.07) | |||
| Localization | 0.988 | |||
| Right colon | 1 | |||
| Left colon | 0.96 (0.55–1.68) | |||
| Rectum | 0.96 (0.55–1.67) | |||
| KRAS Mutation | 0.810 | |||
| No | 1 | |||
| Yes | 1.05 (0.69–1.61) | |||
| NRAS Mutation | 0.352 | |||
| No | 1 | |||
| Yes | 1.58 (0.64–3.93) | |||
| BRAF Mutation | 0.480 | |||
| No | 1 | |||
| Yes | 0.68 (0.21–2.15) | |||
| Presence of RAS/RAF mutation | 0.574 | |||
| No | 1 | |||
| Yes | 1.13 (0.74–1.72) | |||
| Microsatellite Status | 0.517 | |||
| MSS | 1 | |||
| MSI-H | 2.09 (0.28–15.40) | |||
| pT stage | 0.018 | 0.072 | ||
| ypT1-T2 | 1 | 1 | ||
| ypT3-T4 | 2.02 (1.07–3.82) | 2.33 | ||
| pN stage | 0.109 | 0.053 | ||
| ypN0 | 1 | 1 | ||
| ypN+ | 1.43 (0.92–2.24) | 1.74 | ||
| Grade | 0.259 | |||
| 1–2 | 1 | |||
| 3–4 | 1.41 (0.79–2.53) | |||
| Differentiation | 0.626 | |||
| Good and Int | 1 | |||
| Poor | 0.82 (0.35–1.89) | |||
| Tumor Regression Score | 0.066 | |||
| 0–1 | 1 | |||
| 2–3 | 1.57 (0.97–2.52) | |||
| Presence of metastases in One Liver Segment | 0.005 | |||
| Yes | 1 | |||
| No | 1.91 (1.21–3.00) | |||
| Number of Metastases | 0.007 | 0.002 | ||
| ≤3 | 1 | 1 | ||
| >3 | 2.01 (1.24–3.28) | 2.65 | ||
| Largest diameter of Liver metastases | 0.374 | |||
| <30 mm | 1 | |||
| ≥30 mm | 1.22 (0.79–1.86) | |||
| Objective Response | 0.229 | |||
| Absence | 1 | |||
| Presence | 1.67 (0.77–3.63) | |||
| Time to surgery | 0.116 | |||
| <6 months | 1 | |||
| ≥6 months | 1.41 (0.92–2.17) | |||
| Chemotherapy | 0.053 | |||
| Doublet | 1 | 1 | ||
| Doublet + Anti-EGFR | 0.97 (0.44–2.13) | 1.04 | 0.927 | |
| Doublet + Anti-VEGF | 1.84 (0.90–3.77) | 2.22 | 0.056 | |
| Radiotherapy | 0.196 | |||
| Not Received | 1 | |||
| Received | 0.58 (0.25–1.32) | |||
| Adjuvant Chemotherapy | 0.934 | |||
| Not Received | 1 | |||
| Received | 0.97 (0.50–1.88) |
| Univariate Analyses | Multivariate Analyses | |||
|---|---|---|---|---|
| HR (95% Cl) | p | HR (95% CI) | p | |
| Age | 0.587 | |||
| <65 | 1 | |||
| ≥65 | 0.83 (0.42–1.66) | |||
| Gender | 0.852 | |||
| Female | 1 | |||
| Male | 0.95 (0.54–1.65) | |||
| ECOG | 0.873 | |||
| 0 | 1 | |||
| 1 | 0.96 (0.55–1.66) | |||
| Localization | 0.834 | |||
| Right colon | 1 | |||
| Left colon | 0.88 (0.44–1.77) | |||
| Rectum | 0.81 (0.41–1.60) | |||
| KRAS Mutation | 0.398 | |||
| No | 1 | |||
| Yes | 1.27 (0.74–2.18) | |||
| NRAS Mutation | 0.793 | |||
| No | 1 | |||
| Yes | 0.83 (0.20–3.42) | |||
| BRAF Mutation | 0.043 | |||
| No | 1 | |||
| Yes | 0.46 (0–21.85) | |||
| Presence of RAS/RAF mutation | 0.551 | |||
| No | 1 | |||
| Yes | 1.18 (0.69–2.03) | |||
| Microsatellite Status | 0.407 | |||
| MSS | 1 | |||
| MSI-H | 0.05 (0–125.044) | |||
| pT stage | 0.014 | 0.040 | ||
| ypT1-T2 | 1 | 1 | ||
| ypT3-T4 | 2.45 (1.10–5.44) | 12.2 (1.45–16.87) | ||
| pN stage | 0.391 | |||
| ypN0 | 1 | |||
| ypN+ | 1.28 (0.73–2.23) | |||
| Perineural Invasion | 0.264 | |||
| No | 1 | |||
| Yes | 1.42 (0.77–2.61) | |||
| Lymphovascular Invasion | 0.625 | |||
| No | 1 | |||
| Yes | 0.86 (0.47–1.58) | |||
| Grade | 0.044 | |||
| 1–2 | 1 | |||
| 3–4 | 2.14 (1.05–4.36) | |||
| Differentiation | 0.162 | |||
| Good and Intermediate | 1 | |||
| Poor | 1.95 (0.82–4.62) | |||
| Tumor Regression Score | 0.015 | |||
| 0–1 | 1 | |||
| 2–3 | 2.18 (1.17–4.08) | |||
| Presence metastases in One Liver Segment | 0.075 | |||
| No | 1 | |||
| Yes | 1.66 (0.94–2.91) | |||
| Number of Metastases | 0.181 | |||
| ≤3 | 1 | |||
| >3 | 1.54 (0.83–2.84) | |||
| Largest diameter of liver metastases | 0.897 | |||
| <30 mm | 1 | |||
| ≥30 mm | 1.04 (0.60–1.80) | |||
| Objective Response | 0.042 | 0.023 | ||
| Absence | 1 | 1 | ||
| Presence | 0.36 (0.16–0.86) | 0.12 (0.08–0.74) | ||
| Time to surgery | 0.134 | |||
| <6 months | 1 | |||
| ≥6 months | 1.52 (0.88–2.62) | |||
| Chemotherapy | 0.231 | |||
| Doublet | 1 | |||
| Doublet + Anti-EGFR | 0.87 (0.35–2.18) | |||
| Doublet + Anti-VEGF | 1.53 (0.67–3.49) | |||
| Radiotherapy | 0.164 | |||
| Not Received | 1 | |||
| Received | 0.49 (0.18–1.33) | |||
| Adjuvant Chemotherapy | 0.682 | |||
| Not Received | 1 | |||
| Received | 1.19 (0.51–2.80) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceylan, F.; Aktürk Esen, S.; Ünal, O.Ü.; Aslan, F.; Onur, İ.D.; Ateş, Ö.; Demirciler, E.; Ünek, İ.T.; Gülmez, A.; Özen Engin, E.; et al. Long-Term Outcomes of Patients Undergoing Conversion Surgery After Induction Chemotherapy: Turkish Oncology Group Study. Medicina 2025, 61, 776. https://doi.org/10.3390/medicina61050776
Ceylan F, Aktürk Esen S, Ünal OÜ, Aslan F, Onur İD, Ateş Ö, Demirciler E, Ünek İT, Gülmez A, Özen Engin E, et al. Long-Term Outcomes of Patients Undergoing Conversion Surgery After Induction Chemotherapy: Turkish Oncology Group Study. Medicina. 2025; 61(5):776. https://doi.org/10.3390/medicina61050776
Chicago/Turabian StyleCeylan, Furkan, Selin Aktürk Esen, Olçun Ümit Ünal, Ferit Aslan, İlknur Deliktaş Onur, Öztürk Ateş, Erkut Demirciler, İlkay Tuğba Ünek, Ahmet Gülmez, Esra Özen Engin, and et al. 2025. "Long-Term Outcomes of Patients Undergoing Conversion Surgery After Induction Chemotherapy: Turkish Oncology Group Study" Medicina 61, no. 5: 776. https://doi.org/10.3390/medicina61050776
APA StyleCeylan, F., Aktürk Esen, S., Ünal, O. Ü., Aslan, F., Onur, İ. D., Ateş, Ö., Demirciler, E., Ünek, İ. T., Gülmez, A., Özen Engin, E., Taş, S., Gököz Doğu, G., Şimşek, M., Türk, H. M., İnal, A., Şahin, G., Yüksel, H. Ç., Tenekeci, A. K., Hızal, M., ... Uncu, D. (2025). Long-Term Outcomes of Patients Undergoing Conversion Surgery After Induction Chemotherapy: Turkish Oncology Group Study. Medicina, 61(5), 776. https://doi.org/10.3390/medicina61050776